Biosynthesis of Inorganic Nanoparticles: A Fresh Look at the Control of Shape, Size and Composition
Abstract
:1. Introduction
2. Control of Size and Shape of Biologically Synthesized Metallic Nanoparticles
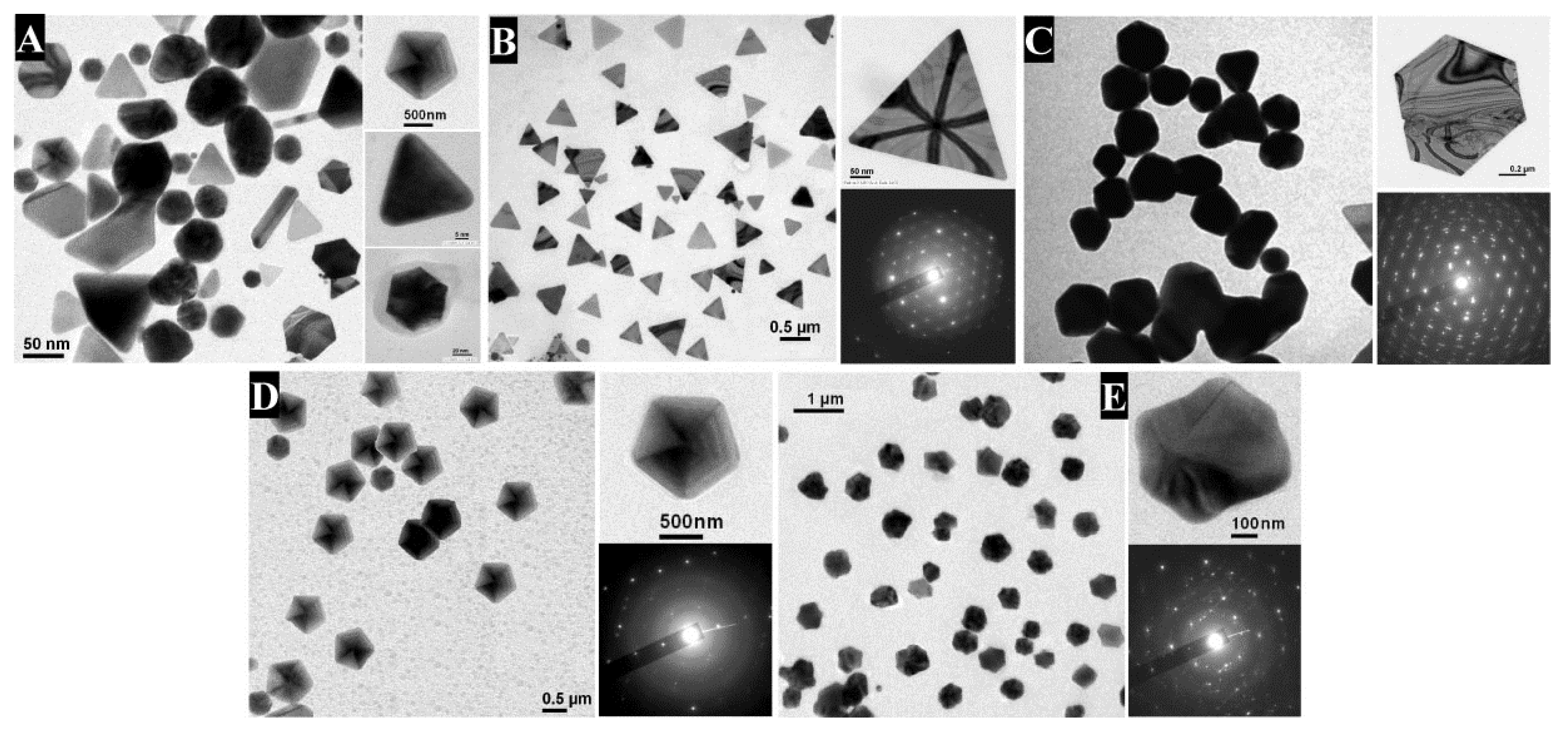
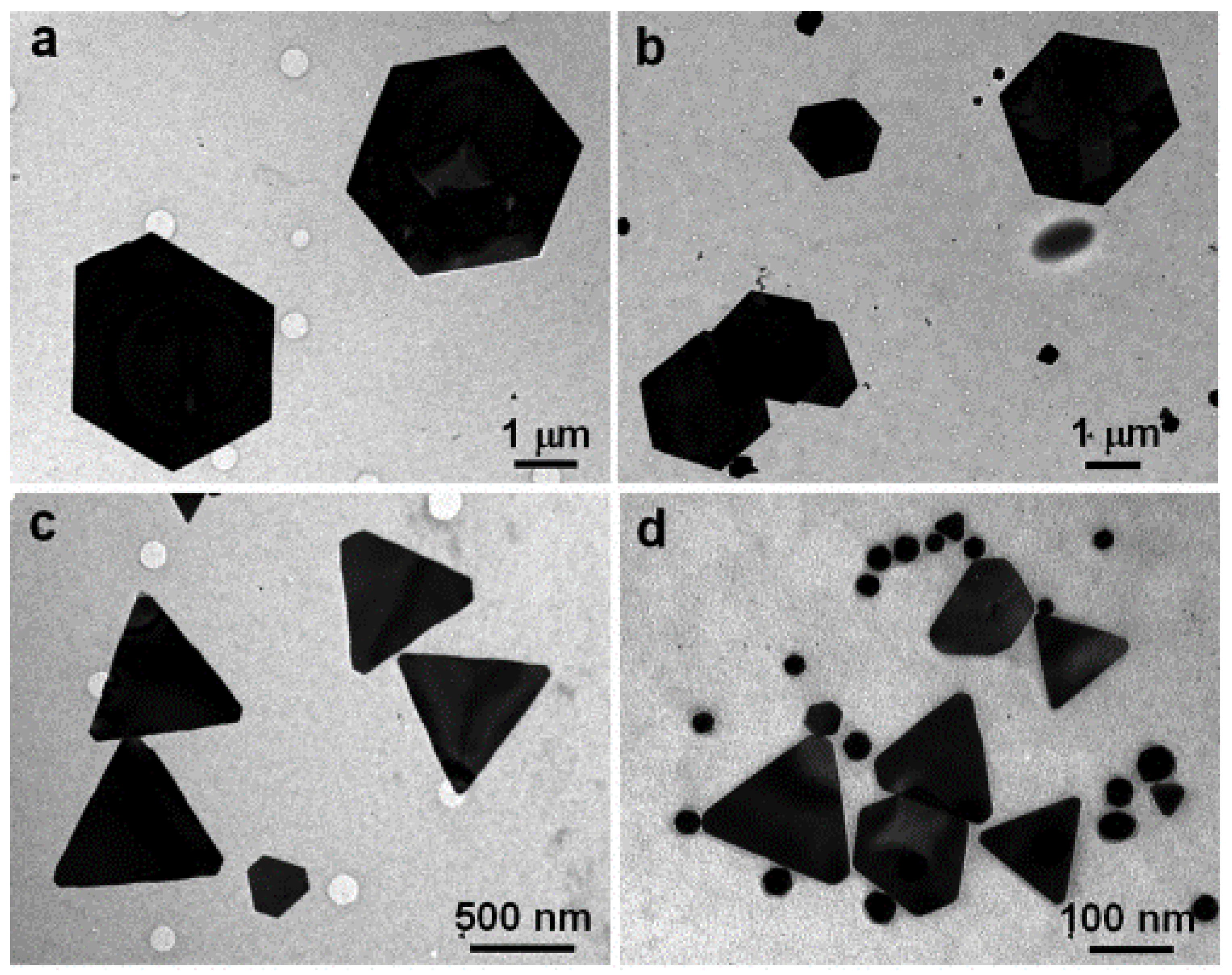
2.1. Gold
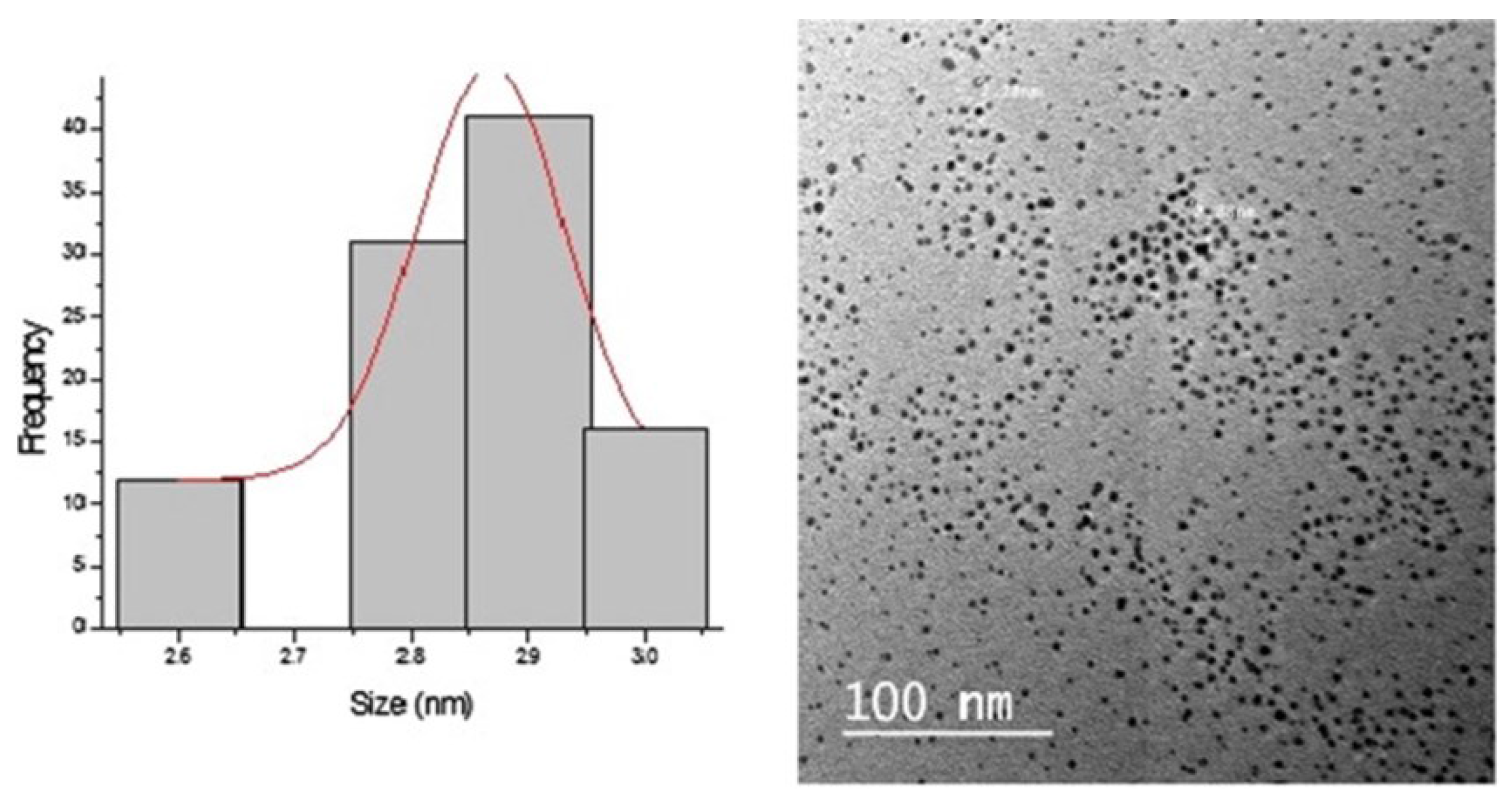
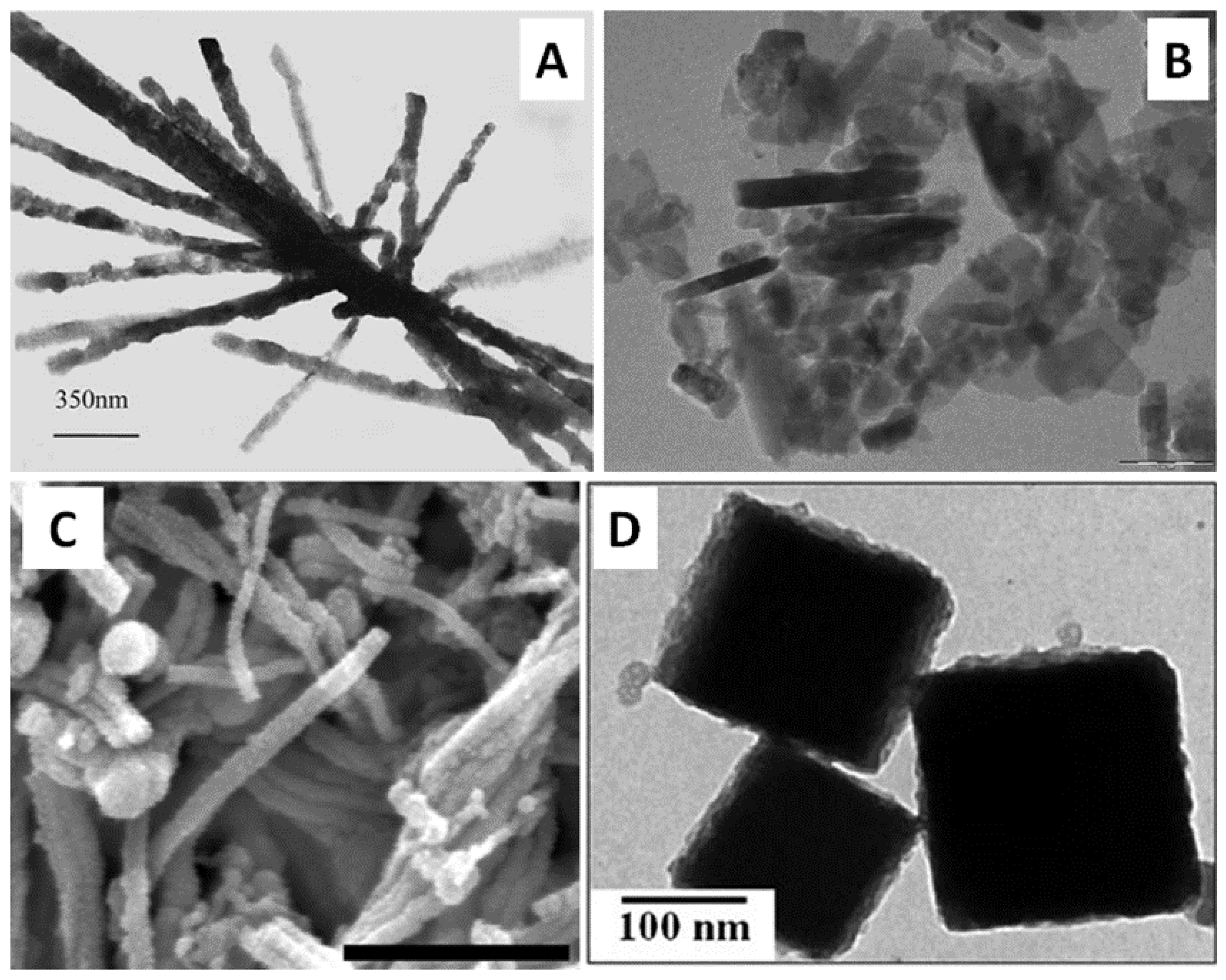
2.2. Silver
2.3. Palladium and Platinum NPs
2.4. Metallic Alloys
3. Control of Size and Shape of Biologically Synthesized Oxide and Carbonate Nanoparticles
4. Control of Size and Shape of Biologically Synthesized Chalcogenide Nanoparticles
5. Conclusion and Perspectives
Conflicts of Interest
References
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of nanoparticles in biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Guo, S.; Wang, E. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today 2011, 6, 240–264. [Google Scholar] [CrossRef]
- Murphy, C.J.; Sau, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 2005, 109, 13857–13870. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, D.; Zhang, J.; Li, Y. Shape-dependent catalytic activity of silver nanoparticles for the oxidation of styrene. Chem. Asian. J. 2006, 1, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, Z.J. Shape control and associated magnetic properties of spinel cobalt ferrite nanocrystals. J. Am. Chem. Soc. 2004, 126, 6164–6168. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Dahoumane, S.A.; Yéprémian, C.; Djédiat, C.; Meyer, M.; Couté, A.; Fiévet, F. ZnO nanoparticles: Synthesis, characterization, and ecotoxicological studies. Langmuir 2010, 26, 6522. [Google Scholar] [CrossRef] [PubMed]
- Soumare, Y.; Garcia, C.; Maurer, T.; Chaboussant, G.; Ott, F.; Fiévet, F.; Piquemal, J.-Y.; Viau, G. Kinetically controlled synthesis of hexagonally close-packed cobalt nanorods with high magnetic coercivity. Adv. Funct. Mater. 2009, 19, 1971–1977. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, X.; Wang, Y.; Xie, S. Shape-controlled synthesis of metal nanocrystals. MRS Bull. 2013, 38, 335–344. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Klaus-Joerger, T.; Joerger, R.; Olsson, E.; Granqvist, C.-G. Bacteria as workers in the living factory: Metal-accumulating bacteria and their potential for materials science. Trend. Biotechnol. 2001, 19, 15–20. [Google Scholar] [CrossRef]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Beeler, E.; Singh, O.V. Extremophiles as sources of inorganic bio-nanoparticles. World J. Microbiol. Biotechnol. 2016, 32, 156. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.; Ingle, A.; Whiteley, C.; Rai, M. Mycogenic metal nanoparticles: Progress and applications. Biotechnol. Lett. 2010, 32, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Sastry, M.; Ahmad, A.; Khan, M.I.; Kumar, R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr. Sci. India 2003, 85, 162–170. [Google Scholar]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Boroumand Moghaddam, A.; Namvar, F.; Moniri, M.; Md Tahir, P.; Azizi, S.; Mohamad, R. Nanoparticles biosynthesized by fungi and yeast: A review of their preparation, properties, and medical applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Dahoumane, S.A.; Wujcik, E.K.; Jeffryes, C. Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzyme Microb. Technol. 2016, 95, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.M.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology—A review. Green Chem. 2017, 19, 552–587. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Mechouet, M.; Alvarez, F.J.; Agathos, S.N.; Jeffryes, C. Microalgae: An outstanding tool in nanotechnology. Bionatura 2016, 1, 196–201. [Google Scholar] [CrossRef]
- Jeffryes, C.; Agathos, S.N.; Rorrer, G. Biogenic nanomaterials from photosynthetic microorganisms. Curr. Opin. Biotechnol. 2015, 33, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, M.B.; Sandhage, K.H.; Naik, R.R. Protein- and peptide-directed syntheses of inorganic materials. Chem. Rev. 2008, 108, 4935–4978. [Google Scholar] [CrossRef] [PubMed]
- Crookes-Goodson, W.J.; Slocik, J.M.; Naik, R.R. Bio-directed synthesis and assembly of nanomaterials. Chem. Soc. Rev. 2008, 37, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.-H.; Shanmugam, V.; Yeh, C.-S. Nanoparticle biosynthesis using unicellular and subcellular supports. NPG Asia Mater. 2015, 7, e209. [Google Scholar] [CrossRef]
- Kitching, M.; Ramani, M.; Marsili, E. Fungal biosynthesis of gold nanoparticles: Mechanism and scale up. Microb. Biotechnol. 2015, 8, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Noruzi, M. Biosynthesis of gold nanoparticles using plant extracts. Bioprocess. Biosyst. Eng. 2015, 38, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schröfel, A.; Kratošová, G.; Šafařík, I.; Šafaříková, M.; Raška, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles—A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Blázquez, M.L.; Muñoz, J.A.; González, F.; Ballester, A. Mechanism and applications of metal nanoparticles prepared by bio-mediated process. Rev. Adv. Sci. Eng. 2014, 3, 199–216. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Guo, Z.; Gu, N. Biological synthesis of gold nanowires using extract of Rhodopseudomonas capsulata. Biotechnol. Prog. 2008, 24, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Philip, D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A 2009, 73, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Das, A.R.; Guha, A.K. Microbial synthesis of multishaped gold nanostructures. Small 2010, 6, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Arockiya Aarthi Rajathi, F.; Arumugam, R.; Saravanan, S.; Anantharaman, P. Phytofabrication of gold nanoparticles assisted by leaves of Suaeda monoica and its free radical scavenging property. J. Photochem. Photobiol. B 2014, 135, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kasthuri, J.; Veerapandian, S.; Rajendiran, N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf. B 2009, 68, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Varma, R.S. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008, 10, 859–862. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, J.Y.; Wang, D.I.; Ting, Y.P. Identification of active biomolecules in the high-yield synthesis of single-crystalline gold nanoplates in algal solutions. Small 2007, 3, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Controlling the optical properties of lemongrass extract synthesized gold nanotriangles and potential application in infrared-absorbing optical coatings. Chem. Mater. 2005, 19, 566–572. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ankamwar, B.; Singh, A.; Ahmad, A.; Sastry, M. Biological synthesis of triangular gold nanoprisms. Nat. Mater. 2004, 3, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xie, J.; Lee, J.Y.; Ting, Y.P.; Chen, J.P. Optimization of high-yield biological synthesis of single-crystalline gold nanoplates. J. Phys. Chem. B 2005, 109, 15256–15263. [Google Scholar] [CrossRef] [PubMed]
- Parial, D.; Patra, H.K.; Roychoudhury, P.; Dasgupta, A.K.; Pal, R. Gold nanorod production by cyanobacteria—A green chemistry approach. J. Appl. Phycol. 2011, 24, 55–60. [Google Scholar] [CrossRef]
- Ghodake, G.; Lee, D.S. Biological synthesis of gold nanoparticles using the aqueous extract of the brown algae Laminaria japonica. J. Nanoelectron. Optoelecton. 2011, 6, 268–271. [Google Scholar] [CrossRef]
- Sharma, B.; Purkayastha, D.D.; Hazra, S.; Gogoi, L.; Bhattacharjee, C.R.; Ghosh, N.N.; Rout, J. Biosynthesis of gold nanoparticles using a freshwater green alga, Prasiola crispa. Mater. Lett. 2014, 116, 94–97. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Yéprémian, C.; Djédiat, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. A global approach of the mechanism involved in the biosynthesis of gold colloids using micro-algae. J. Nanopart. Res. 2014, 16:2607. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Djédiat, C.; Yéprémian, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. Species selection for the design of gold nanobioreactor by photosynthetic organisms. J. Nanopart. Res. 2012, 14:883. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Yéprémian, C.; Djédiat, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. Improvement of kinetics, yield, and colloidal stability of biogenic gold nanoparticles using living cells of Euglena gracilis microalga. J. Nanopart. Res. 2016, 18:79. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Djédiat, C.; Yéprémian, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. Recycling and adaptation of Klebsormidium flaccidum microalgae for the sustained production of gold nanoparticles. Biotechnol. Bioeng. 2012, 109, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Shukla, S.P.; Sandeep, K.P.; Singh, A.R.; Sharma, N. Evaluation of the performance of an algal bioreactor for silver nanoparticle production. J. Appl. Phycol. 2015, 27, 285–291. [Google Scholar] [CrossRef]
- Sicard, C.; Brayner, R.; Margueritat, J.; Hémadi, M.; Couté, A.; Yéprémian, C.; Djédiat, C.; Aubard, J.; Fiévet, F.; Livage, J.; et al. Nano-gold biosynthesis by silica-encapsulated micro-algae: A “living” bio-hybrid material. J. Mater. Chem. 2010, 20, 9342–9347. [Google Scholar] [CrossRef]
- Spedalieri, C.; Sicard, C.; Perullini, M.; Brayner, R.; Coradin, T.; Livage, J.; Bilmes, S.A.; Jobbágy, M. Silica@proton-alginate microreactors: A versatile platform for cell encapsulation. J. Mater. Chem. B 2015, 3, 3189–3194. [Google Scholar] [CrossRef]
- Lin, L.; Wang, W.; Huang, J.; Li, Q.; Sun, D.; Yang, X.; Wang, H.; He, N.; Wang, Y. Nature factory of silver nanowires: Plant-mediated synthesis using broth of Cassia fistula leaf. Chem. Eng. J. 2010, 162, 852–858. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, T.; Paliwal, C.; Maurya, R.; Ghosh, A.; Mishra, S. Green synthesis, characterization and antioxidant potential of silver nanoparticles biosynthesized from de-oiled biomass of thermotolerant oleaginous microalgae Acutodesmus dimorphus. RSC Adv. 2016, 6, 72269–72274. [Google Scholar] [CrossRef]
- Das, V.L.; Thomas, R.; Varghese, R.T.; Soniya, E.V.; Mathew, J.; Radhakrishnan, E.K. Extracellular synthesis of silver nanoparticles by the bacillus strain CS 11 isolated from industrialized area. 3 Biotech 2013, 4, 121–126. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Wijesekera, K.; Filipe, C.D.; Brennan, J.D. Stoichiometrically controlled production of bimetallic gold-silver alloy colloids using micro-alga cultures. J. Colloid Interface Sci. 2014, 416, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Shabnam, N.; Sharmila, P.; Kim, H.; Pardha-Saradhi, P. Light mediated generation of silver nanoparticles by spinach thylakoids/chloroplasts. PLoS ONE 2016, 11, e0167937. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, J.Y.; Wang, D.I.C.; Ting, Y.P. Silver nanoplates: From biological to biomimetic synthesis. ACS Nano 2007, 1, 429–439. [Google Scholar] [CrossRef] [PubMed]
- AbdelRahim, K.; Mahmoud, S.Y.; Ali, A.M.; Almaary, K.S.; Mustafa, A.E.; Husseiny, S.M. Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J. Biol. Sci. 2017, 24, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Dauthal, P.; Mukhopadhyay, M. Biosynthesis of palladium nanoparticles using Delonix regia leaf extract and its catalytic activity for nitro-aromatics hydrogenation. Ind. Eng. Chem. Res. 2013, 52, 18131–18139. [Google Scholar] [CrossRef]
- Farhadi, K.; Pourhossein, A.; Forough, M.; Molaei, R.; Abdi, A.; Siyami, A. Biosynthesis of highly dispersed palladium nanoparticles using Astraglmanna aqueous extract. J. Chin. Chem. Soc. 2013, 60, 1144–1149. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, Q.; Li, Q.; Song, H. The biosynthesis of palladium nanoparticles by antioxidants in Gardenia jasminoides ellis: Long lifetime nanocatalysts for p-nitrotoluene hydrogenation. Nanotechnology 2009, 20, 385601. [Google Scholar] [CrossRef] [PubMed]
- Omajali, J.B.; Mikheenko, I.P.; Merroun, M.L.; Wood, J.; Macaskie, L.E. Characterization of intracellular palladium nanoparticles synthesized by Desulfovibrio desulfuricans and Bacillus benzeovorans. J. Nanopart. Res. 2015, 17, 264. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Barberousse, H.; Hemadi, M.; Djédjat, C.; Yéprémian, C.; Coradin, T.; Livage, J.; Fiévet, F.; Couté, A. Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme-mediated route. J. Nanosci. Nanotechnol. 2007, 7, 2696–2708. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Biosynthesis of silver and platinum nanoparticles using orange peel extract: Characterisation and applications. IET Nanobiotechnol. 2015, 9, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Riddin, T.L.; Gericke, M.; Whiteley, C.G. Analysis of the inter- and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology 2006, 17, 3482–3489. [Google Scholar] [PubMed]
- Govindaraju, K.; Basha, S.K.; Kumar, V.G.; Singaravelu, G. Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) geitler. J. Mater. Sci. 2008, 43, 5115–5122. [Google Scholar] [CrossRef]
- Roychoudhury, P.; Ghosh, S.; Pal, R. Cyanobacteria mediated-green synthesis of gold-silver nanoalloy. J. Plant. Biochem. Biotechnol. 2016, 25, 73–78. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Roy, N.; Laskar, R.A.; Sk, I.; Basu, S.; Mandal, D.; Begum, N.A. Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani Jacq) leaves. Colloids Surf. B 2011, 82, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, G.; Qian, Q.; Cui, D. Chloroplasts-mediated biosynthesis of nanoscale Au-Ag alloy for 2-butanone assay based on electrochemical sensor. Nanoscale Res. Lett. 2012, 7, 475. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Extracellular biosynthesis of bimetallic Au-Ag alloy nanoparticles. Small 2005, 1, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Castro-Longoria, E.; Vilchis-Nestor, A.R.; Avalos-Borja, M. Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surf. B 2011, 83, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Pradeep, T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Hosseinkhani, B.; Sobjerg, L.S.; Rotaru, A.E.; Emtiazi, G.; Skrydstrup, T.; Meyer, R.L. Microbially supported synthesis of catalytically active bimetallic Pd-Au nanoparticles. Biotechnol. Bioeng. 2012, 109, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, Y.; Hong, Z.; Cai, K.; Zhao, F.; Han, H. Microbial synthesis of highly dispersed PdAu alloy for enhanced electrocatalysis. Sci. Adv. 2016, 2, e1600858. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, H.; Pu, S.; Zhang, W.; Liao, X.; Shi, B. One-step room-temperature synthesis of Au@Pd core-shell nanoparticles with tunable structure using plant tannin as reductant and stabilizer. Green Chem. 2011, 13, 950–957. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, G.; Huang, J.; Wang, H.; Li, Q. Plant-mediated fabrication and surface enhanced raman property of flower-like Au@Pd nanoparticles. Materials 2014, 7, 1360–1369. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Abboud, Y.; Saffaj, T.; Chagraoui, A.; El Bouari, A.; Brouzi, K.; Tanane, O.; Ihssane, B. Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata). Appl. Nanosci. 2013, 4, 571–576. [Google Scholar] [CrossRef]
- Mahdavi, M.; Namvar, F.; Ahmad, M.B.; Mohamad, R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 2013, 18, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Rautaray, D.; Bharde, A.; Ahire, K.; Sanyal, A.; Ahmad, A.; Sastry, M. Fungus-mediated biosynthesis of silica and titania particles. J. Mater. Chem. 2005, 15, 2583–2589. [Google Scholar] [CrossRef]
- Bansal, V.; Rautaray, D.; Ahmad, A.; Sastry, M. Biosynthesis of zirconia nanoparticles using the fungus Fusarium oxysporum. J. Mater. Chem. 2004, 14, 3303–3305. [Google Scholar] [CrossRef]
- Li, P.; Wu, C.; Wu, Q.; Li, J.; Li, H. Biosynthesis of different morphologies of CaCO3 nanomaterial assisted by thermophilic strains HEN-Qn1. J. Nanopart. Res. 2008, 11, 903–908. [Google Scholar] [CrossRef]
- Pandimurugan, R.; Thambidurai, S. Seaweed-ZnO composite for better antibacterial properties. J. Appl. Polym. Sci. 2014, 131, 40948. [Google Scholar] [CrossRef]
- Pandimurugan, R.; Thambidurai, S. Novel seaweed capped ZnO nanoparticles for effective dye photodegradation and antibacterial activity. Adv. Powder Technol. 2016, 27, 1062–1072. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Djédiat, C.; Yéprémian, C.; Couté, A.; Fiévet, F.; Brayner, R. Design of magnetic akaganeite-cyanobacteria hybrid biofilms. Thin Solid Films 2010, 518, 5432–5436. [Google Scholar] [CrossRef]
- Brayner, R.; Yéprémian, C.; Djédiat, C.; Coradin, T.; Herbst, F.; Livage, J.; Fiévet, F.; Couté, A. Photosynthetic microorganism-mediated synthesis of akaganeite (beta-FeOOH) nanorods. Langmuir 2009, 25, 10062–10067. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Coradin, T.; Beaunier, P.; Grenèche, J.M.; Djédiat, C.; Yéprémian, C.; Couté, A.; Fiévet, F. Intracellular biosynthesis of superparamagnetic 2-lines ferri-hydrite nanoparticles using Euglena gracilis microalgae. Colloids Surf. B 2012, 93, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kumeria, T.; Santos, A.; Forward, P.; Lambert, M.F.; Losic, D. Iron oxide nanowires from bacteria biofilm as an efficient visible-light magnetic photocatalyst. ACS Appl. Mater. Interfaces 2016, 8, 20110–20119. [Google Scholar] [CrossRef] [PubMed]
- Bharde, A.; Rautaray, D.; Bansal, V.; Ahmad, A.; Sarkar, I.; Yusuf, S.M.; Sanyal, M.; Sastry, M. Extracellular biosynthesis of magnetite using fungi. Small 2006, 2, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Dahoumane, S.A.; Nguyen, J.N.-L.; Yéprémian, C.; Djédiat, C.; Couté, A.; Fiévet, F. Ecotoxicological studies of CdS nanoparticles on photosynthetic microorganisms. J. Nanosci. Nanotechnol. 2011, 11, 1852–1858. [Google Scholar] [CrossRef]
- Shakouri-Arani, M.; Salavati-Niasari, M. Synthesis and characterization of cadmium sulfide nanocrystals in the presence of a new sulfur source via a simple solvothermal method. New J. Chem. 2014, 38, 1179. [Google Scholar] [CrossRef]
- Yang, H.; Huang, C.; Li, X.; Shi, R.; Zhang, K. Luminescent and photocatalytic properties of cadmium sulfide nanoparticles synthesized via microwave irradiation. Mater. Chem. Phys. 2005, 90, 155–158. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Girao, A.V.; Trindade, T.; Costa, M.C.; Duarte, A.; Rocha-Santos, T. Biological synthesis of nanosized sulfide semiconductors: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 8283–8302. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.Y.; Mao, C.; Gao, X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Iverson, B.L. Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Ponnanikajamideen, M.; Malarkodi, C.; Malini, M.; Annadurai, G. Microbe-mediated synthesis of antimicrobial semiconductor nanoparticles by marine bacteria. J. Nanostruct. Chem. 2014, 4. [Google Scholar] [CrossRef]
- Bai, H.J.; Zhang, Z.M.; Guo, Y.; Yang, G.E. Biosynthesis of cadmium sulfide nanoparticles by photosynthetic bacteria Rhodopseudomonas palustris. Colloids Surf. B 2009, 70, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Borovaya, M.; Pirko, Y.; Krupodorova, A.; Naumenko, A.; Blume, Y.; Yemets, A. Biosynthesis of cadmium sulphide quantum dots by using Pleurotus ostreatus (Jacq.) P. Kumm. Biotechnol. Biotechnol. Equip. 2015, 29, 1156–1163. [Google Scholar] [CrossRef]
- Reyes, L.; Gomez, I.; Garza, M.T. Biosynthesis of cadmium sulfide nanoparticles by the fungi Fusarium sp. Int. J. Nanotechnol. 2009, 1, 90–95. [Google Scholar] [CrossRef]
- Prasad, K.S.; Amin, T.; Katuva, S.; Kumari, M.; Selvaraj, K. Synthesis of water soluble CdS nanoparticles and study of their DNA damage activity. Arab. J. Chem. 2014. [Google Scholar] [CrossRef]
- Borovaya, M.N.; Naumenko, A.P.; Matvieieva, N.A.; Blume, Y.B.; Yemets, A.I. Biosynthesis of luminescent CdS quantum dots using plant hairy root culture. Nanoscale Res. Lett. 2014, 9:686. [Google Scholar] [CrossRef] [PubMed]
- Al-Shalabi, Z.; Stevens-Kalceff, M.A.; Doran, P.M. Application of Solanum lycopersicum (tomato) hairy roots for production of passivated CdS nanocrystals with quantum dot properties. Biochem. Eng. J. 2014, 84, 36–44. [Google Scholar] [CrossRef]
- Mandal, R.P.; Sekh, S.; Sen Sarkar, N.; Chattopadhyay, D.; De, S. Algae mediated synthesis of cadmium sulphide nanoparticles and their application in bioremediation. Mater. Res. Express 2016, 3:055007. [Google Scholar] [CrossRef]
- Rao, M.D.; Pennathur, G. Green synthesis and characterization of cadmium sulphide nanoparticles from Chlamydomonas reinhardtii and their application as photocatalysts. Mater. Res. Bull. 2017, 85, 64–73. [Google Scholar] [CrossRef]
- MubarakAli, D.; Gopinath, V.; Rameshbabu, N.; Thajuddin, N. Synthesis and characterization of CdS nanoparticles using C-phycoerythrin from the marine cyanobacteria. Mater. Lett. 2012, 74, 8–11. [Google Scholar] [CrossRef]
- Dunleavy, R.; Lu, L.; Kiely, C.J.; McIntosh, S.; Berger, B.W. Single-enzyme biomineralization of cadmium sulfide nanocrystals with controlled optical properties. Proc. Natl. Acad. Sci. USA 2016, 113, 5275–5280. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Lu, Z.; Cui, X.; Qiao, Y.; Guo, J.; Anderson, J.M.; Li, C.M. Extracellular microbial synthesis of biocompatible CdTe quantum dots. Acta Biomater. 2010, 6, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Hao, N.; Yang, Y.; Zhao, D. Biosynthesis of biocompatible cadmium telluride quantum dots using yeast cells. Nano Res. 2010, 3, 481–489. [Google Scholar] [CrossRef]
- Syed, A.; Ahmad, A. Extracellular biosynthesis of CdTe quantum dots by the fungus Fusarium oxysporum and their anti-bacterial activity. Spectrochim. Acta A 2013, 106, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.-H.; Cui, R.; Zhang, Z.-L.; Yu, X.; Xie, Z.; Shi, Y.-B.; Pang, D.-W. Uniform fluorescent nanobioprobes for pathogen detection. ACS Nano 2014, 8, 5116–5124. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, R.; Zhang, P.; Chen, B.-B.; Tian, Z.-Q.; Li, L.; Hu, B.; Pang, D.-W.; Xie, Z.-X. Mechanism-oriented controllability of intracellular quantum dots formation: The role of glutathione metabolic pathway. ACS Nano 2013, 7, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, M.G.; Yoo, B.; Myung, N.V.; Maeng, J.; Lee, T.; Dohnalkova, A.C.; Fredrickson, J.K.; Sadowsky, M.J.; Hur, H.G. Biogenic formation of photoactive arsenic-sulfide nanotubes by Shewanella sp. strain HN-41. Proc. Natl. Acad. Sci. USA 2007, 104, 20410–20415. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Lee, J.H.; Kim, M.G.; Myung, N.V.; Fredrickson, J.K.; Sadowsky, M.J.; Hur, H.G. Biogenic formation of As-S nanotubes by diverse Shewanella strains. Appl. Environ. Microbiol. 2009, 75, 6896–6899. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Flynn, C.E.; Hayhurst, A.; Sweeney, R.; Qi, J.; Georgiou, G.; Iverson, B.; Belcher, A.M. Viral assembly of oriented quantum dot nanowires. Proc. Natl. Acad. Sci. USA 2003, 100, 6946–6951. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahoumane, S.A.; Jeffryes, C.; Mechouet, M.; Agathos, S.N. Biosynthesis of Inorganic Nanoparticles: A Fresh Look at the Control of Shape, Size and Composition. Bioengineering 2017, 4, 14. https://doi.org/10.3390/bioengineering4010014
Dahoumane SA, Jeffryes C, Mechouet M, Agathos SN. Biosynthesis of Inorganic Nanoparticles: A Fresh Look at the Control of Shape, Size and Composition. Bioengineering. 2017; 4(1):14. https://doi.org/10.3390/bioengineering4010014
Chicago/Turabian StyleDahoumane, Si Amar, Clayton Jeffryes, Mourad Mechouet, and Spiros N. Agathos. 2017. "Biosynthesis of Inorganic Nanoparticles: A Fresh Look at the Control of Shape, Size and Composition" Bioengineering 4, no. 1: 14. https://doi.org/10.3390/bioengineering4010014





