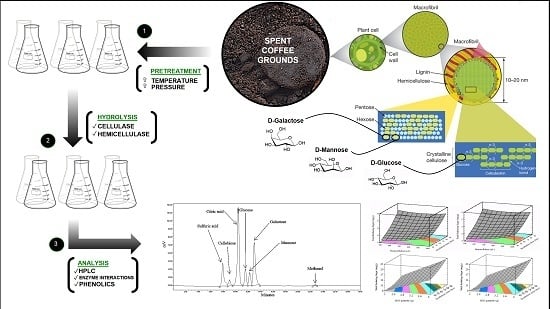
An Investigation into Spent Coffee Waste as a Renewable Source of Bioactive Compounds and Industrially Important Sugars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Acquisition and Preparation
2.3. Proximate Composition
2.3.1. Moisture Content
2.3.2. pH
2.3.3. Ash Content
2.3.4. Minerals and Trace Elements
2.3.5. Soluble Protein
2.3.6. Total Lipids
2.4. Enzymatic Hydrolysis of Spent Espresso Coffee Lignocellulose
2.4.1. Preliminary Study
2.4.2. Central Composite Design
2.5. Analysis of Reducing Sugars
2.5.1. The DNS Assay
2.5.2. HPLC-RI
2.6. Analysis of Caffeine, Polyphenols and Antioxidant Activities
2.6.1. Total Flavonoids Content (TFC) Assay
2.6.2. Total Polyphenol Content (TPC) Assay
2.6.3. DPPH Assay
2.6.4. FRAP Assay
2.6.5. Analysis of Caffeine Using HPLC-DAD
2.7. Statistical Analysis
3. Results and Discussions
3.1. Proximate Analysis of SEGs
3.2. Optimization of Variables to Maximize Lignocellulose Saccharification
3.3. Optimization of Reducing Sugar Yield
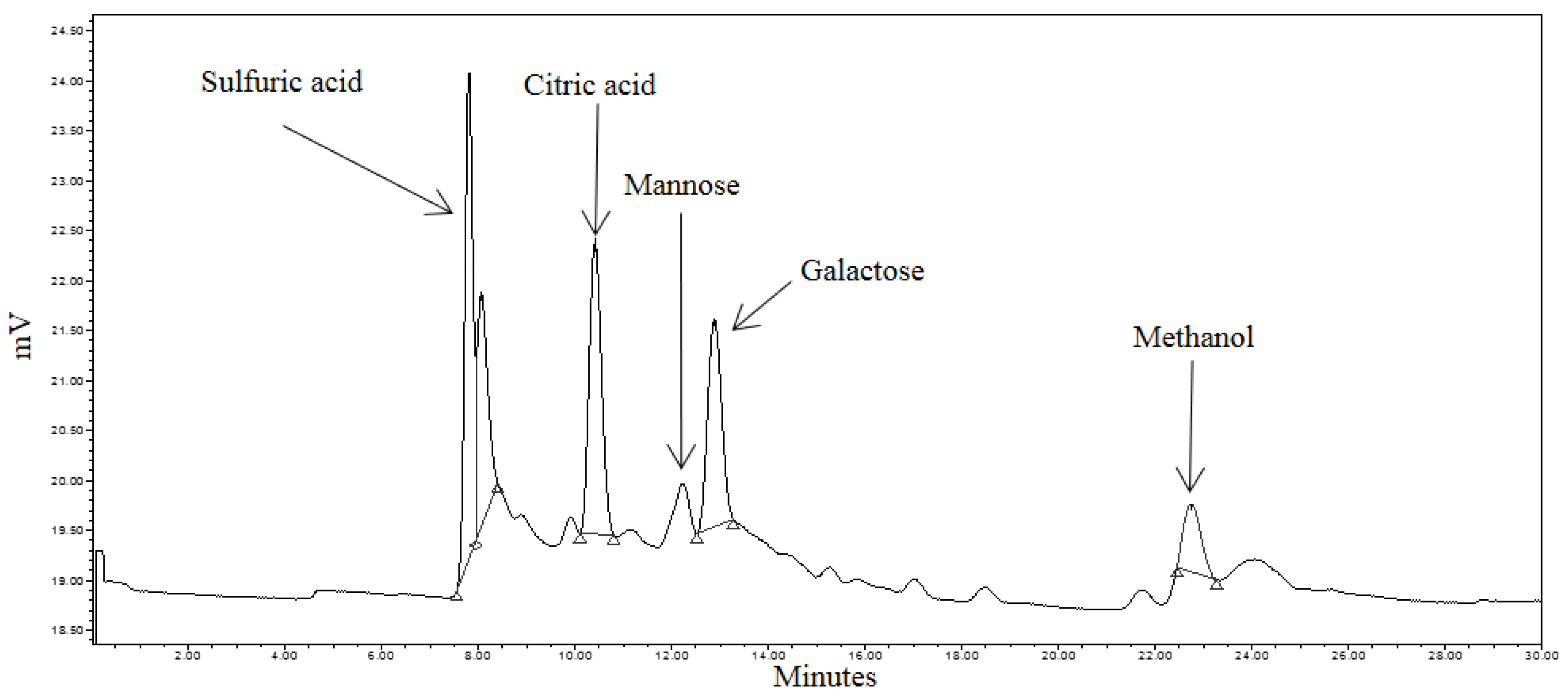
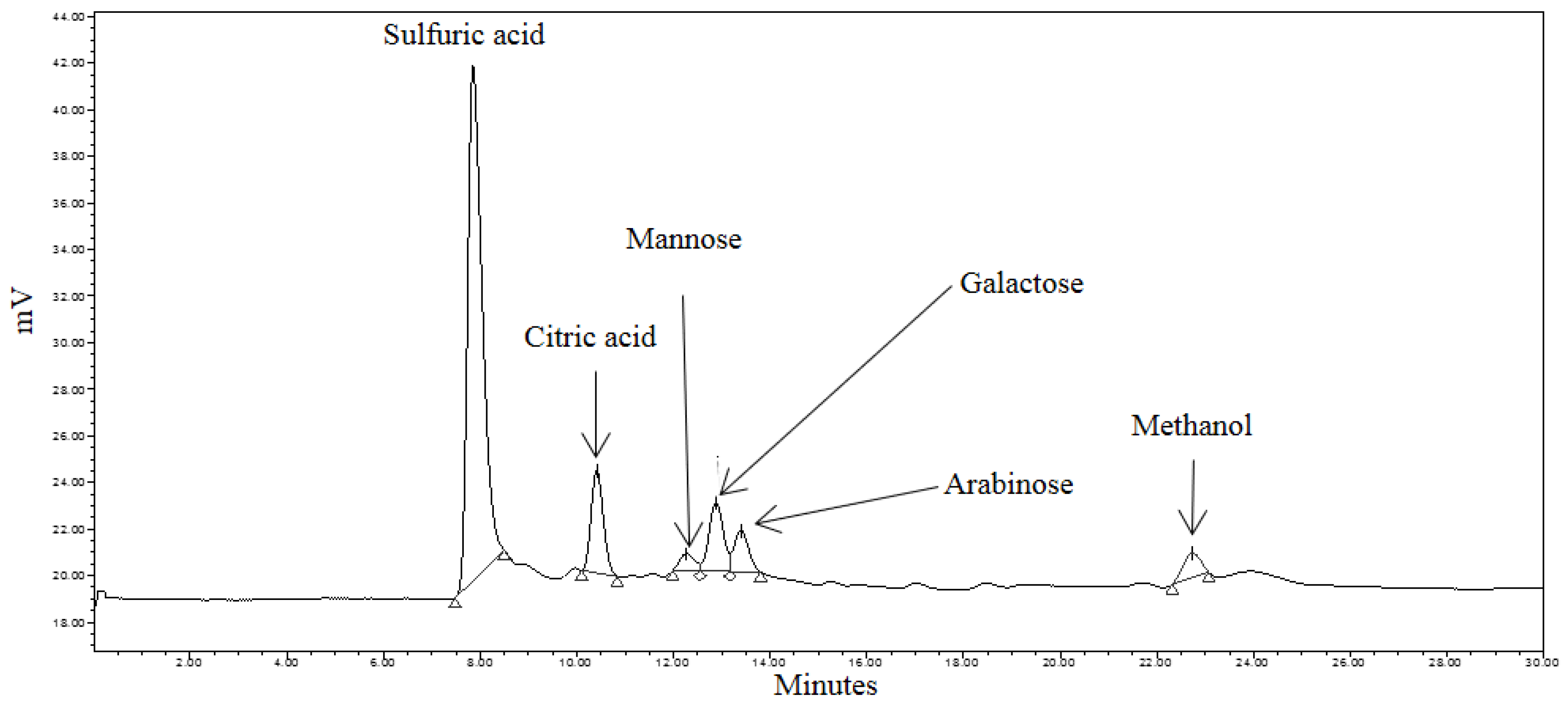
3.4. Identification of Reducing Sugars
3.5. Caffeine, Polyphenols and Antioxidant Activities
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Coffee Organization. Trade Statistics. Available online: http://www.ico.org/trade_statistics.asp (accessed on 3 February 2015).
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Cardoso, M.M.; Fernandes, L.; Oliveira, M.; Mendes, E.; Baptista, P.; Morais, S.; Casal, S. Espresso coffee residues: A valuable source of unextracted compounds. J. Agric. Food. Chem. 2012, 60, 7777–7784. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.E.; Dahlgren, R.A.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems—A review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pretreatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N. Enzymatic Hydrolysis of Cellulose: Experimental and Modeling Studies. Available online: http://orbit.dtu.dk/files/5466093/Afhandling%20A4%20format.pdf (accessed on 12 September 2014).
- Van Dyk, J.; Pletschke, B. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Qi, B.; Chen, X.; Shen, F.; Su, Y.; Wan, Y. Optimization of enzymatic hydrolysis of wheat straw pretreated by alkaline peroxide using response surface methodology. Ind. Eng. Chem. Res. 2009, 48, 7346–7353. [Google Scholar] [CrossRef]
- Gupta, S.; Jaiswal, A.K.; Abu-Ghannam, N. Optimization of fermentation conditions for the utilization of brewing waste to develop a nutraceutical rich liquid product. Ind. Crops Prod. 2013, 44, 272–282. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. Evaluation of ultrasound assisted potassium permanganate pre-treatment of spent coffee waste. Bioresour. Technol. 2017. [Google Scholar] [CrossRef]
- Association of Analytical Chemists (AOAC). Official Methods of Analysis; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Szymczycha-Madeja, A.; Welna, M.; Pohl, P. Fast method of elements determination in slim coffees by ICP OES. Food Chem. 2014, 146, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- De Melo, M.M.; Barbosa, H.M.; Passos, C.P.; Silva, C.M. Supercritical fluid extraction of spent coffee grounds: Measurement of extraction curves, oil characterization and economic analysis. J. Supercrit. Fluids 2014, 86, 150–159. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Gupta, S.; Abu-Ghannam, N. Optimisation of lactic acid fermentation of york cabbage for the development of potential probiotic products. Int. J. Food Sci. Technol. 2012, 47, 1605–1612. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Effect of hydrothermal processing on colour, antioxidant and free radical scavenging capacities of edible Irish brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 2485–2493. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Rajauria, G.; Abu-Ghannam, N.; Gupta, S. Effect of different solvents on polyphenolic content, antioxidant capacity and antibacterial activity of Irish York cabbage. J. Food Biochem. 2012, 36, 344–358. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recy. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Heidelberg, Germany, 2009. [Google Scholar]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Bicho, N.C.; Leitão, A.E.; Ramalho, J.C.; Lidon, F.C. Identification of chemical clusters discriminators of the roast degree in arabica and robusta coffee beans. Eur. Food Res. Technol. 2011, 233, 303–311. [Google Scholar] [CrossRef]
- Kostenberg, D.; Marchaim, U. Anaerobic digestion and horticultural value of solid waste from manufacture of instant coffee. Environ. Technol. 1993, 14, 973–980. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S.; Mendonça, J.C.; Barros-Júnior, M.C. Proximate composition and fatty acids profile of green and roasted defective coffee beans. LWT-Food Sci. Technol. 2006, 39, 235–239. [Google Scholar] [CrossRef]
- Jooste, T.; García-Aparicio, M.; Brienzo, M.; Van Zyl, W.; Görgens, J. Enzymatic hydrolysis of spent coffee ground. Appl. Biochem. Biotechnol. 2013, 169, 2248–2262. [Google Scholar] [CrossRef] [PubMed]
- Redgwell, R.J.; Trovato, V.; Curti, D.; Fischer, M. Effect of roasting on degradation and structural features of polysaccharides in arabica coffee beans. Carbohydr. Res. 2002, 337, 421–431. [Google Scholar] [CrossRef]
- Cheong, M.W.; Tong, K.H.; Ong, J.J.M.; Liu, S.Q.; Curran, P.; Yu, B. Volatile composition and antioxidant capacity of arabica coffee. Food Res. Int. 2013, 51, 388–396. [Google Scholar] [CrossRef]
- Hečimović, I.; Belščak-Cvitanović, A.; Horžić, D.; Komes, D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011, 129, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]

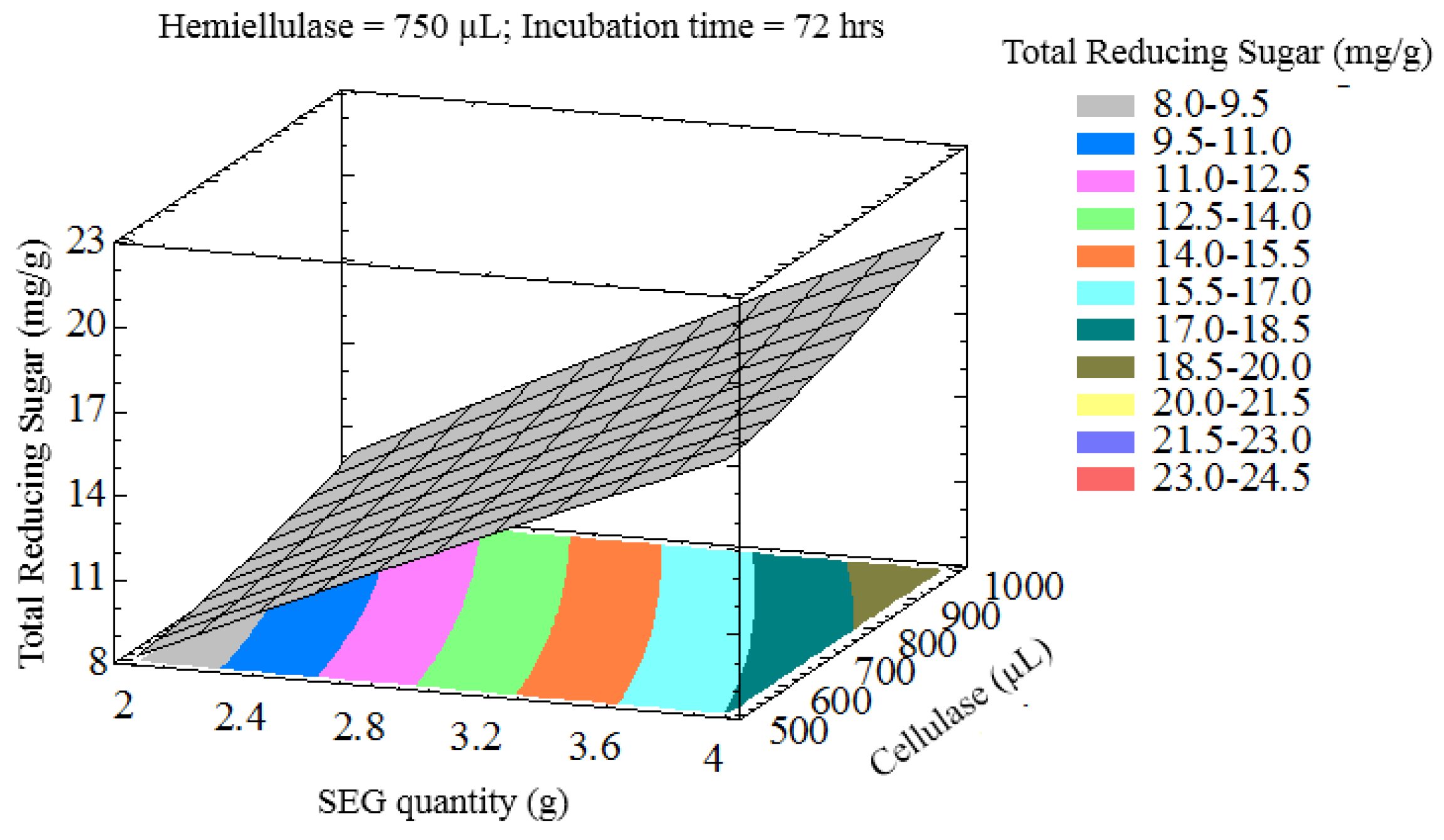
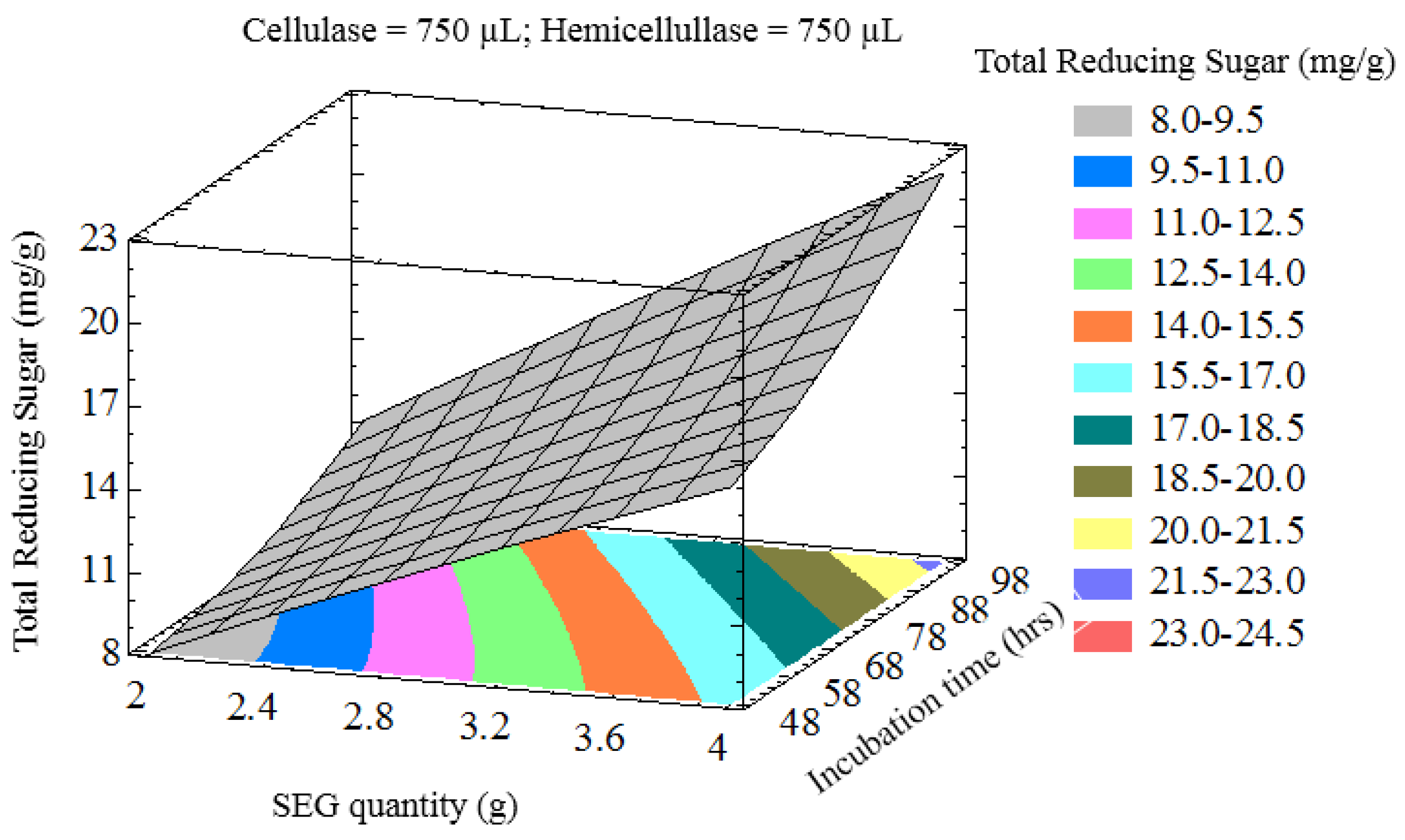
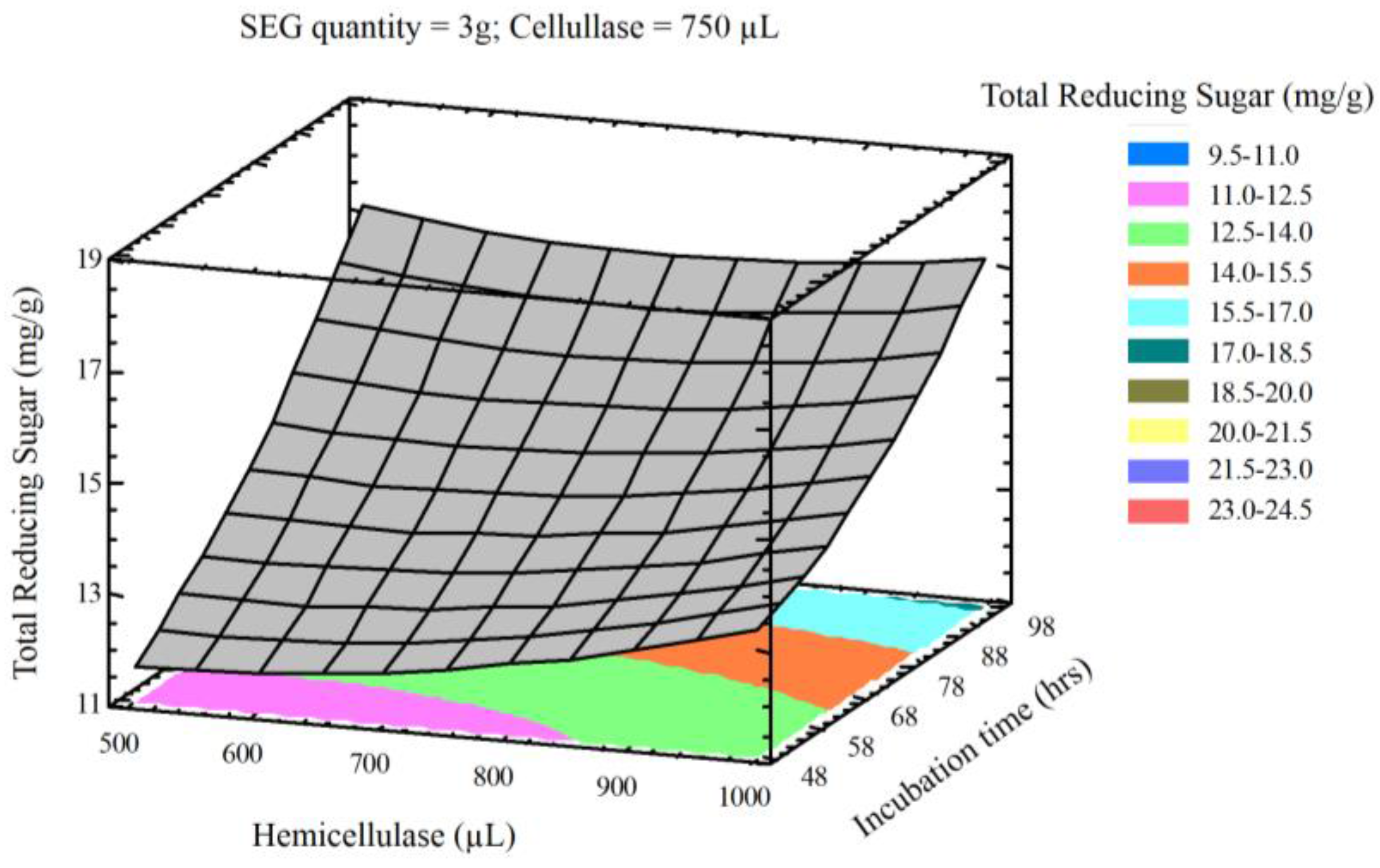

| Process Variables | Coded Values | ||||
|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | |
| Χ1: SEG Quantity (g) | 1 | 2 | 3 | 4 | 5 |
| Χ2: Cellulase (µL) | 250 | 500 | 750 | 1000 | 1250 |
| Χ3: Hemicellulase (µL) | 250 | 500 | 750 | 1000 | 1250 |
| Χ4: Incubation Time (h) | 24 | 48 | 72 | 36 | 120 |
| Trace Elements | Concentration (mg·(100 g)−1) |
|---|---|
| Potassium | 258.2 ± 23.66 |
| Phosphorus | ND |
| Magnesium | 49.6 ± 3.34 |
| Calcium | 37.2 ± 2.89 |
| Manganese | 1.8 ± 0.06 |
| Copper | 1.2 ± 0.19 |
| Sodium | 1.1 ± 0.01 |
| Iron | 0.9 ± 0.02 |
| Zinc | 0.1 ± 0.03 |
| Experiment | Process Variables * | Concentration (mg·mL−1) | ||||
|---|---|---|---|---|---|---|
| Χ1 (g) | Χ2 (µL) | Χ3 (µL) | Χ4 (h) | Predicted | Observed | |
| 1 | 3 | 750 | 750 | 120 | 20.38 | 18.37 |
| 2 | 3 | 750 | 750 | 72 | 13.76 | 12.76 |
| 3 | 3 | 250 | 750 | 72 | 12.35 | 10.94 |
| 4 | 4 | 1000 | 500 | 96 | 23.69 | 26.05 |
| 5 | 4 | 1000 | 500 | 48 | 17.77 | 17.79 |
| 6 | 5 | 750 | 750 | 72 | 22.44 | 19.65 |
| 7 | 4 | 500 | 500 | 48 | 13.70 | 14.57 |
| 8 | 4 | 1000 | 1000 | 48 | 18.97 | 20.80 |
| 9 | 3 | 750 | 750 | 72 | 13.76 | 14.12 |
| 10 | 2 | 500 | 1000 | 48 | 8.32 | 7.76 |
| 11 | 4 | 1000 | 1000 | 96 | 23.38 | 23.24 |
| 12 | 4 | 500 | 1000 | 96 | 21.56 | 23.20 |
| 13 | 2 | 500 | 1000 | 96 | 11.27 | 12.14 |
| 14 | 3 | 1250 | 750 | 72 | 17.42 | 16.14 |
| 15 | 2 | 500 | 500 | 96 | 10.71 | 10.69 |
| 16 | 2 | 500 | 500 | 48 | 6.24 | 7.27 |
| 17 | 1 | 750 | 750 | 72 | 3.88 | 3.97 |
| 18 | 4 | 500 | 1000 | 72 | 15.79 | 16.55 |
| 19 | 3 | 750 | 750 | 96 | 13.76 | 13.91 |
| 20 | 3 | 750 | 250 | 72 | 14.48 | 13.09 |
| 21 | 2 | 1000 | 500 | 96 | 12.59 | 12.72 |
| 22 | 3 | 750 | 750 | 72 | 13.76 | 14.24 |
| 23 | 2 | 1000 | 1000 | 96 | 12.27 | 13.21 |
| 24 | 3 | 750 | 1250 | 72 | 16.25 | 14.94 |
| 25 | 3 | 750 | 750 | 24 | 11.51 | 10.82 |
| 26 | 2 | 1000 | 500 | 48 | 9.50 | 9.66 |
| 27 | 2 | 1000 | 1000 | 48 | 10.69 | 10.65 |
| 28 | 4 | 500 | 500 | 96 | 20.99 | 21.92 |
| Process Variables | Limit Values | Optimum Values | |
|---|---|---|---|
| Low | High | ||
| SEG Quantity (g) | 1 | 5 | 4.97 |
| Cellulase (µL) | 250 | 1250 | 1246.07 |
| Hemicellulase (µL) | 250 | 1250 | 250 |
| Incubation Time (h) | 24 | 120 | 120 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scully, D.S.; Jaiswal, A.K.; Abu-Ghannam, N. An Investigation into Spent Coffee Waste as a Renewable Source of Bioactive Compounds and Industrially Important Sugars. Bioengineering 2016, 3, 33. https://doi.org/10.3390/bioengineering3040033
Scully DS, Jaiswal AK, Abu-Ghannam N. An Investigation into Spent Coffee Waste as a Renewable Source of Bioactive Compounds and Industrially Important Sugars. Bioengineering. 2016; 3(4):33. https://doi.org/10.3390/bioengineering3040033
Chicago/Turabian StyleScully, Damhan S., Amit K. Jaiswal, and Nissreen Abu-Ghannam. 2016. "An Investigation into Spent Coffee Waste as a Renewable Source of Bioactive Compounds and Industrially Important Sugars" Bioengineering 3, no. 4: 33. https://doi.org/10.3390/bioengineering3040033