Photocurable Bioink for the Inkjet 3D Pharming of Hydrophilic Drugs
Abstract
:1. Introduction
2. Materials and Methods
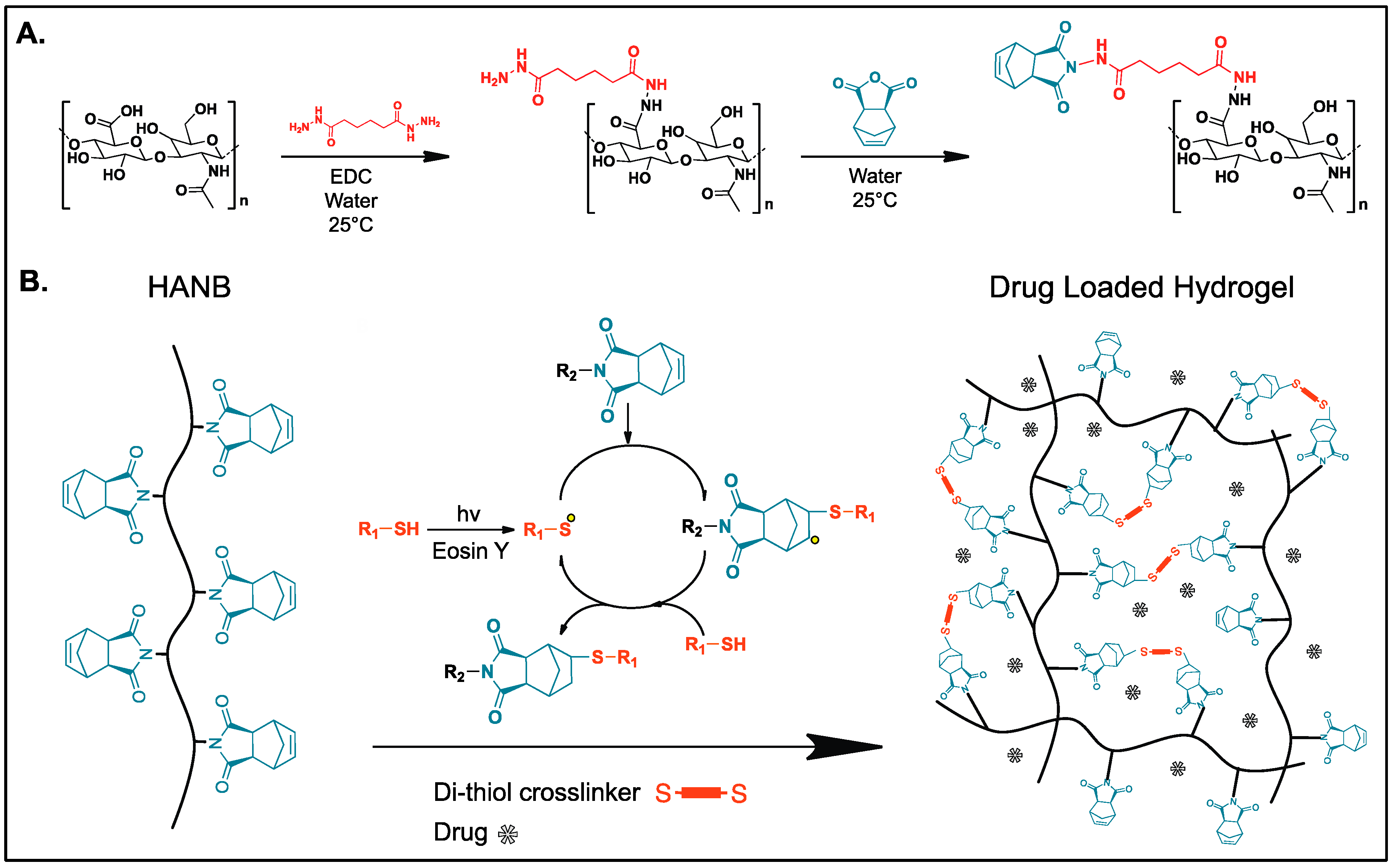
2.1. Norbornene Functionalized Hyaluronic Acid (HANB) Synthesis
2.2. Photocurable Formula Preparation
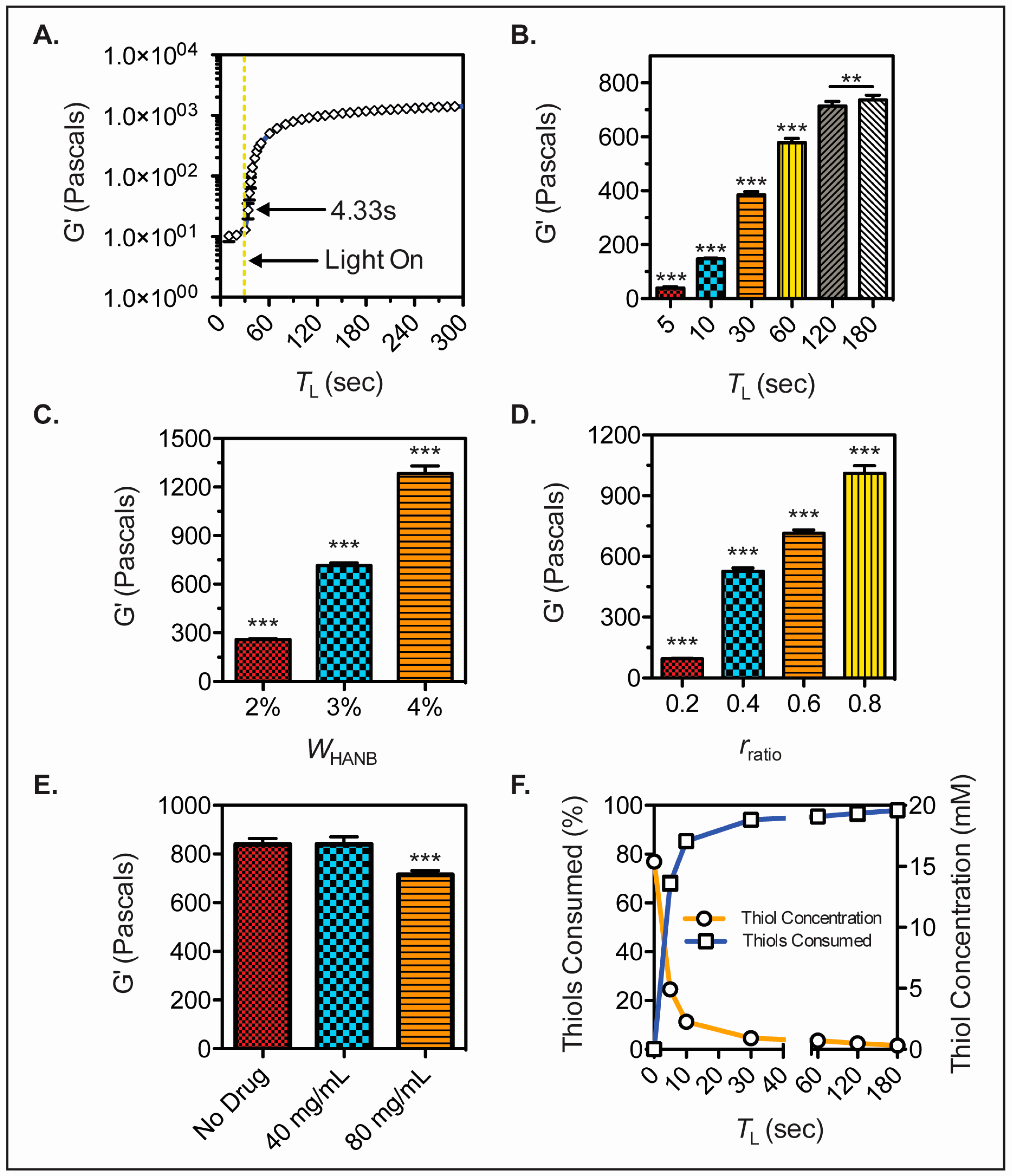
2.3. Gelation and Mechanical Properties
2.4. Thiols Consumption
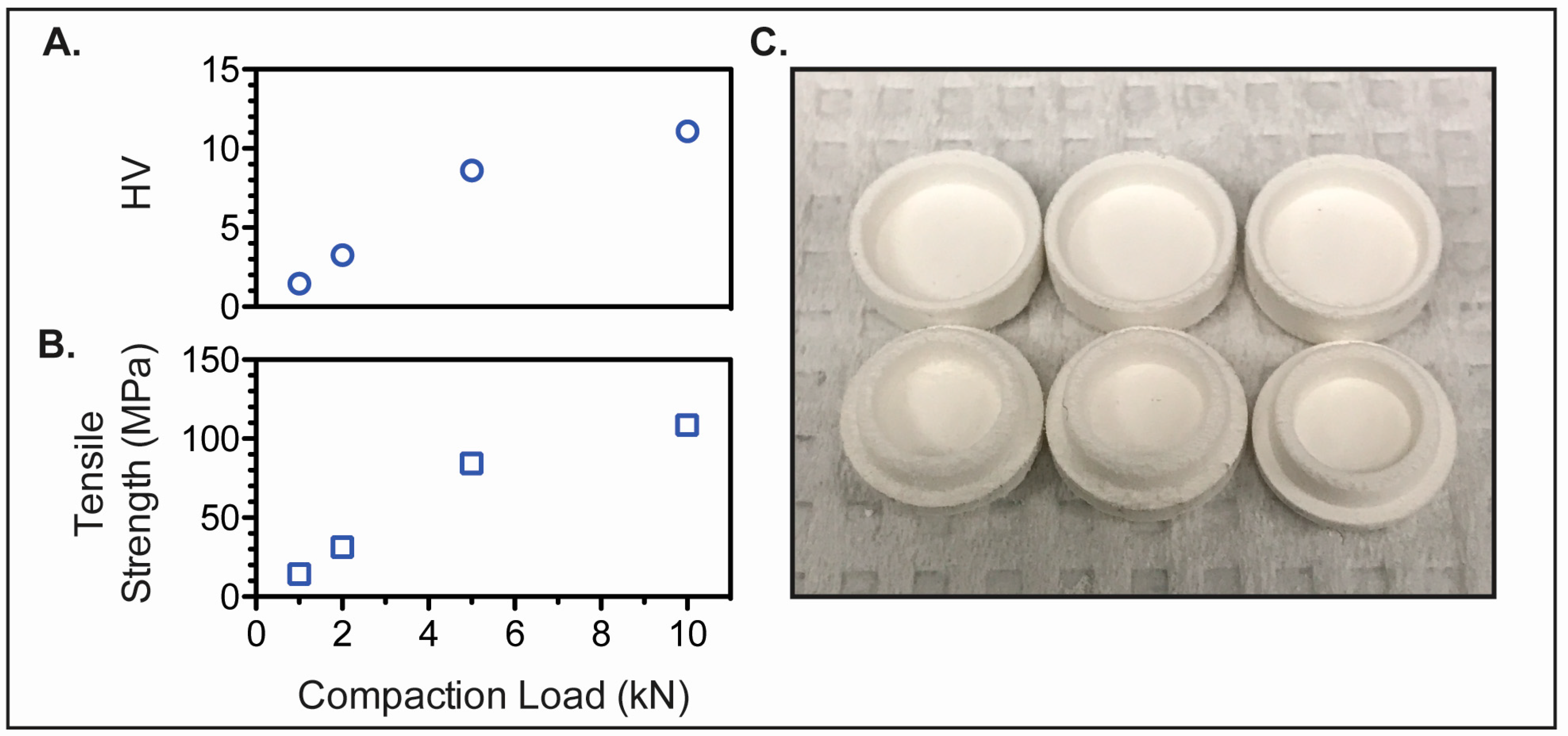
2.5. Preform Tablet Fabrication
2.6. Drug Dispensing and Release Kinetics
2.7. Hydrogel Drying
2.8. Statistical Analysis
3. Results and Discussion
3.1. HANB Synthesis and Gelation
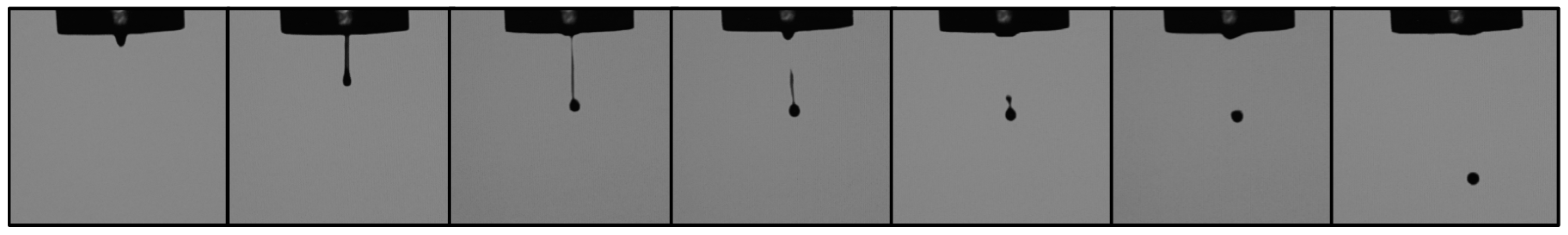
3.2. Hydrogel Characterization and Droplet Formation
3.3. Preform Tablet Fabrication
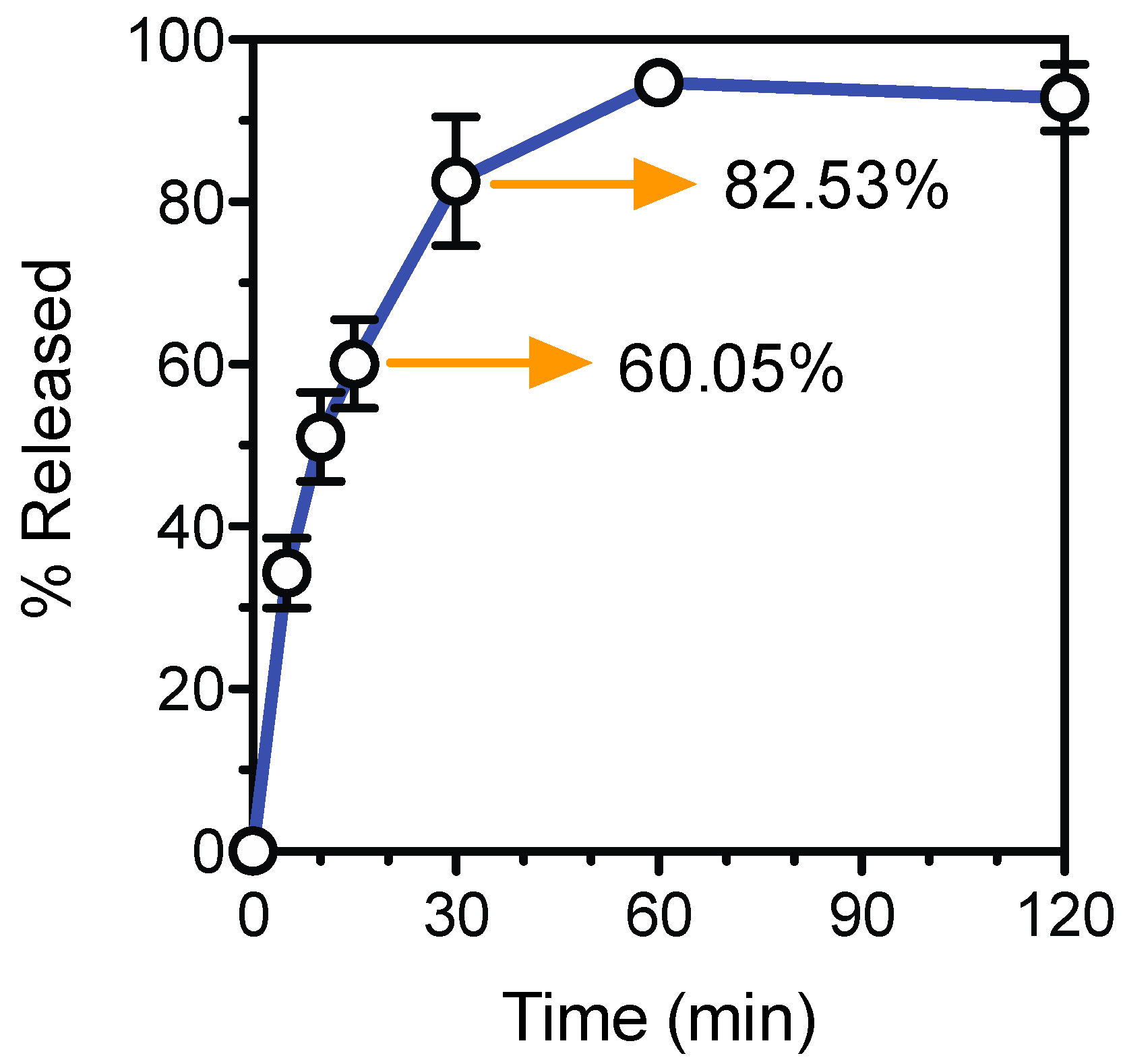
3.4. Tablet Fabrication and Dissolution Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cohen, J.S. Dose discrepancies between the physicians’ desk reference and the medical literature, and their possible role in the high incidence of dose-related adverse drug events. Arch. Intern. Med. 2001, 161, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Florence, A.T.; Lee, V.H.L. Personalised medicines: More tailored drugs, more tailored delivery. Int. J. Pharm. 2011, 415, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Schork, N.J. Personalized medicine: Time for one-person trials. Nature 2015, 520, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Vélez, G.F.; Wu, B.M. 3D pharming: Direct printing of personalized pharmaceutical tablets abstract powder bed inkjet 3D printing. Polym. Sci. 2016, 1, 1–10. [Google Scholar]
- Wu, B.M.; Borland, S.W.; Giordano, R.A.; Cima, L.G.; Sachs, E.M.; Cima, M.J. Solid free-form fabrication of drug delivery devices. J. Control. Release 1996, 40, 77–87. [Google Scholar] [CrossRef]
- Sun, Y.; Soh, S. Printing tablets with fully customizable release profiles for personalized medicine. Adv. Mater. 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Wang, J.; Buanz, A.; Martinez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D printing of medicines: Engineering novel oral devices with unique design and drug release characteristics. Mol. Pharm. 2015, 4077–4084. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet printing for pharmaceutics—A review of research and manufacturing. Int. J. Pharm. 2015, 494, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Verkouteren, R.M.; Verkouteren, J.R. Inkjet metrology: High-accuracy mass measurements of microdroplets produced by a drop-on-demand dispenser. Anal. Chem. 2009, 81, 8577–8584. [Google Scholar] [CrossRef] [PubMed]
- Alomari, M.; Mohamed, F.H.; Basit, A.W.; Gaisford, S. Personalised dosing: Printing a dose of one’s own medicine. Int. J. Pharm. 2015, 494, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.D.; Grande-Allen, K.J. Review. Hyaluronan: A powerful tissue engineering tool. Tissue Eng. Part A 2006, 12, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-H.; Zimmerman, S.C. Orthogonality in organic, polymer, and supramolecular chemistry: From Merrifield to click chemistry. Chem. Commun. 2013, 49, 1679–1695. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.; Mirmira, R.G.; Lin, C.-C. Visible light-initiated interfacial thiol-norbornene photopolymerization for forming an islet surface conformal coating. J. Mater. Chem. B 2015, 3, 170–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tompson, D.J.; Vearer, D. Steady-state pharmacokinetic properties of a 24-hour prolonged-release formulation of ropinirole: Results of two randomized studies in patients with Parkinson’s disease. Clin. Ther. 2007, 29, 2654–2666. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Kim, D.; Moon, J.; Jang, D.; Kim, D.; Moon, J. Influence of fluid physical properties on ink-jet printability. Langmuir 2009, 25, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Cole-Parmer Instrument Company. Available online: https://pim-resources.coleparmer.com/instruction manual/surface-tension-apparatus-instruction-manual.pdf (accessed on 12 January 2017).
- Zustiak, S.P.; Leach, J.B. Hydrolytically degradable poly (ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules 2010, 11, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, W.M.; Kim, I.L.; Burdick, J.A. Synthesis and orthogonal photopatterning of hyaluronic acid hydrogels with thiol-norbornene chemistry. Biomaterials 2013, 34, 9803–9811. [Google Scholar] [CrossRef] [PubMed]
- Gudapatia, H.; Deyb, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Pilaniya, K.; Chandrawanshi, H.K.; Pilaniya, U.; Manchandani, P.; Jain, P.; Singh, N. Recent trends in the impurity profile of pharmaceuticals. J. Adv. Pharm. Technol. Res. 2010, 1, 302–310. [Google Scholar] [PubMed]
- Rowe, R.C.; Sheskey, P.J.; Paul, J.; Weller, P.J. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009; pp. 206–208. [Google Scholar]
- Bi, Y.X.; Sunada, H.; Yonezawa, Y.; Danjo, K. Evaluation of rapidly disintegrating tablets prepared by a direct compression method. Drug Dev. Ind. Pharm. 1999, 25, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Broilo, T.L.; Heitz, C.; Oliveira, G.D.E.; Oliveira, H.W.D.E.; Maris, S.; Nobre, W.; Silva, D.N. Dimensional error of selective laser sintering, three-dimensional printing and PolyJet models in the reproduction of mandibular anatomy. J. Cranio-Maxillofac. Surg. 2009, 37, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Ionita, C.N.; Mokin, M.; Varble, N.; Bednarek, D.R.; Xiang, J.; Snyder, K.V.; Siddiqui, A.H.; Levy, E.I.; Meng, H.; Stroke, T. Challenges and limitations of patient-specific vascular phantom fabrication using 3D Polyjet printing. Proc. SPIE Int. Soc. Opt. Eng. 2014, 9038, 90380M. [Google Scholar] [PubMed]

| Nozzle Diameter (mm) | Density (kg/m3) | Surface Tension (mN/m) | Viscosity (mPa·s) | Z | Swelling Ratio |
|---|---|---|---|---|---|
| 0.08 | 1042 | 51.47 | 8.38 | 7.82 | 21.14 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Vélez, G.F.; Linsley, C.S.; Craig, M.C.; Wu, B.M. Photocurable Bioink for the Inkjet 3D Pharming of Hydrophilic Drugs. Bioengineering 2017, 4, 11. https://doi.org/10.3390/bioengineering4010011
Acosta-Vélez GF, Linsley CS, Craig MC, Wu BM. Photocurable Bioink for the Inkjet 3D Pharming of Hydrophilic Drugs. Bioengineering. 2017; 4(1):11. https://doi.org/10.3390/bioengineering4010011
Chicago/Turabian StyleAcosta-Vélez, Giovanny F., Chase S. Linsley, Madison C. Craig, and Benjamin M. Wu. 2017. "Photocurable Bioink for the Inkjet 3D Pharming of Hydrophilic Drugs" Bioengineering 4, no. 1: 11. https://doi.org/10.3390/bioengineering4010011







