Fluorometric In Situ Monitoring of an Escherichia coli Cell Factory with Cytosolic Expression of Human Glycosyltransferase GalNAcT2: Prospects and Limitations
Abstract
:1. Introduction
2. Material and Methods
2.1. Strain
2.2. Pre-Cultivation
2.3. Bioreactor Culture
2.4. Batch Cultivation with EnPresso® B Medium
2.5. Offline Analytics
2.6. Purification of Soluble Human Glycosyltransferase
2.7. Human Glycosyltransferase Activity Assay
2.8. Online Data Collection
2.9. Chemometric Modeling
3. Results and Discussion
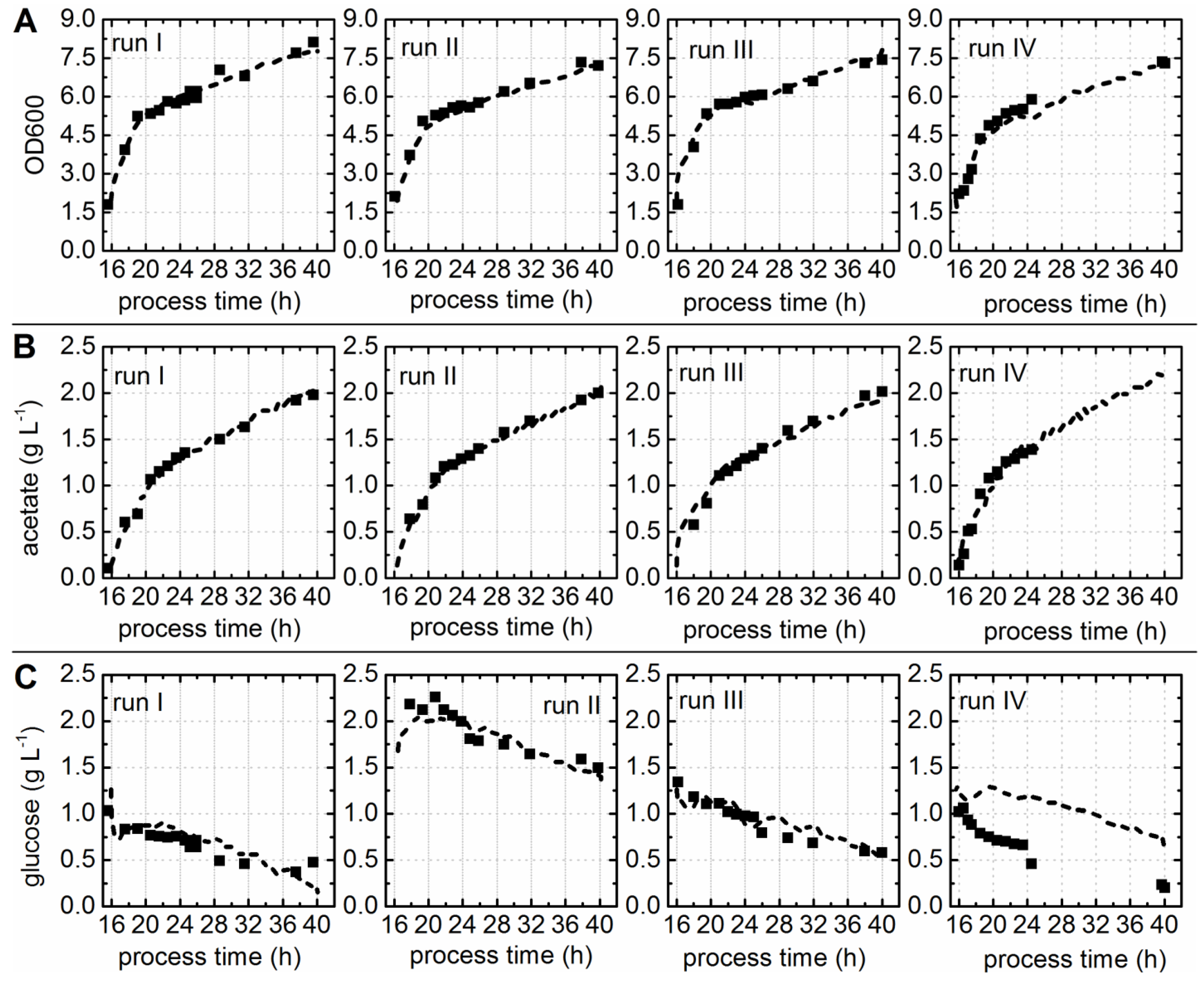
3.1. EnPresso® B Batch Cultivations and Chemometric Modeling of Process Parameters
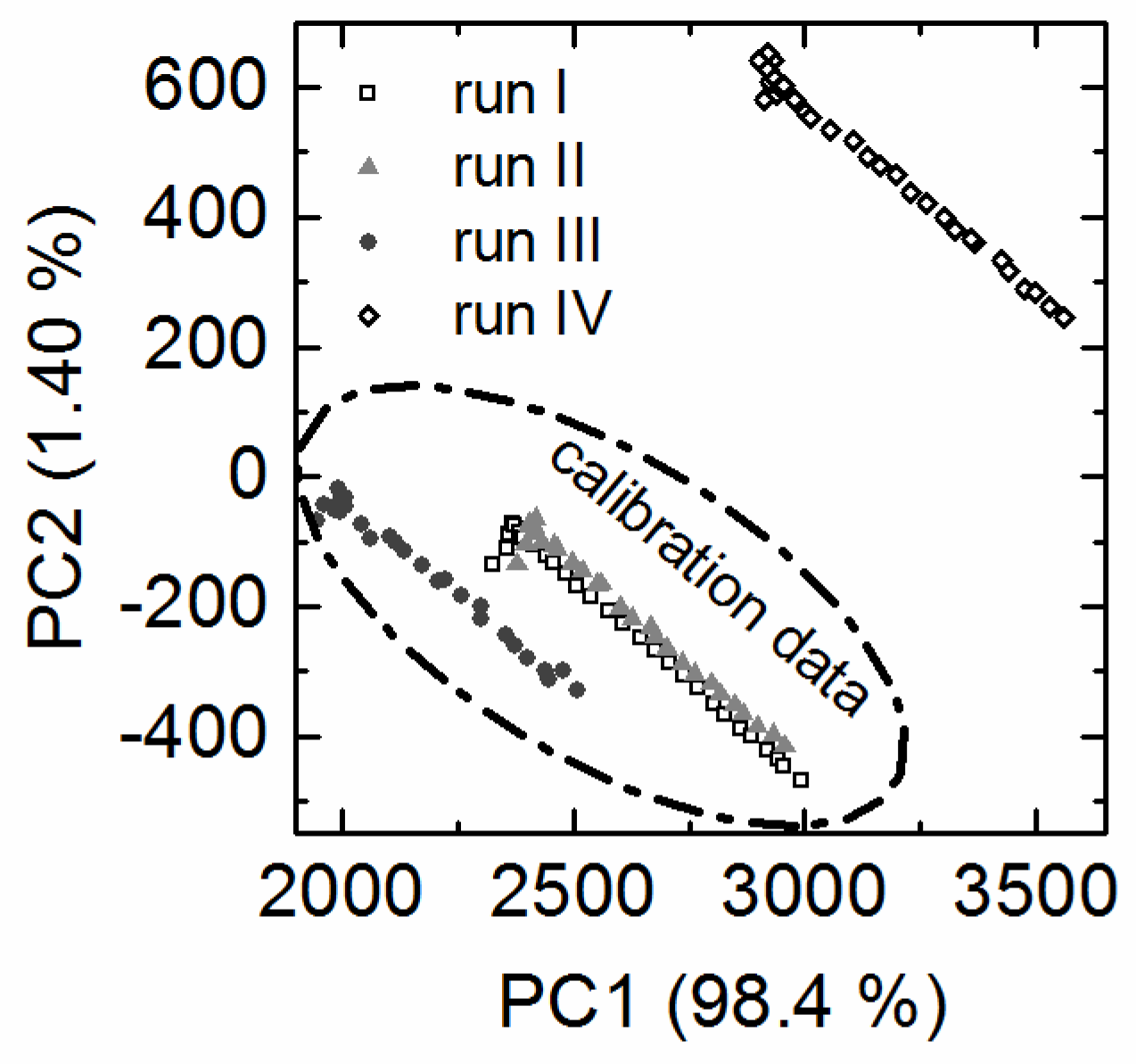
3.2. Overall Batch Behavior Evaluated by Principal Component Analysis
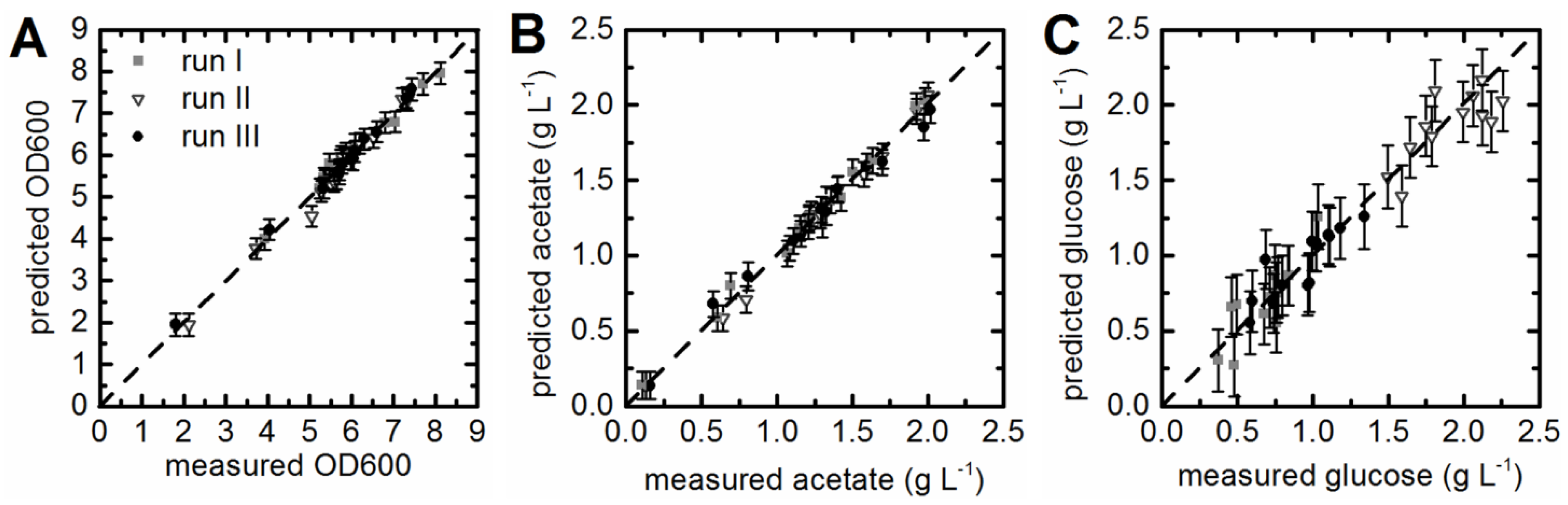
3.3. Prediction of Acetate Concentrations and Optical Density
3.4. Prediction of Substrate Concentrations
3.5. Chemometric Modeling of the Cell Factory’s Efficiency
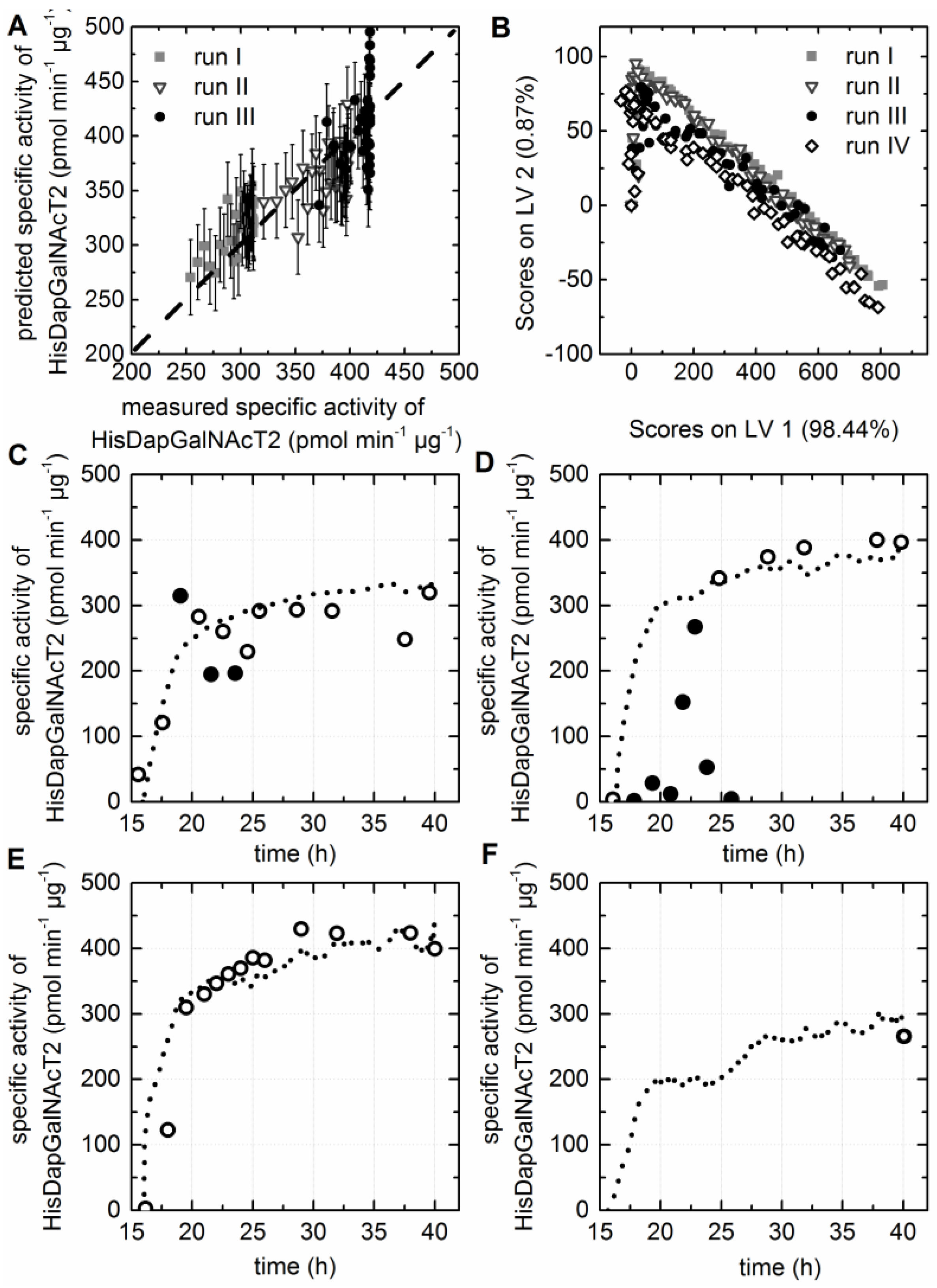
3.6. Prediction of Soluble Protein to Biomass Ratio
3.7. Prediction of the Specific Activity of the HisDapGalNAcT2
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lobstein, J.; Emrich, C.A.; Jeans, C.; Faulkner, M.; Riggs, P.; Berkmen, M. Shuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm. Microb. Cell Fact. 2012, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.W.; Ho, S.Y.; Hogg, P.J. Disulfide bond acquisition through eukaryotic protein evolution. Mol. Biol. Evol. 2011, 28, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Stock, J.; Rauch, B.; Roseman, S. Periplasmic space in salmonella typhimurium and Escherichia coli. J. Biol. Chem. 1977, 252, 7850–7861. [Google Scholar] [PubMed]
- Ritz, D.; Lim, J.; Reynolds, C.M.; Poole, L.B.; Beckwith, J. Conversion of a peroxiredoxin into a disulfide reductase by a triplet repeat expansion. Science 2001, 294, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.J.; Åslund, F.; Beckwith, J. Disulfide bond formation in the Escherichia coli cytoplasm: An in vivo role reversal for the thioredoxins. EMBO J. 1998, 17, 5543–5550. [Google Scholar] [CrossRef] [PubMed]
- Lauber, J.; Handrick, R.; Leptihn, S.; Dürre, P.; Gaisser, S. Expression of the functional recombinant human glycosyltransferase galnact2 in Escherichia coli. Microb. Cell Fact. 2015, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Hortsch, R.; Weuster-Botz, D. Growth and recombinant protein expression with Escherichia coli in different batch cultivation media. Appl. Microbiol. Biotechnol. 2011, 90, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.; van den Heuvel, J.; Zidek, N.; Rinas, U. Minimizing inclusion body formation during recombinant protein production in Escherichia coli at bench and pilot plant scale. Enzym. Microb. Technol. 2004, 34, 235–241. [Google Scholar] [CrossRef]
- Luchner, M.; Striedner, G.; Cserjan-Puschmann, M.; Strobl, F.; Bayer, K. Online prediction of product titer and solubility of recombinant proteins in Escherichia coli fed-batch cultivations. J. Chem. Technol. Biotechnol. 2015, 90, 283–290. [Google Scholar] [CrossRef]
- Cruz, M.V.; Sarraguça, M.C.; Freitas, F.; Lopes, J.A.; Reis, M.A.M. Online monitoring of P(3HB) produced from used cooking oil with near-infrared spectroscopy. J. Biotechnol. 2015, 194, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, C.; Marose, S.; Nielsen, H.O.; Scheper, T. 2-dimensional fluorescence spectroscopy for on-line bioprocess monitoring. Sens. Actuators B Chem. 1998, 51, 273–277. [Google Scholar] [CrossRef]
- Mazarevica, G.; Diewok, J.; Baena, J.R.; Rosenberg, E.; Lendl, B. On-line fermentation monitoring by mid-infrared spectroscopy. Appl. Spectrosc. 2004, 58, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Jayaraman, G.; Kökpinar, Ö.; Rinas, U.; Hitzmann, B. On-line monitoring of recombinant bacterial cultures using multi-wavelength fluorescence spectroscopy. Biochem. Eng. J. 2011, 58–59, 133–139. [Google Scholar] [CrossRef]
- Schwab, K.; Amann, T.; Schmid, J.; Handrick, R.; Hesse, F. Exploring the capabilities of fluorometric online monitoring on cho cell cultivations producing a monoclonal antibody. Biotechnol. Prog. 2016. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.P.; Portugal, C.A.M.; Carinhas, N.; Dias, J.M.L.; Crespo, J.P.; Alves, P.M.; Carrondo, M.J.T.; Oliveira, R. In situ 2D fluorometry and chemometric monitoring of mammalian cell cultures. Biotechnol. Bioeng. 2009, 102, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Mercier, S.M.; Diepenbroek, B.; Dalm, M.C.; Wijffels, R.H.; Streefland, M. Multivariate data analysis as a pat tool for early bioprocess development data. J. Biotechnol. 2013, 167, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Duggan, D.E.; Bowman, R.L.; Brodie, B.B.; Udenfriend, S. A spectrophotofluorometric study of compounds of biological interest. Arch. Biochem. Biophys. 1957, 68, 1–14. [Google Scholar] [CrossRef]
- Marose, S.; Lindemann, C.; Scheper, T. Two-dimensional fluorescence spectroscopy: A new tool for on-line bioprocess monitoring. Biotechnol. Prog. 1998, 14, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Haack, M.B.; Eliasson, A.; Olsson, L. On-line cell mass monitoring of saccharomyces cerevisiae cultivations by multi-wavelength fluorescence. J. Biotechnol. 2004, 114, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Hisiger, S.; Jolicoeur, M. A multiwavelength fluorescence probe: Is one probe capable for on-line monitoring of recombinant protein production and biomass activity? J. Biotechnol. 2005, 117, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Ganzlin, M.; Marose, S.; Lu, X.; Hitzmann, B.; Scheper, T.; Rinas, U. In situ multi-wavelength fluorescence spectroscopy as effective tool to simultaneously monitor spore germination, metabolic activity and quantitative protein production in recombinant aspergillus niger fed-batch cultures. J. Biotechnol. 2007, 132, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.M.; Solle, D.; Hitzmann, B.; Ayub, M.A.Z. Chemometric modeling and two-dimensional fluorescence analysis of bioprocess with a new strain of klebsiella pneumoniae to convert residual glycerol into 1,3-propanediol. J. Ind. Microbiol. Biotechnol. 2012, 39, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Hantelmann, K.; Kollecker, M.; Hull, D.; Hitzmann, B.; Scheper, T. Two-dimensional fluorescence spectroscopy: A novel approach for controlling fed-batch cultivations. J. Biotechnol. 2006, 121, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Ödman, P.; Johansen, C.L.; Olsson, L.; Gernaey, K.V.; Lantz, A.E. On-line estimation of biomass, glucose and ethanol in saccharomyces cerevisiae cultivations using in-situ multi-wavelength fluorescence and software sensors. J. Biotechnol. 2009, 144, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Van Dat Nguyen, F.H.; Salo, K.E.; Enlund, E.; Zhang, C.; Ruddock, L.W. Pre-expression of a sulfhydryl oxidase significantly increases the yields of eukaryotic disulfide bond containing proteins expressed in the cytoplasm of E. coli. Microb. Cell Fact. 2011, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Wise, B.M.; Gallagher, N.B.; Bro, R.; Shaver, J.M. PLS Toolbox 3.0; Eigenvector Research Inc.: Manson, WA, USA, 2003; Volume 171. [Google Scholar]
- Wold, S. Chemometrics; what do we mean with it, and what do we want from it? Chemom. Intell. Lab. Syst. 1995, 30, 109–115. [Google Scholar] [CrossRef]
- Panula-Perälä, J.; Siurkus, J.; Vasala, A.; Wilmanowski, R.; Casteleijn, M.G.; Neubauer, P. Enzyme controlled glucose auto-delivery for high cell density cultivations in microplates and shake flasks. Microb. Cell Fact. 2008, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Diaz Ricci, J.C.; Hernández, M.E. Plasmid effects on Escherichia coli metabolism. Crit. Rev. Biotechnol. 2000, 20, 79–108. [Google Scholar] [CrossRef] [PubMed]

| Target | LV | R2cal | R2CV | RMSEC | RMSEP |
|---|---|---|---|---|---|
| Glucose concentration (g·L−1) | 3 | 0.93 | 0.88 | 0.14 | 0.19 |
| Acetate concentration (g·L−1) | 4 | 0.99 | 0.97 | 0.05 | 0.08 |
| OD600 | 4 | 0.99 | 0.97 | 0.02 | 0.24 |
| Specific activity GalNAcT2 (pmol·min−1·µg−1) | 3 | 0.65 | 0.59 | 30.7 | 33.5 |
| Ratio of soluble GalNAcT2 / Dry matter (µg w/w) | 3 | 0.86 | 0.74 | 4 × 10−4 | 5.5 × 10−4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwab, K.; Lauber, J.; Hesse, F. Fluorometric In Situ Monitoring of an Escherichia coli Cell Factory with Cytosolic Expression of Human Glycosyltransferase GalNAcT2: Prospects and Limitations. Bioengineering 2016, 3, 32. https://doi.org/10.3390/bioengineering3040032
Schwab K, Lauber J, Hesse F. Fluorometric In Situ Monitoring of an Escherichia coli Cell Factory with Cytosolic Expression of Human Glycosyltransferase GalNAcT2: Prospects and Limitations. Bioengineering. 2016; 3(4):32. https://doi.org/10.3390/bioengineering3040032
Chicago/Turabian StyleSchwab, Karen, Jennifer Lauber, and Friedemann Hesse. 2016. "Fluorometric In Situ Monitoring of an Escherichia coli Cell Factory with Cytosolic Expression of Human Glycosyltransferase GalNAcT2: Prospects and Limitations" Bioengineering 3, no. 4: 32. https://doi.org/10.3390/bioengineering3040032





