3.2. Scanning Electron Microscopy
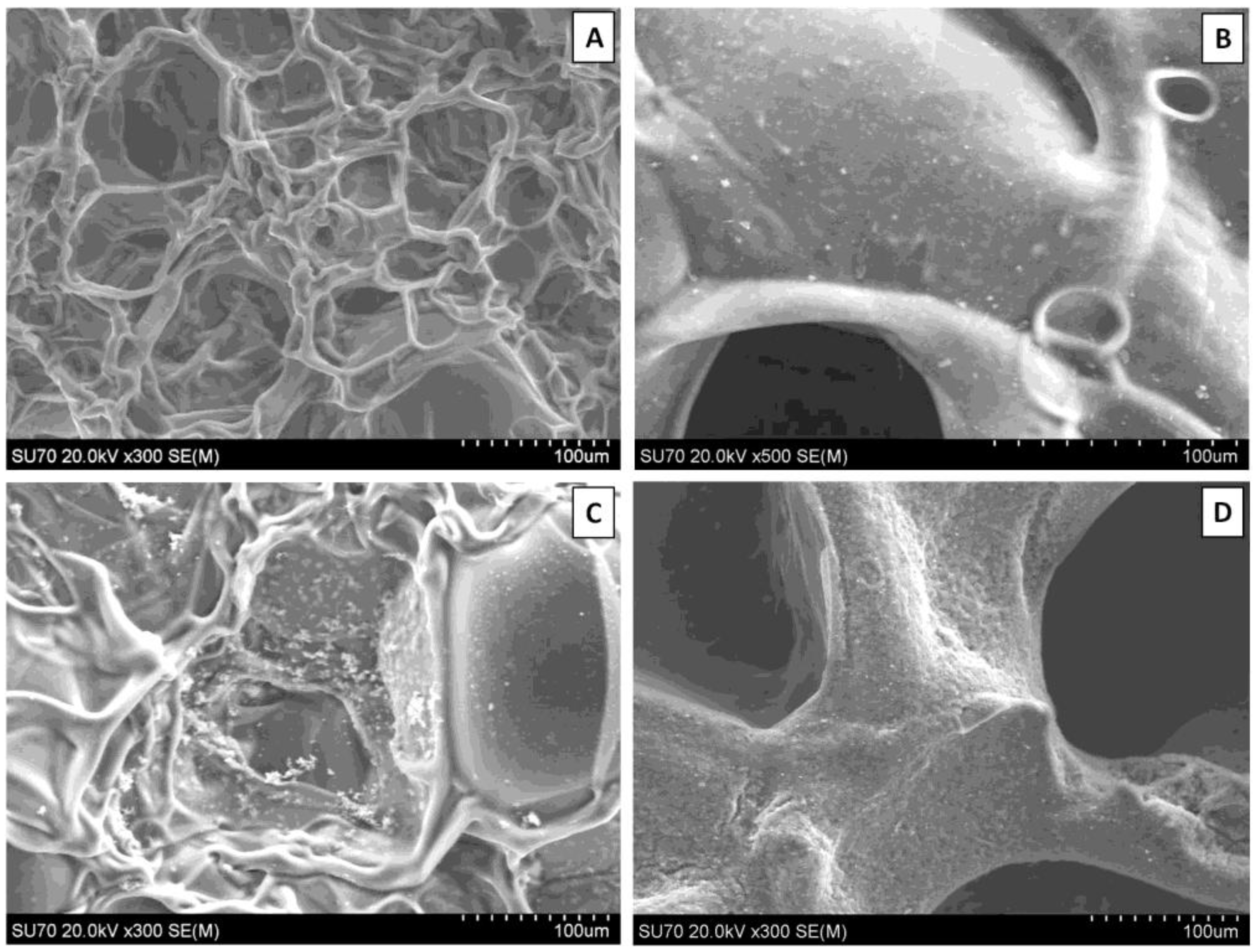
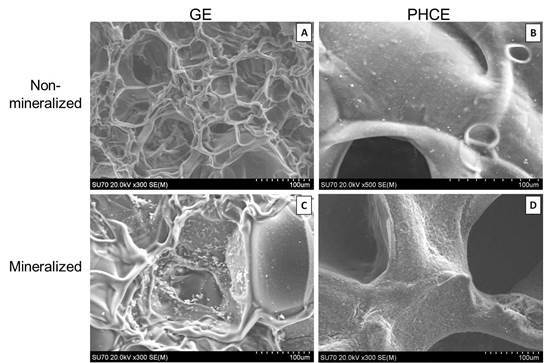
SEM revealed surface differences between GE and PHCE scaffolds, as well as mineralized and non-mineralized scaffolds (
Figure 2). GE scaffolds appear to have a smoother material surface with smaller pores while PHCE scaffolds contain a rougher surface with larger pores. Since GE scaffolds are only composed of gelatin, it was expected that they exhibit a more uniform surface composition. The addition of growth factors and nanofillers for PHCE scaffolds altered the scaffold characteristics by having a presence of the added molecules on the surface as well as a larger pore size. It is hypothesized that the small spheres and rougher surface visualized in
Figure 2(B) is attributed to the incorporated nanofillers (HA and CW) present on or near the surface of the scaffold. After mineralization, GE-M scaffolds showed small clusters of mineral nucleation while PHCE-M scaffolds exhibited a more uniform attraction. One of the aims of this study is to evaluate differences between mineralized and non-mineralized scaffold protein release, mechanical properties, and cellular response to understand any added benefit to the nucleation of crystals to the scaffold surface.
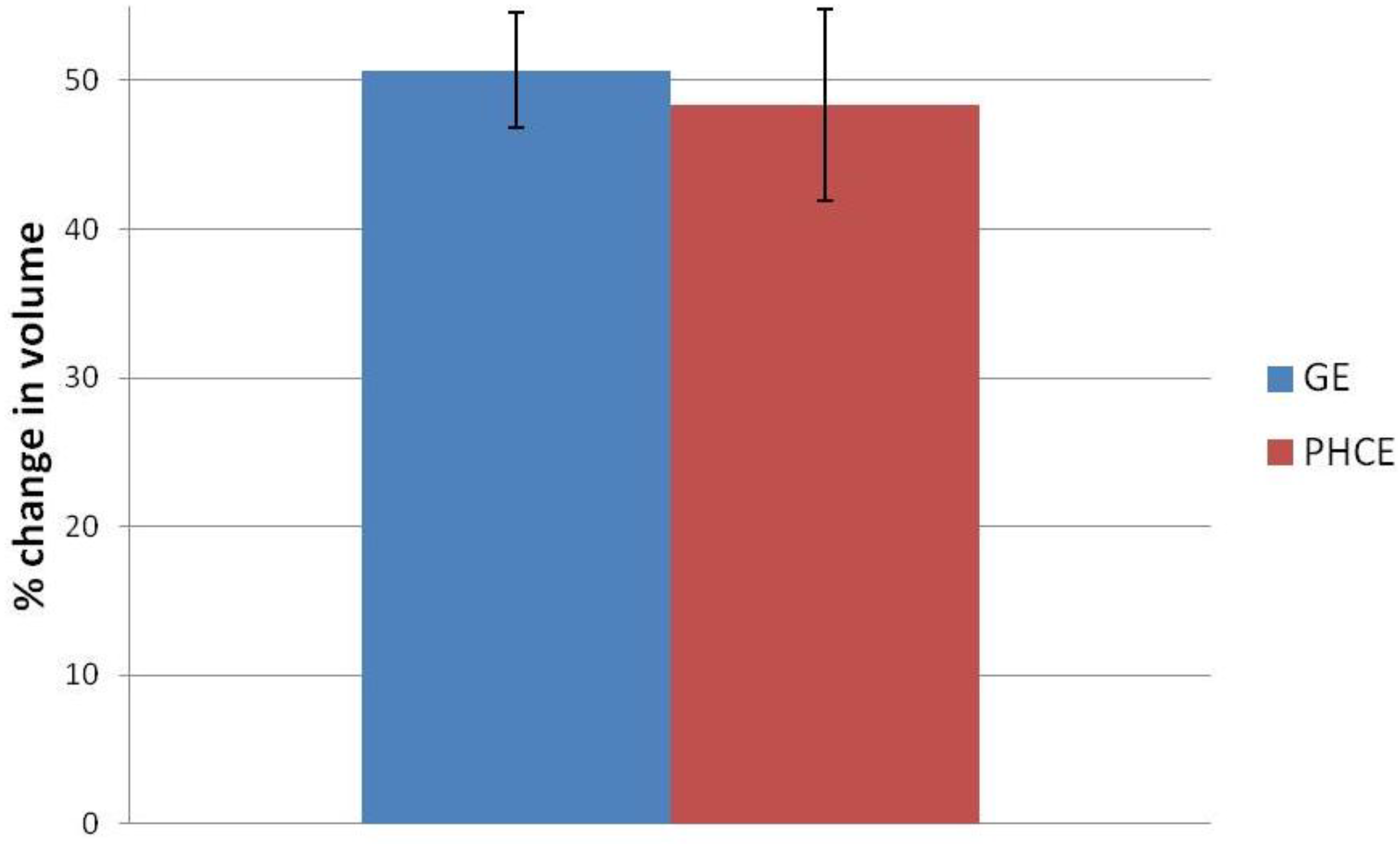
Figure 1.
Swelling percentage of gelatin+EDC (GE) and PRGF+HA+CW+EDC (PHCE) scaffolds upon hydration. All scaffolds swelled up to approximately 50% of their original volume with no statistical difference between compositions.
Figure 1.
Swelling percentage of gelatin+EDC (GE) and PRGF+HA+CW+EDC (PHCE) scaffolds upon hydration. All scaffolds swelled up to approximately 50% of their original volume with no statistical difference between compositions.
Figure 2.
Scanning electron microscope (SEM) images of (A) GE, (B) PHCE, (C) GE-M, and (D) PHCE-M scaffold surfaces (Scale bars at 100 µm). Non-mineralized GE sponges (A) had smaller pores and a more uniform/smooth surface compared to PHCE sponges (B) which exhibited larger pores and a coarser surface as a result of the incorporated nanofillers. GE scaffolds sparsely nucleated crystals upon mineralization (C) while PHCE scaffolds uniformly nucleated a layer of minerals on the surface (D).
Figure 2.
Scanning electron microscope (SEM) images of (A) GE, (B) PHCE, (C) GE-M, and (D) PHCE-M scaffold surfaces (Scale bars at 100 µm). Non-mineralized GE sponges (A) had smaller pores and a more uniform/smooth surface compared to PHCE sponges (B) which exhibited larger pores and a coarser surface as a result of the incorporated nanofillers. GE scaffolds sparsely nucleated crystals upon mineralization (C) while PHCE scaffolds uniformly nucleated a layer of minerals on the surface (D).
3.4. Scaffold Protein Release
In this study, the Pierce BCA assay was used to quantify amounts of general protein released from the lyophilized composite gelatin sponges into solution. This data provides some insight into scaffold degradation in that as the scaffold degrades, it releases its protein contents.
In order to understand the degree to which the scaffold is breaking down, we first must know the original total protein content of the 6 mm discs. The original total protein of each disc was quantified by allowing non-cross-linked gelatin (G) and gelatin+PRGF+HA+CW (PHC) scaffolds to fully degrade in PBS. There was no statistical difference (p < 0.05) between the average original total protein content of the G and PHC scaffolds (
Figure 4). It was expected that the PRGF containing scaffolds would record higher initial protein values since it was an additive to the existing gelatin solution. However, the small amounts of PRGF added (3.33 mg/mL) may not be enough protein to record detectable differences when compared to the majority component of gelatin (30 mg/mL). It was hypothesized that if a higher concentration of PRGF was added to the scaffolds, then a detectable increase in protein content would be observed.
Figure 4.
Original total protein content of non-cross-linked scaffolds. All scaffolds reported statistically similar values or original protein incorporation.
Figure 4.
Original total protein content of non-cross-linked scaffolds. All scaffolds reported statistically similar values or original protein incorporation.
Knowing that the G and PHC scaffolds contain the same amount of total protein, we can now directly compare GE, GE-M, PHCE, and PHCE-M release kinetics over 21 days without standardization.
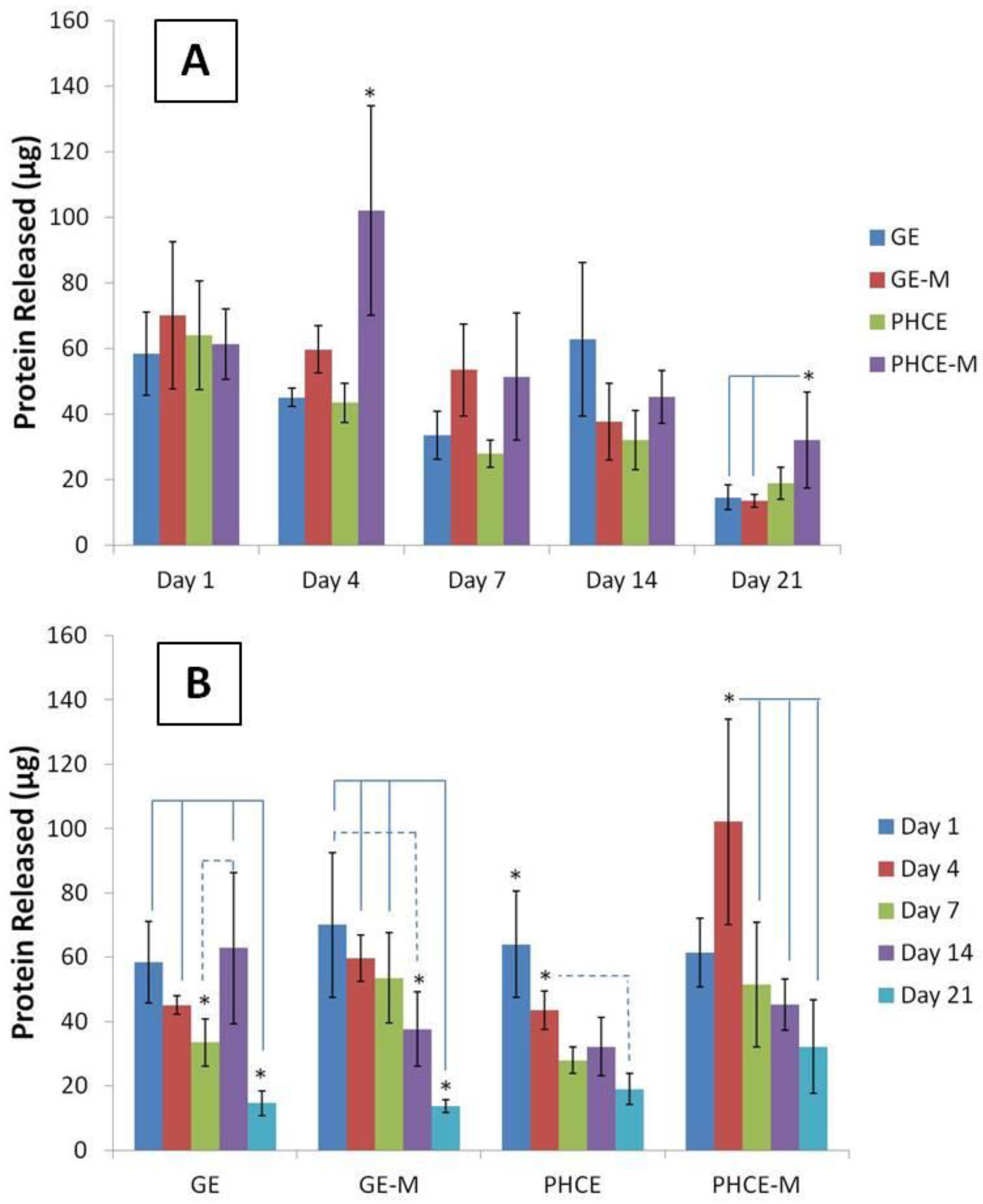
Figure 5(A) displays scaffold protein release quantification over 21 days such that a comparison can be made between which scaffolds released the most protein per time point. Interestingly, all scaffold types released similar amounts of protein (no significant difference, p < 0.05) on days 1, 7, and 14. On the other hand, PHCE-M scaffolds released significantly more protein (p < 0.05) on days 4 and 21 when compared to GE, GE-M, PHCE and GE, GE-M scaffolds, respectively. This data suggests that PHCE-M scaffolds were degrading and releasing their contents at a faster rate than all other scaffolds, particularly on days 4 and 21.
Figure 5(B) contains the same data, but is displayed to examine the protein release kinetics of a given scaffold composition over 21 days incubation in PBS. For GE scaffolds, there was a significant decrease (p < 0.05) in release on day 21 when compared to days 1, 4, and 14. However, a significant increase (p < 0.05) in release was observed between days 7 and 14. GE-M scaffolds exhibited a similar significant decrease (p < 0.05) in release on day 21 when compared to days 1, 4, and 7. Day 14 release from GE-M was also significantly lower than day 1. This suggests that most of the release of GE-M scaffolds occurs within the first 7 days. For PHCE scaffolds, day 1 release was significantly higher (p < 0.05) than all other time points. Day 4 PHCE release was also significantly higher (p < 0.05) than day 21. This data supports that there is a bulk release of PHCE scaffolds on day 1, followed by sustained release of protein at lower doses. For PHCE-M scaffolds, protein release on day 4 was significantly higher (p < 0.05) than on days 7, 14, and 21. These results suggest that for PHCE-M scaffolds, there is normal release on day 1, while day 4 releases more compared to the following time points. Overall, these results show that each scaffold has its own protein release profile which indicates that mineralization can alter the scaffold degradation.
Figure 5.
Comparison of protein released (A) by scaffolds at a given time point and (B) over time, specific to each scaffold. PHCE sponges released significantly more protein on days 4 and 21 compared to all other scaffolds. Overall, protein released from all scaffolds significantly decreased by day 21 suggesting the majority of release occurs at earlier time points.
Figure 5.
Comparison of protein released (A) by scaffolds at a given time point and (B) over time, specific to each scaffold. PHCE sponges released significantly more protein on days 4 and 21 compared to all other scaffolds. Overall, protein released from all scaffolds significantly decreased by day 21 suggesting the majority of release occurs at earlier time points.
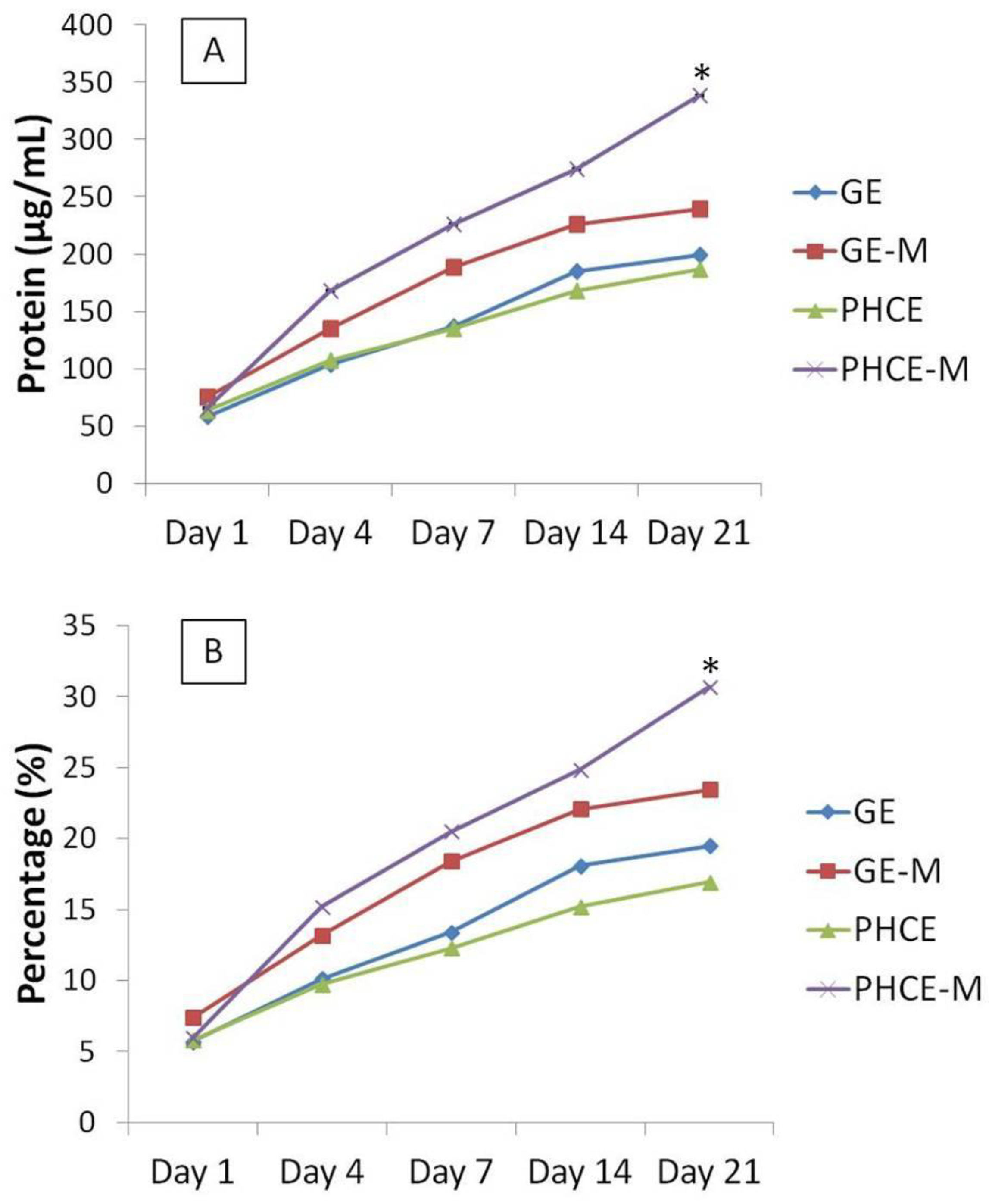
The cumulative protein release as a concentration and percent of total protein released from each scaffold was summed and graphed (
Figure 6). These graphs illustrate that starting on day 4, PHCE-M releases more protein overall compared to all other scaffold types. Specifically looking at
Figure 6(B), we are able to demonstrate that PHCE-M scaffolds are releasing 5% of its total protein content between days 14 and 21 while all other scaffold types are only releasing approximately 1%. This suggests that PHCE-M scaffolds are capable of sustained release of high quantities of protein even at later time points.
In order to gain a relative understanding of protein quantity released by each scaffold, day 21 cumulative released amounts were compared to non-cross-linked original total protein content to determine the percentage of protein released (
Figure 6(B)). GE, GE-M, PHCE, and PHCE-M released 19.5%, 21.4%, 16.9%, 30.7%, respectively. Cumulative protein release from PHCE-M scaffolds was significantly higher (p < 0.05) than all other scaffolds. GE-M cumulative release was also significantly higher (p < 0.05) than only PHCE release. These results show that PHCE-M scaffolds are capable of 30.7% release of its incorporated protein within the first 21 days and that mineralization of PHCE scaffolds increases protein release. The increase in mineralized scaffold protein release could be attributed to the gaps that form during mineralization between the inorganic particles and the polymer matrix which can provide channels for the penetration of water [
29]. Continuous elevated sustained release of protein is ideal for tissue engineering scaffolds, rendering PHCE-M scaffolds a viable candidate for tissue regeneration constructs.
Figure 6.
Cumulative protein release measured as (A) concentration and (B) percent of total protein from scaffolds over 21 days. PHCE-M sponges released the most protein from day 14 to 21. The continuous release of more protein by PHCE-M scaffolds resulted in a significantly higher percentage of total released contents (30.7%).
Figure 6.
Cumulative protein release measured as (A) concentration and (B) percent of total protein from scaffolds over 21 days. PHCE-M sponges released the most protein from day 14 to 21. The continuous release of more protein by PHCE-M scaffolds resulted in a significantly higher percentage of total released contents (30.7%).
3.5. Growth Factor Analysis
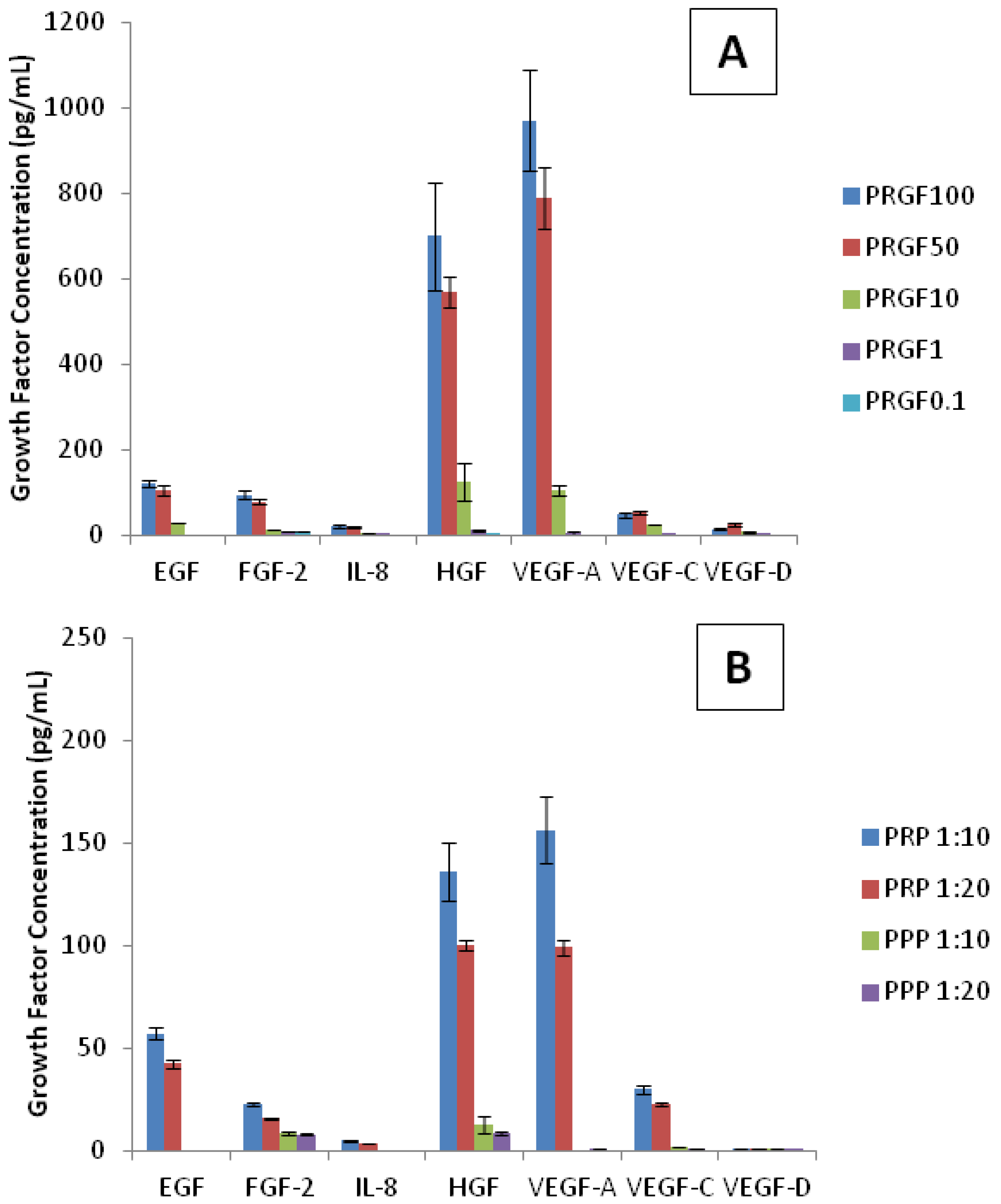
The previous generic protein release studies were conducted to gain an understanding of how the scaffolds degrade and release their inherent proteins. The aim of this section was to determine what specific proteins were incorporated within the scaffolds and subsequently present in the releasate. First, understanding which growth factors are present in liquid PRP and PRGF (lyophilized PRP) are important when analyzing cellular responses to scaffolds. Although platelet poor plasma (PPP) was not used in this study, a growth factor analysis of PPP was performed to understand the composition and difference between PRP and PPP. In this study, a PRGF, PRP, and PPP growth factor profile curve was obtained from dilutions of pure PRGF (100, 50, 10, 1, and 0.1 mg/mL), PRP (1:10 and 1:20), and PPP (1:10 and 1:20) in DI water. Although these profiles do not encompass all growth factors present in PRGF, PRP, and PPP, it does provide an analysis of the several key factors (
Figure 7). Of the analytes used, only two growth factors (G-CSF and FGF-1) were not detected and therefore not present in PRGF, PRP, or PPP. The highest concentrations of growth factors detected in PRGF and PRP were HGF and VEGF-A. Lower amounts of EGF, FGF-2, and VEGF-C were also present. IL-8 and VEGF-D were detected at even lower concentrations. As expected, the dilutions of PRGF and PRP resulted in low recorded values for all growth factors. However, PRGF (100 and 50 mg/mL) and PRP (1:10 and 1:20) provide a strong profile of growth factors present. It was expected that PRGF and PRP report the same growth factor profile since PRGF is a lyophilized version of PRP. This demonstrates that of the factors analyzed, PRP lyophilization does not affect the growth factors present in PRGF. It was noticed that the concentrations for each growth factor within PRP (1:10 and 1:20) more closely resemble the PRGF values at 10 mg/mL dilution. Since PRP is a liquid, it is measured in volume when administered clinically and experimentally. Alternatively, PRGF is measured as a weight/volume concentration since it is a powdered substance. This analysis suggests that the concentrations of growth factors present in a 1:10 and 1:20 dilution of PRP is comparable that found in 10 mg/mL of PRGF.
Table 1 presents the mean value of all growth factor concentrations within PRGF, PRP, and PPP dilutions with PRP (1:10 and 1:20) and PRGF (10 mg/mL) highlighted to show the comparison of similar growth factor concentrations. As previously mentioned, PRGF components have positive effects on angiogenesis and bone remodeling both
in vivo and
in vitro. PPP was not expected to contain high concentrations of growth factors since there are a low number of platelets. The analysis of PPP dilutions revealed the low presence of FGF-2, HGF, VEGF-A, VEGF-C, and VEGF-D. The growth factor profile of PPP (1:10) more closely resembles that of PRGF (1 and 0.1 mg/mL), both highly diluted solutions. The PPP growth factor analysis profile is also presented in
Table 1.
Table 1.
Average growth factor concentration (pg/mL) in preparations rich in growth factors (PRGF), platelet-rich plasma (PRP), and platelet-poor plasma (PPP) dilutions. The average of the lowest standard is presented as the minimum level of detection (Min Detection). 6 mm PHC discs reported traces of epidermal growth factor (EGF). All other factors were present in low undetectable concentrations.
Table 1.
Average growth factor concentration (pg/mL) in preparations rich in growth factors (PRGF), platelet-rich plasma (PRP), and platelet-poor plasma (PPP) dilutions. The average of the lowest standard is presented as the minimum level of detection (Min Detection). 6 mm PHC discs reported traces of epidermal growth factor (EGF). All other factors were present in low undetectable concentrations.
| EGF | FGF-2 | IL-8 | HGF | VEGF-A | VEGF-C | VEGF-D |
|---|
| PRGF100 | 121.0 | 93.6 | 20.5 | 700.2 | 970.8 | 48.1 | 13.8 |
| PRGF50 | 105.3 | 78.0 | 19.3 | 569.2 | 789.8 | 51.9 | 25.6 |
| PRGF10 | 28.9 | 0.0 | 3.9 | 124.3 | 105.6 | 25.6 | 7.2 |
| PRGF1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| PRGF0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| PRP 1:10 | 57.3 | 22.8 | 4.9 | 135.8 | 156.4 | 30.1 | 0.0 |
| PRP 1:20 | 42.4 | 15.7 | 3.2 | 100.4 | 99.3 | 22.7 | 0.0 |
| PPP 1:10 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| PPP 1:20 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| PHC | 13.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Min Detection | 2.7 | 13.7 | 1.4 | 27.2 | 13.7 | 6.8 | 6.9 |
Figure 7.
Growth factor analysis of (A) PRGF, (B) PRP and PPP dilutions. The highest concentrations of growth factors detected in PRGF and PRP were hepatocyte growth factor (HGF) and vascular endothelial growth factor A (VEGF-A). As expected, dilutions of PRGF and PRP recorded lower values for all growth factors.
Figure 7.
Growth factor analysis of (A) PRGF, (B) PRP and PPP dilutions. The highest concentrations of growth factors detected in PRGF and PRP were hepatocyte growth factor (HGF) and vascular endothelial growth factor A (VEGF-A). As expected, dilutions of PRGF and PRP recorded lower values for all growth factors.
Multiplexer analysis of the uncross-linked PHC scaffolds provides the growth factor profile of the original loaded content of each scaffold. There were detectable amounts of only EGF in PHC scaffolds with a concentration ranging between PRGF 1 mg/mL and PRGF 10 mg/mL (
Table 1). The reported PHC scaffold concentrations of growth factors are expected since 3.33 mg/mL PRGF was used to fabricate PHCE scaffolds. These results confirm that PRGF can successfully be incorporated within lyophilized gelatin sponges and the presence/concentrations of growth factors (specifically EGF) are maintained. Multiplexer analysis of PHCE and PHCE-M scaffold release over 21 days showed no quantifiable traces of the above growth factors. It was expected that some growth factors would be detectable as the scaffold degraded and released its contents. This absence of measurable growth factors from scaffold release could be a result of a highly diluted sample since 6 mm discs were incubated in 1 mL of PBS to assess degradation. In this instance, growth factors released from the scaffold become diluted in the high volume of PBS which may result in no detection (as evident of the dilutions of pure PRGF in
Figure 7 and
Table 1). It is also possible that there was not enough PRGF originally incorporated within the PHCE scaffolds such that any release is in small amounts and therefore not detected by the multiplexer. On the other hand, it could be stated that there are no detectable growth factors in the releasate because PRGF is simply not being released. This would be a result of the PRGF components being sequestered by HA since HA has the ability to bind a variety of molecules and there are equal amounts of HA and PRGF [
30]. The non-release of PRGF can potentially be an added benefit in that the scaffold is able to maintain the incorporated growth factors and constantly provide a bioactive surface for cells to attach, proliferate, and remodel. Future studies are required and will focus on which of these situations is occurring with lyophilized gelatin-PRGF sponges. This will be conducted by degrading the scaffold in less solution (to remove the possibility of severe dilution) and by incorporating higher initial concentrations of PRGF.
3.6. Uniaxial Compression Testing
Uniaxial compression testing using a platen system was used to record peak load (N) and Young’s modulus (MPa) for compression of scaffolds incubated in media for up to 21 days. Day 4 of mechanical testing was not reported as the mechanical properties showed more significant changes weekly.
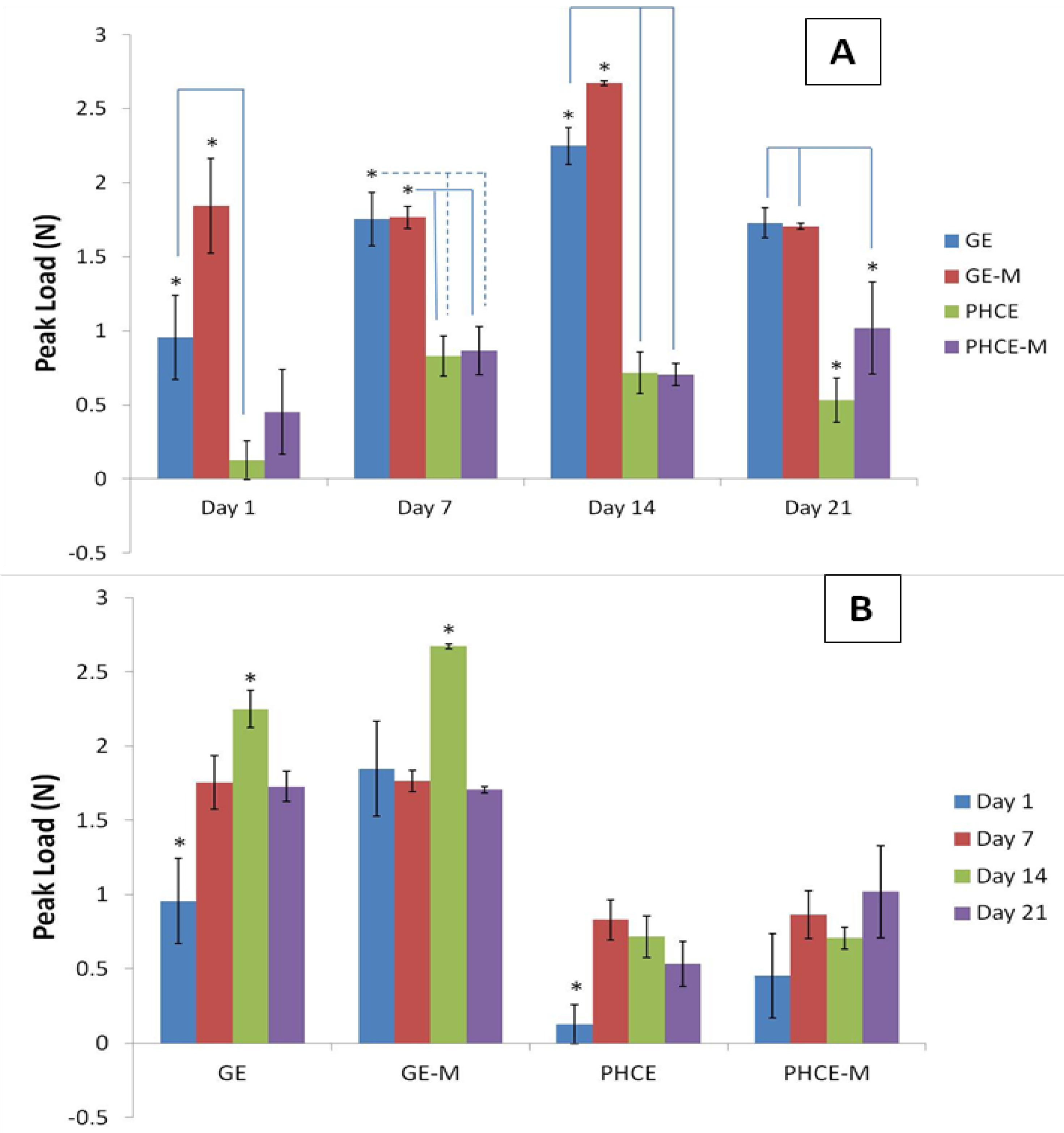
Figure 8(A) reports and analyzes the peak loads of each scaffold type at a given time point. At days 1 and 14, GE-M scaffolds exhibited significantly higher (p < 0.05) peak loads than all other scaffolds. On days 7 and 21, GE and GE-M had significantly higher (p < 0.05) peak values than PHCE and PHCE-M scaffolds. This data suggests that throughout 21 days, PHCE and PHCE-M scaffolds consistently report significantly lower (p < 0.05) peak load values (0.1–1.0 N) when compared to GE and GE-M scaffolds (1.0–2.7 N). It was expected that the sponges incorporated with nanofillers would exhibit higher mechanical properties. The authors speculate that the concentration of the added factors was too high to enhance the peak load of the sponges. Future studies are being considered where incrementing concentrations of nanofillers are added to scaffolds to determine if there is a threshold such that an access of nanofillers begins having adverse effects on mechanical properties. For the purpose of this study, the above results are acceptable and within the same magnitude of values comparable to properties found in the early stages of bone healing.
Figure 8.
Peak load of scaffolds (A) at a given time point and (B) over 21 days incubation in media. Over 21 days, PHCE and PHCE-M scaffolds reported lower peak load values than mineralized and non-mineralized gelatin sponges.
Figure 8.
Peak load of scaffolds (A) at a given time point and (B) over 21 days incubation in media. Over 21 days, PHCE and PHCE-M scaffolds reported lower peak load values than mineralized and non-mineralized gelatin sponges.
The same data was graphed with the axis reversed to compare peak values of a given scaffold throughout 21 days incubation (
Figure 8(B)). For GE scaffolds, day 1 and day 14 produced the significantly lowest and highest (p < 0.05) values, respectively. For GE-M scaffolds, incubation for 14 days in media produced the highest (p < 0.05) peak load value compared to all other days. Day 1 for PHCE scaffolds was significantly lower (p < 0.05) than all other days while PHCE-M scaffolds showed no significant changes (p < 0.05) in peak load throughout 21 days. With the exception of PHCE-M scaffolds, this data shows that scaffolds have lower peak loads at earlier time points (days 1 and 7) when compared to later time points (day 14).
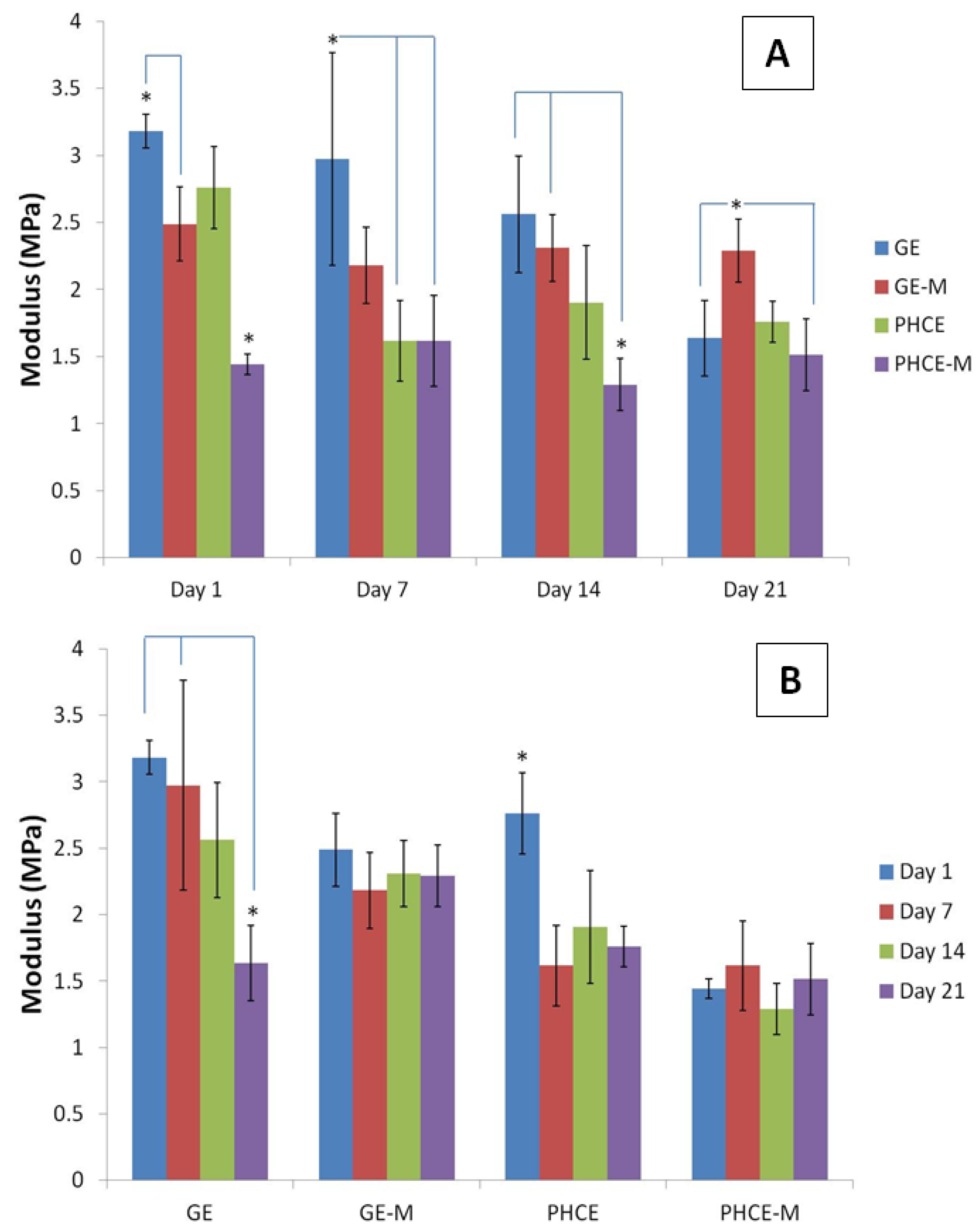
Figure 9(A) reports and analyzes the compressive modulus of each scaffold type at a given time point. After 1 day incubation, PHCE-M scaffolds had a significantly lower (p < 0.05) modulus than all other scaffold types. In fact, GE scaffolds had significantly higher (p < 0.05) values than GE-M and PHCE-M. For day 7, GE scaffolds reported a significantly higher (p < 0.05) modulus than both PHCE and PHCE-M scaffolds. After 14 days incubation, GE and GE-M scaffolds had significantly higher moduli when compared to PHCE-M scaffolds. Day 14 shows a higher modulus for GE-M scaffolds when compared to GE and PHCE-M scaffolds. Overall PHCE-M scaffolds report lower Young’s modulus values (1.3–1.6 MPa) when compared to GE and GE-M scaffolds (1.6–3.2 MPa). One aim of this study is to create a scaffold with similar mechanical properties to that of native bone healing tissue. The first stages of bone healing involve granulation tissue and hyaline cartilage (soft tissue) formation before any mineralization occurs. The Young’s modulus of cartilage in the literature is at similar low values of 0.5–0.8 MPa while mature bone and other materials are in the GPa range [
31]. Zhang
et al. report similar low compressive Young’s modulus values (0.3–3.0 MPa) for gelatin-hyaluronic acid composite freeze-dried sponges intended for soft tissue engineering applications [
28]. The low range of modulus values of these scaffolds provide a mechanical microenvironment similar to that found in the early stages of bone healing.
Evaluating statistical differences in Young’s modulus of a given scaffold over 21 days will provide a different viewpoint on how each scaffold’s properties change over time. To achieve this, the same modulus data was graphed with the legend and x-axis switched and statistics were performed (
Figure 9(B)). The modulus for GE and PHCE scaffolds significantly decreased (p < 0.05) after 21 days incubation in media while their mineralized counterparts showed no significant changes (p < 0.05) in modulus within the same time period. This suggests that mineralized scaffolds maintain their modulus throughout incubation while non-mineralized scaffolds show a decrease. It is also noticed from
Figure 9(A) that the initial modulus (Day 1) of mineralized scaffolds is significantly lower (p < 0.05) than non-mineralized scaffolds. This could attribute to the decrease in modulus as seen in non-mineralized scaffolds since they start off at higher values.
Figure 9.
Young’s modulus for compression of (A) scaffolds at a given time point and (B) a given scaffold over 21 days incubation in media. Overall PHCE-M scaffolds report lower Young’s modulus values when compared to GE and GE-M scaffolds.
Figure 9.
Young’s modulus for compression of (A) scaffolds at a given time point and (B) a given scaffold over 21 days incubation in media. Overall PHCE-M scaffolds report lower Young’s modulus values when compared to GE and GE-M scaffolds.
3.7. Cell Attachment and Infiltration
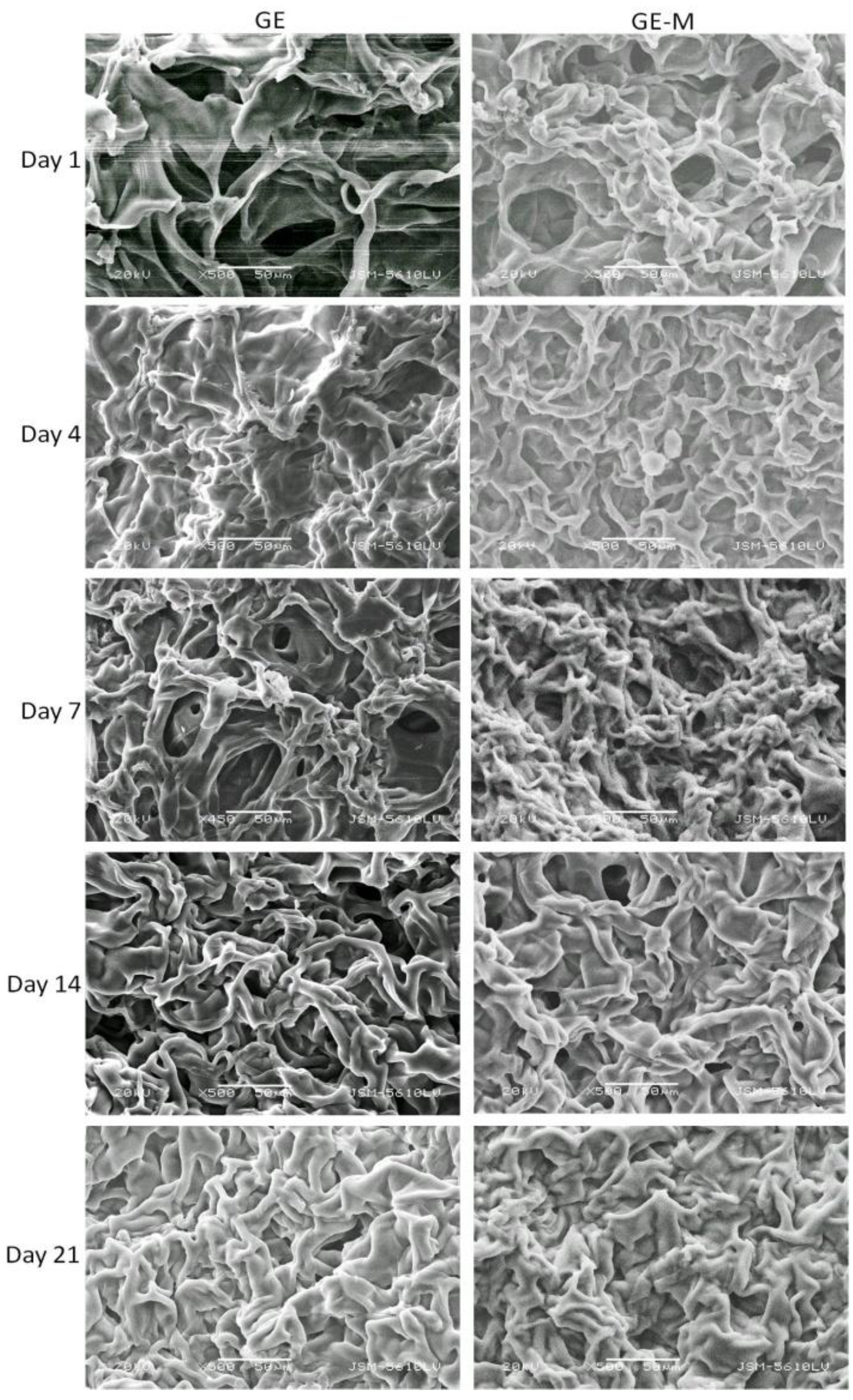
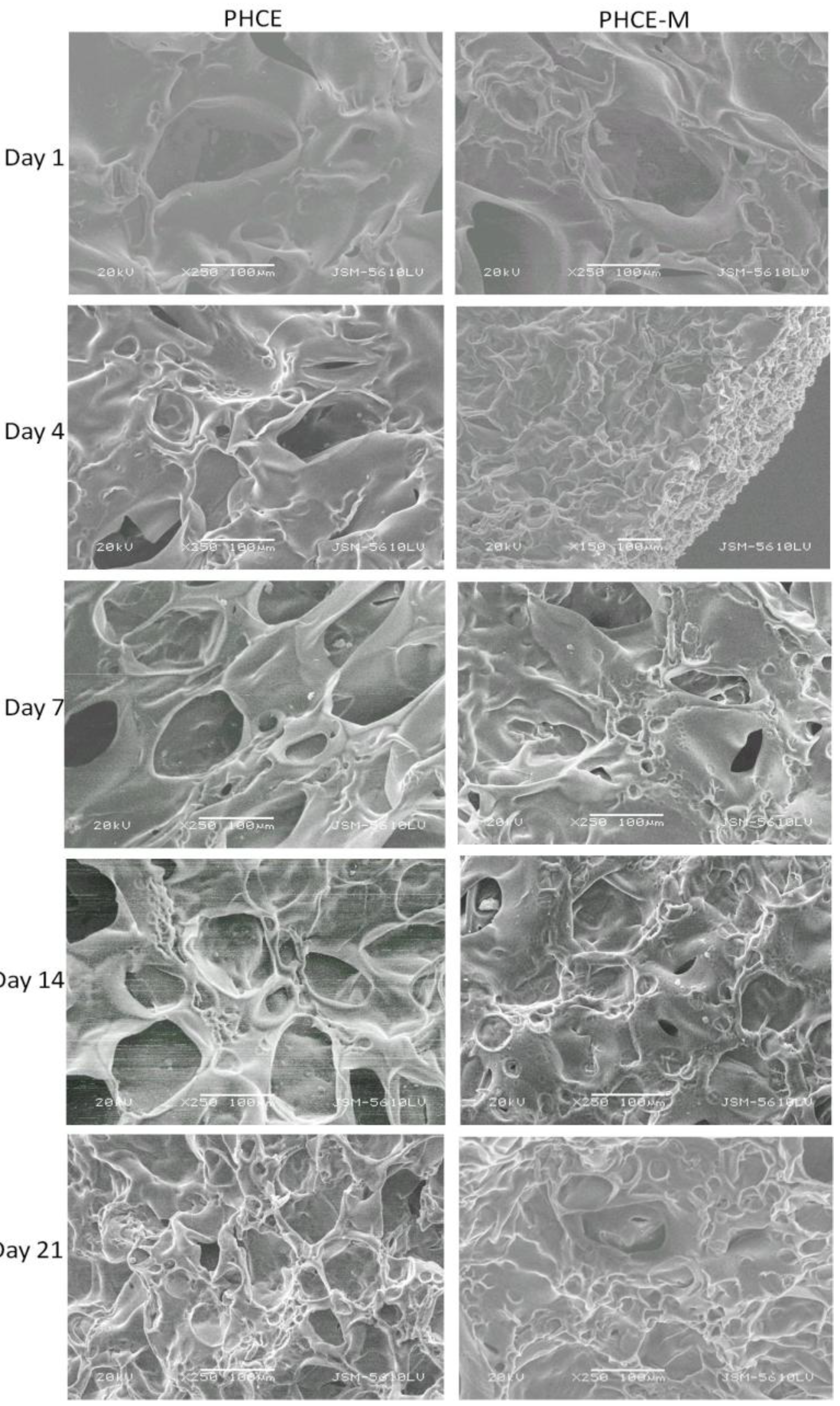
Scanning electron microscopy was performed on GE, GE-M, PHCE, and PHCE-M scaffolds after dynamic cell seeding and subsequent static culture for 1, 4, 7, 14, and 21 days (
Figure 10 and
Figure 11). Scaffolds were subjected to ethanol dehydration in order to image cellularized scaffolds. Ethanol dehydration is a procedure commonly used to prepare cellular scaffolds for imaging. However, since these sponges are hygroscopic in nature, dehydration caused the scaffolds to shrink upon preparation for imaging. Therefore, the images presented in this section are not representative of a fully hydrated swollen scaffold that cells are in contact with. Although this is the case, basic observations can still be made. One noticeable difference with the images presented is the visual appearance of no cells attached to any scaffolds at any time point. In the previous study, cells attached more as spheres and were easily visualized via SEM [
24]. It is plausible that the dynamic cell seeding technique caused the cells to attach to and integrate with the scaffold better than static cell seeding (where spheres of cells were visualized). It is also possible that there are more cells within the scaffold rather than on the surface, in which case will not be captured via SEM. Since mechanical stress is a factor for bone development, it has been shown that osteoblasts respond differently to mechanical stimuli when compared to a static environment [
32,
33]. Future studies could focus on the degree of cell attachment and infiltration into the scaffold under dynamic and static culture conditions. For GE, GE-M, PHCE, and PHCE-M scaffolds, it was also observed that the scaffold became less porous over 21 days. This could be due to the remodeling of the scaffold by the cells, however, more in depth cell experiments analyzing the surface and entire cellular scaffold will need to be conducted for any conclusive results.
Figure 10.
SEM of cellularized GE and GE-M scaffolds. No discernible cells on surface although scaffold becomes less porous over time. Scale bars at 50 µm.
Figure 10.
SEM of cellularized GE and GE-M scaffolds. No discernible cells on surface although scaffold becomes less porous over time. Scale bars at 50 µm.
Figure 11.
SEM of cellularized PHCE and PHCE-M scaffolds. No discernible cells on surface although scaffold becomes less porous over time. Scale bars at 100 µm.
Figure 11.
SEM of cellularized PHCE and PHCE-M scaffolds. No discernible cells on surface although scaffold becomes less porous over time. Scale bars at 100 µm.
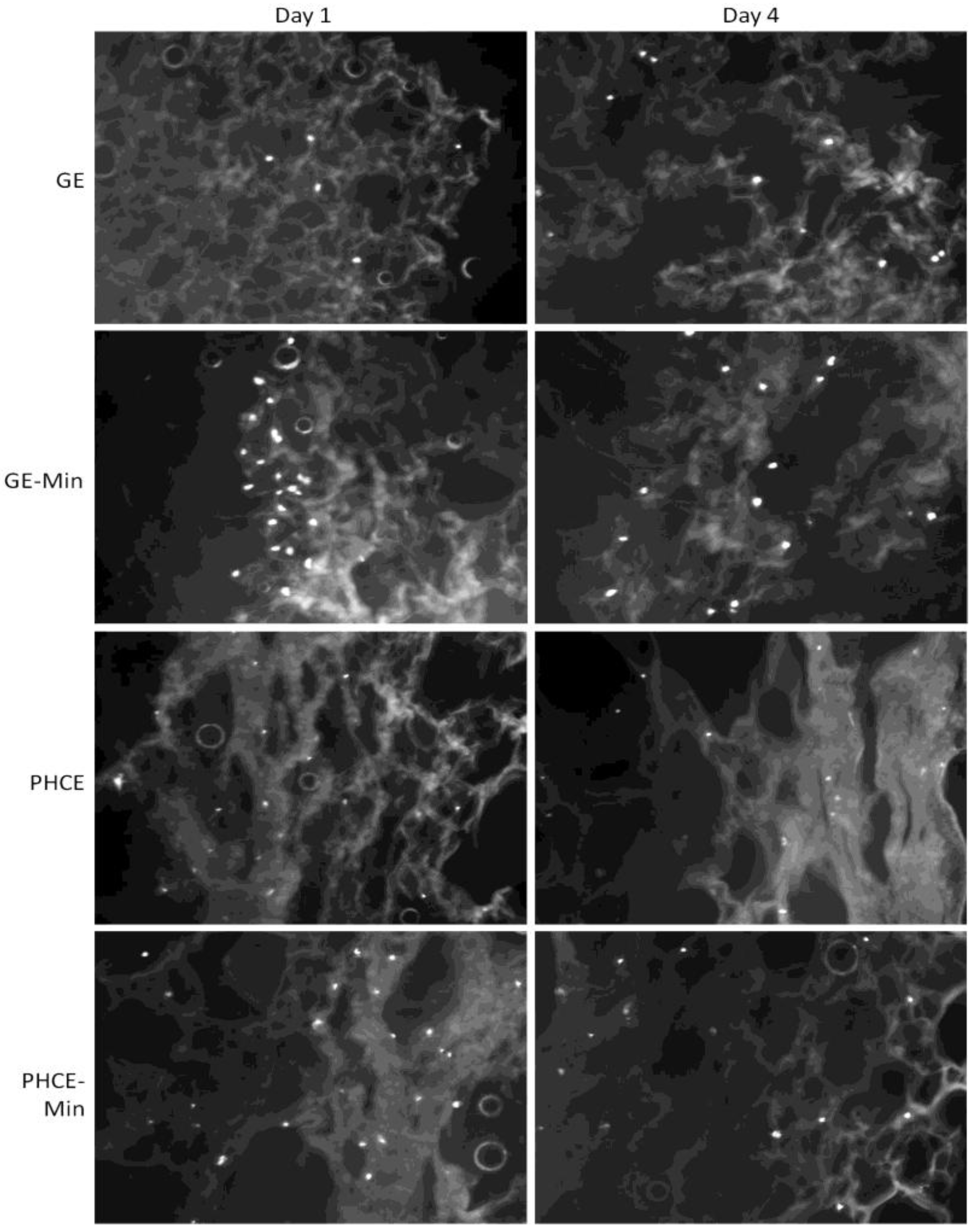
Cell attachment and more importantly, infiltration into all scaffolds, was analyzed via DAPI staining after dynamic seeding followed by static culture for 1 and 4 days (
Figure 12). The control GE scaffolds attached fewer cells than all other scaffold types, but did show appropriate cell infiltration after days 1 and 4. Cell infiltration into GE scaffolds is most likely attributed to the dynamic cell seeding method, since in the previous study, cells statically seeded on GE scaffolds primarily remained on the surface [
24]. A visual increase in cell attachment at the surface was observed for GE-M scaffolds suggesting that the minerals nucleated on the scaffold surface provide additional cell adhesion sites for osteoblasts. After 4 days in culture, cells were present throughout the entire scaffold indicating successful cell migration. Both PHCE and PHCE-M showed a large number of cells present throughout the scaffold on days 1 and 4. This increased cell attachment and infiltration was also reported for PHCE scaffolds in the previous study using the static cell seeding method [
24]. This suggests that PHCE scaffolds increase initial cell attachment and infiltration independent of the cell seeding technique. Although there is no visual difference of cell attachment or infiltration between PHCE and PHCE-M scaffolds, there is a noticeable increase in initial cell attachment and infiltration when compared to GE and GE-M scaffolds on day 1. To confirm the cell attachment and infiltration trends mentioned above, cell density per field of view (10× magnification) for each 60 μm thick section was calculated. On day 1, GE, GE-M, PHCE, and PHCE-M scaffolds attached 5, 23, 21, 26 cells/image, respectively. Although GE-M scaffolds had a comparable cell density to PHCE and PHCE-M, DAPI revealed that cells on GE-M sponges were restricted to the surface while PRGF containing sponges enhanced initial infiltration. After 4 days in culture, cell densities for GE, GE-M, PHCE, and PHCE-M were 10, 15, 17, and 15 cells/image, respectively. Although GE scaffolds had a lower cell density, all scaffolds promoted the infiltration of cells after 4 days in culture. As previously mentioned, imaging cell nuclei via DAPI staining becomes increasingly difficult the longer cells are cultured on sponges. This could attribute to the decrease in cell density noticed on day 4 for scaffolds with high initial densities. Even though cells on scaffolds are not clearly visible on the SEM images (
Figure 11), DAPI results confirm the presence and viability of cells throughout the scaffolds (
Figure 12).
Figure 12.
4′-6-diamidino-2-phenylindole (DAPI) staining of mineralized and non-mineralized GE and PHCE scaffolds after dynamic cell seeding and subsequent static culture for 1 and 4 days. Increases in cell attachment (GE-M) and attachment/infiltration (PHCE and PHCE-M) are observed. All images taken at 10× magnification.
Figure 12.
4′-6-diamidino-2-phenylindole (DAPI) staining of mineralized and non-mineralized GE and PHCE scaffolds after dynamic cell seeding and subsequent static culture for 1 and 4 days. Increases in cell attachment (GE-M) and attachment/infiltration (PHCE and PHCE-M) are observed. All images taken at 10× magnification.