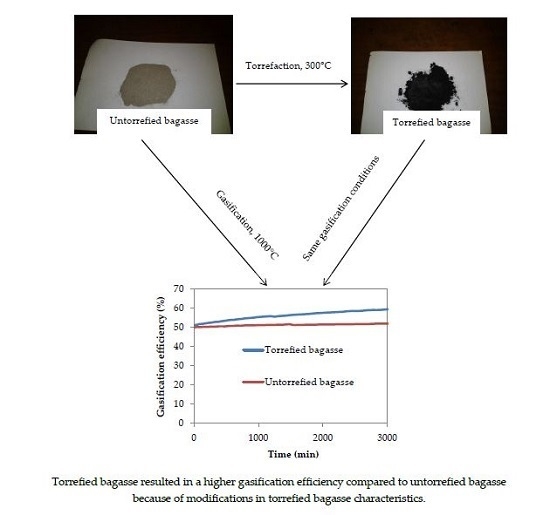
Influence of Torrefaction on the Conversion Efficiency of the Gasification Process of Sugarcane Bagasse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Torrefaction Process
2.3. Product Characterization
2.3.1. Analysis of Gaseous Products
2.3.2. Solid Product Analysis
2.3.3. Proximate and Ultimate Analysis
2.3.4. Calorific Value Determination
2.3.5. Thermogravimetric Analysis
2.3.6. Scanning Electron Microscopic Analysis
2.3.7. Gasification Simulation
3. Results
3.1. Product Distribution from Bagasse Torrefaction
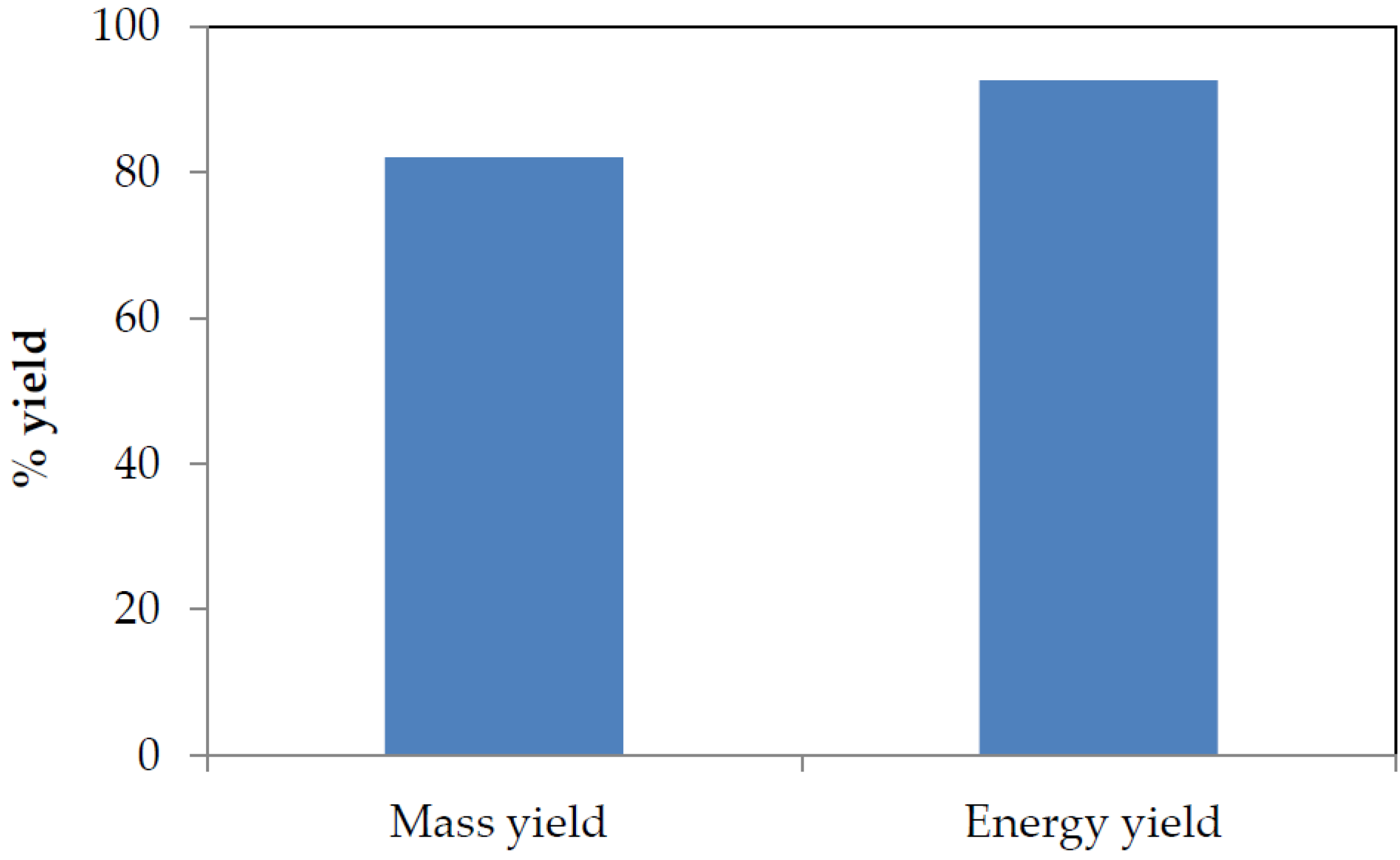
3.2. Mass and Energy Yield
3.3. Physical, Chemical and Calorific Value Analyses
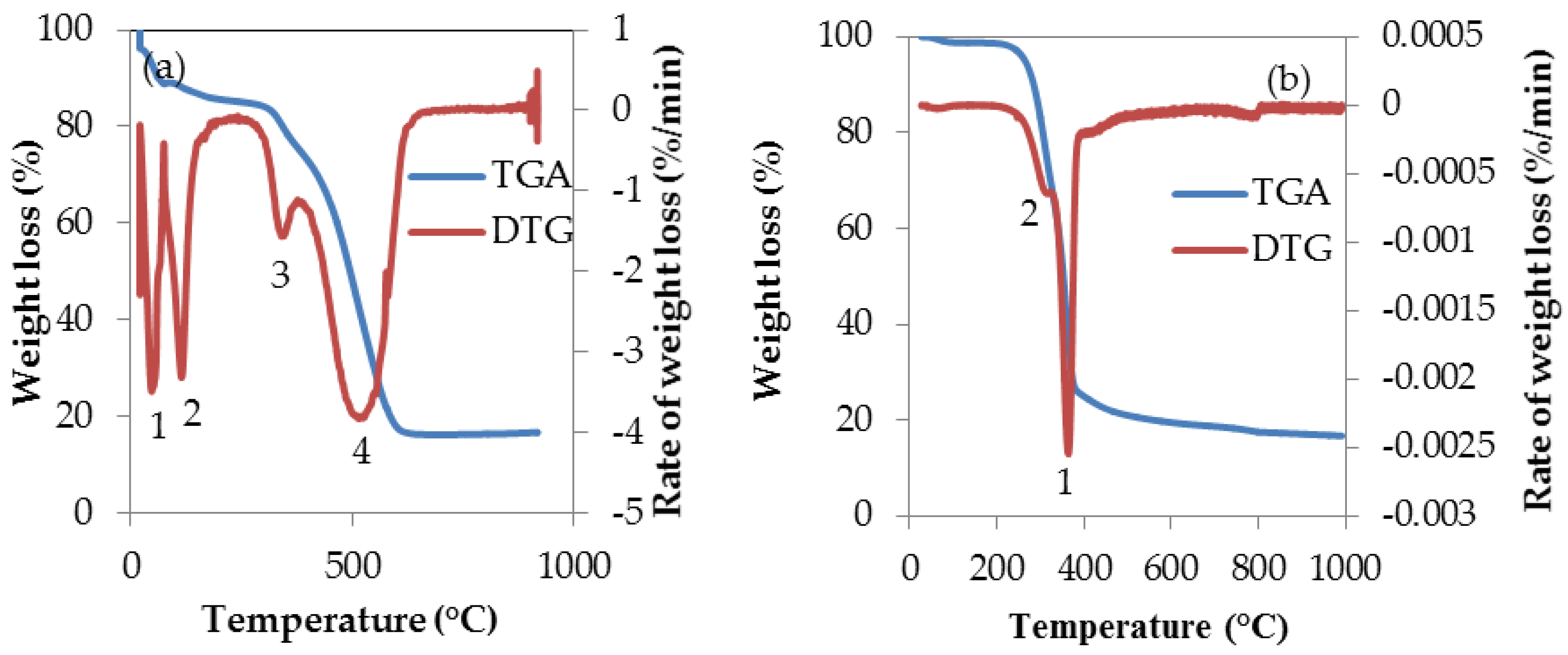
3.4. Thermal Characteristics
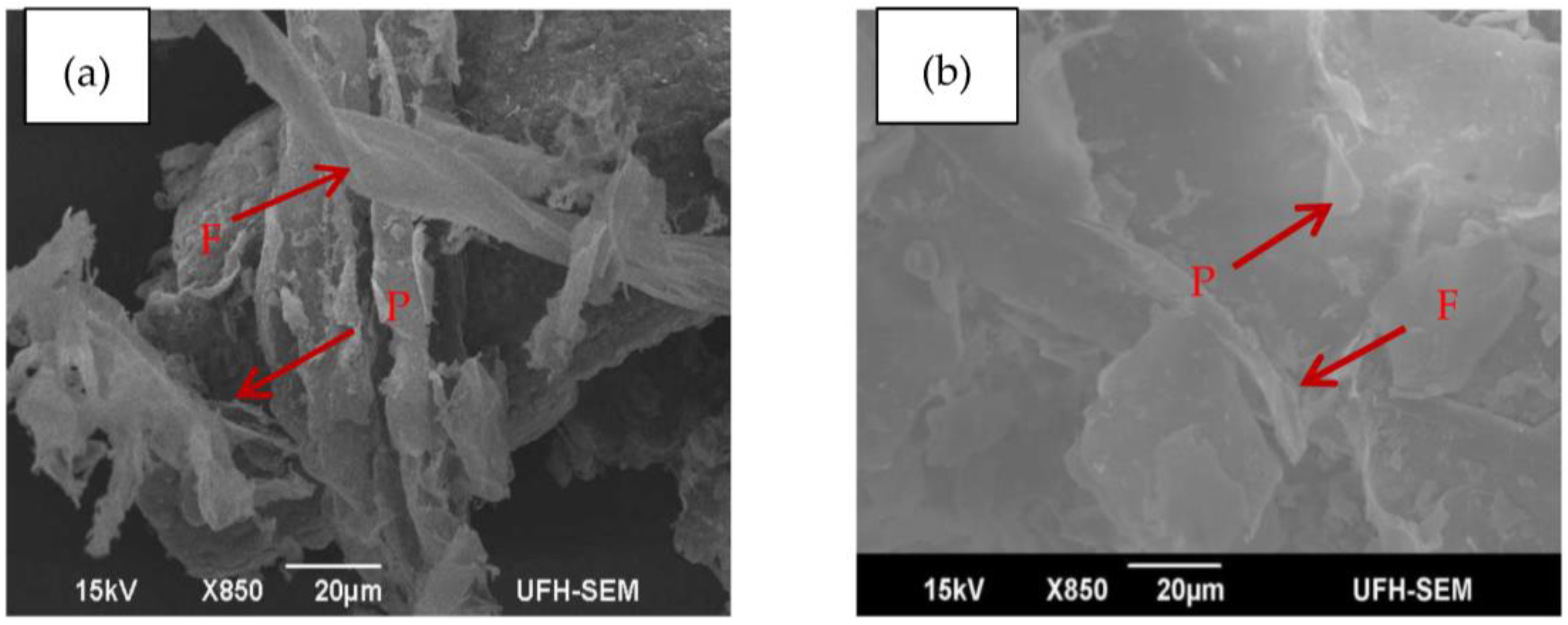
3.5. Morphological Characterization
3.6. Gasification Simulation Process
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GHG | Greenhouse gas |
| FC | Fixed carbon |
| MC | Moisture content |
| VMC | Volatile matter content |
| HHV | Higher heating value, (solid) MJ·kg−1 (gas) MJ·Nm−3 |
| SCB | Sugarcane bagasse |
| CV | Calorific value |
| GC | Gas chromatography |
| W1 | Weight of biomass prior to torrefaction |
| W2 | Weight of biomass after torrefaction |
| CVgas | Calorific value of gas |
| CVfuel | Calorific value of fuel |
| TGA | Thermogravimetric analysis |
| DTG | Derivative of thermogravimetry |
| RW | Rate of weight loss |
| F | Fibre structure |
| P | Pith |
| ca. | Approximately |
References
- GIZ. Renewable Energies and Energy Efficiency for a Greener Future, 2016. Available online: https://www.giz.de/en/worldwide/17790.html (accessed on 3 December 2014).
- International Energy Outlook. Chapter 4-“Coal”. Energy Information Administration, 2009. Available online: http://en.wikipedia.org/wiki/Coal_in_South_Africa (accessed on 28 January 2015).
- Balat, M. Influence of coal as an energy source on environmental pollution. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 29, 581–589. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Principles and practice of biomass fast pyrolysis processes for liquids. J. Anal. Appl. Pyrolysis 1999, 51, 3–22. [Google Scholar] [CrossRef]
- Faaij, A. Modern biomass conversion technologies. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 335–367. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Peacocke, G.V.C. Fast pyrolysis processes for biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Demirbas, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Srinivasan, R.; Yu, F.; Steele, P.; Li, Q.; Mitchell, B. Effect of acid, alkali, and steam explosion pretreatments on characteristics of bio-oil produced from pinewood. Energy Fuels 2011, 25, 3758–3764. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Effect of thermal pretreatment on equilibrium moisture content of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.M.; Steele, P.H.; Ingram, L. Characterization of fast pyrolysis bio-oils produced from pretreated pine wood. Appl. Biochem. Biotechnol. 2009, 154, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Zwart, R.W.R.; Boerrigter, H.; van der Drift, A. The impact of biomass pretreatment on the feasibility of overseas biomass conversion to Fischer-Tropsch products. Energy Fuels 2006, 20, 2192–2197. [Google Scholar] [CrossRef]
- Van der Stelta, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Phanphanich, M.; Mani, S. Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Cheng, W.Y.; Lu, K.M.; Huang, Y.P. An evaluation on improvement of pulverized biomass property for solid fuel through torrefaction. Appl. Energy 2011, 88, 3636–3644. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Williams, A.; Waldron, D.J. An investigation of the grindability of two torrefied energy crops. Fuel 2010, 89, 3911–3918. [Google Scholar] [CrossRef]
- Repellin, V.; Govin, A.; Rolland, M.; Guyonnet, R. Energy requirement for fine grinding of torrefied wood. Biomass Bioenergy 2010, 34, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Chew, J.J.; Doshi, V. Recent advances in biomass pretreatment-torrefaction fundamentals and technology. Renew. Sustain. Energy Rev. 2011, 15, 4212–4222. [Google Scholar] [CrossRef]
- Parikh, J.; Channiwala, S.A.; Ghosal, G.K. A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 2005, 84, 487–494. [Google Scholar] [CrossRef]
- Chen, W.H.; Kuo, P.C. Isothermal torrefaction kinetics of hemicellulose, cellulose, lignin and xylan using thermogravimetric analysis. Energy 2011, 36, 6451–6460. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Shield, I.; Williams, P.T. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Wannapeera, J.; Fungtammasan, B.; Worasuwannarak, N. Effects of temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrolysis 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Lu, K.M.; Lee, W.J.; Chen, W.H.; Liu, S.H.; Lin, T.C. Torrefaction and low temperature carbonization of oil palm fiber and eucalyptus in nitrogen and air atmospheres. Bioresour. Technol. 2012, 123, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Du, S.W.; Tsai, C.H.; Wang, Z.Y. Torrefied biomasses in a drop tube furnace to evaluate their utility in blast furnaces. Bioresour. Technol. 2012, 111, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, J.S.; Liu, B.J.; Mei, Q.F.; Luo, Z.Y. Influence of torrefaction pretreatment on biomass gasification technology. Chin. Sci. Bull. 2011, 56, 1449–1456. [Google Scholar] [CrossRef]
- Rousset, P.; Aguiar, C.; Labbe, N.; Commandre, J.M. Enhancing the combustible properties of bamboo by torrefaction. Bioresour. Technol. 2011, 102, 8225–8231. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.H.; Bi, H.T.; Sokhansanj, S.; Lim, J.C. A study of particle size effect on biomass torrefaction and densification. Energy Fuels 2012, 26, 3826–3839. [Google Scholar] [CrossRef]
- Chen, W.H.; Hsu, H.C.; Lu, K.M.; Lee, W.J.; Lin, T.C. Thermal pretreatment of wood (Lauan) block by torrefaction and its influence on the properties of the biomass. Energy 2011, 36, 3012–3021. [Google Scholar] [CrossRef]
- Uemura, Y.; Omar, W.N.; Tsutsui, T.; Yusup, S.B. Torrefaction of oil palm wastes. Fuel 2011, 90, 2585–2591. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Legros, R.; Bi, X.T.; Lim, C.J.; Sokhansanj, S. Torrefaction of sawdust in a fluidized bed reactor. Bioresour. Technol. 2012, 103, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, Y.H.; Lee, S.M.; Lee, H.W. Optimizing the torrefaction of mixed softwood by response surface methodology for biomass upgrading to high energy density. Bioresour. Technol. 2012, 116, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Dorde, M. Investigation of Torrefaction Process Parameters and Characterisation of Torrefied Biomass. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2012. [Google Scholar]
- Johannes, T. Pyrolysis of Sugarcane Bagasse. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2010. [Google Scholar]
- Chang, S.; Zhao, Z.; Zheng, A.; He, F.; Huang, Z.; Li, H. Characterisation of products from torrefaction of sprucewood and bagasse in an auger reactor. Energy Fuels 2012, 26, 7009–7017. [Google Scholar] [CrossRef]
- Pimchuai, A.; Dutta, A.; Basu, P. Torrefaction of agriculture residue to enhance combustible properties. Energy Fuels 2010, 24, 4638–4645. [Google Scholar] [CrossRef]
- Bourgois, J.; Guyonnet, R. Characterization and analysis of torrefied wood. Wood Sci. Technol. 1988, 22, 143–155. [Google Scholar] [CrossRef]
- Pentananunt, R.; Rahman, A.N.M.M.; Bhattacharya, S.C. Upgrading of biomass by means of torrefaction. Energy 1990, 15, 1175–1179. [Google Scholar] [CrossRef]
- Anukam, A.; Mamphweli, S.; Meyer, E.; Okoh, O. Computer simulation of the mass and energy balance during gasification of sugarcane bagasse. J. Energy 2014, 1–15. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.J.; Kuang, J.H.; Zhang, Y.L.; Luo, Y.H. Pretreatment of agricultural residues for co-gasification via torrefaction. J. Anal. Appl. Pyrolysis 2009, 86, 331–337. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Couhert, C.; Salvador, S.; Commandré, J.M. Impact of torrefaction on syngas production from wood. Fuel 2009, 88, 2286–2290. [Google Scholar] [CrossRef]
- Boyd, T.; de Vries, D.; Kempthorne, H.; Wearing, J.; Wolff, I. Mass and Energy Balance for Torrefied Pellet Production. In Proceedings of the UBC Biomass Pelletization Workshop, Vancouver, BC, Canada, 17–18 May 2011.
- Melin, S. Torrefied Wood—A New Emerging Energy Carrier; Clean Coal Power Coalition of Canada: Calgary, AB, Canada, 2011. [Google Scholar]
- Ostermeijer, G. Possibilities and Bottlenecks for Long Distance Transport of Torrefied Materials–Biomass a Challenge for Quality Control and Logistics Systems 2011; Peterson Control Union Group: Rotterdam, The Netherlands, 2011. [Google Scholar]
- Luo, X. Torrefaction of Biomass—A Comparative and Kinetic Study of Thermal Decomposition for Norway Spruce Stump, Poplar and Fuel Tree Chips. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2011. [Google Scholar]
- Bergman, P.C.A.; Boersma, A.R.; Kiel, J.H.A.; Prins, M.J.; Ptasinski, K.J.; Janssen, F.G.G.J. Torrefied Biomass for Entrained-Flow Gasification of Biomass; Report ECN-C-05-026; Energy Research Centre of the Netherlands (ECN): Petten, The Netherlands, 2005. [Google Scholar]
- Chen, W.H.; Lu, K.M.; Lee, W.J.; Liu, S.H.; Lin, T.C. Non-oxidative and oxidative torrefaction characterization and SEM observations of fibrous and ligneous biomass. Appl. Energy 2014, 114, 104–113. [Google Scholar] [CrossRef]
- Tanger, P.; Field, J.L.; Jahn, C.E.; de Foort, M.W.; Leach, J.E. Biomass for thermochemical conversion: Targets and challenges. Rev. Artic. Front. Plant Sci. 2013, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Leco Corporation. Moisture, Volatile Matter, Ash, and Fixed Carbon Determination-Solid Fuel Characterization Measurements in Coke. Organic Application Note 2010, Form No. 203-821-381. Available online: http://www.leco.co.za/wp-content/uploads/2012/02/TGA701_COKE_203-821-381.pdf (accessed on 15 April 2015).
- ASTM Standards. ASTM D 5142-04: Standard Test Method for Proximate Analysis of the Analysis Sample of Coal and Coke by Instrumental Procedures; ASTM Standards: West Conshohocken, PA, USA, 2008; Volume 05.06. [Google Scholar]
- Sheng, C.; Azevedo, J.L.T. Estimating the higher heating value of biomass fuels from basic analysis data. Biomass Bioenergy 2005, 28, 499–507. [Google Scholar] [CrossRef]
- Chen, J. Kinetic Engineering Modeling of Co-Current Moving Bed Gasification Reactors for Carbonaceous Material; Cornell University: New York, NY, USA, 1986. [Google Scholar]
- Jayah, T. Evaluation of a Downdraft Wood Gasifier for Tea Manufacturing in Sri Lanka; Melbourne University: Victoria, Australia, 2002. [Google Scholar]
- Mamphweli, S. Implementation of a 150KVA Biomass Gasifier System for Community Economic Empowerment in South Africa. Ph.D. Thesis, University of Fort Hare, Alice, South Africa, 2010. [Google Scholar]
- Bjerketvedt, D.; Bakke, J.; Wingerden, K. Gas explosion handbook. J. Hazard. Mater. 1997, 52, 1–150. [Google Scholar] [CrossRef]
- Fossum, M.; Beyer, R. Co-Combustion: Biomass Fuel Gas and Natural Gas; SINTEF Energy Research: Trondheim, Norway, 1998. [Google Scholar]
- Anukam, A.; Mamphweli, S.; Reddy, P.; Okoh, O.; Meyer, E. An investigation into the impact of reaction temperature on various parameters during torrefaction of sugarcane bagasse relevant to gasification. J. Chem. 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.C.A.; Boersman, A.R.; Zwart, R.W.R.; Kiel, J.H.A. Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations. “BIOCOAL”; Renewable Energy in the Netherlands; Energy Research Centre of the Netherlands (ECN): Petten, The Netherlands, 2005. [Google Scholar]
- Bourgois, J.P.; Doat, J. Torrefied Wood from Temperate and Tropical Species: Advantages and Prospects. In Bioenergy 84, Proceedings of the International Conference on Bioenergy, Goteborg, Sweden, 15–21 June 1984; Egneus, H., Ellegard, A., O’Keefe, P., Kristofferson, L., Eds.; Elsevier Applied Science: London, UK, 1984; pp. 153–159. [Google Scholar]
- Antal, M.J. Biomass pyrolysis: A review of the literature. Part I carbohydrate pyrolysis. Adv. Sol. Energy 1983, 11, 61–111. [Google Scholar]
- Mansaray, K.G.; Ghaly, A.E. Thermal degradation of rice husks in nitrogen atmosphere. Bioresour. Technol. 1998, 65, 13–20. [Google Scholar] [CrossRef]
- Xue, G.; Kwapinska, M.; Kwapinski, W.; Czajka, K.M.; Kennedy, J.; Leahy, J.J. Impact of torrefaction on properties of Miscanthus giganteus relevant to gasification. Fuel 2014, 121, 189–197. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood-Part 2. Analysis of products. J. Anal. Appl. Pyrolysis 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. The thermal performance of the polysaccharides extracted from hardwood: Cellulose and hemicellulose. Carbohydr. Polym. 2010, 82, 39–45. [Google Scholar] [CrossRef]
- White, R.H.; Dietenberger, M.A. Wood Products: Thermal Degradation and Fire. In The Encyclopedia of Materials: Science Technology; Elsevier Science Ltd.: London, UK, 2001; pp. 9712–9716. [Google Scholar]
- Lapuerta, M.; Hernandez, J.J.; Pazo, A.; Lopez, J. Gasification and co-gasification of biomass wastes: Effect of the biomass origin and the gasifier operating conditions. Fuel Process. Technol. 2008, 89, 828–837. [Google Scholar] [CrossRef]
- Pinto, F.; Franco, C.; André, R.N.; Miranda, M.; Gulyurtlu, I.; Cabrita, I. Co-gasification study of biomass mixed with plastic wastes. Fuel 2002, 81, 291–297. [Google Scholar] [CrossRef]
- Franco, C.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. The study of reactions influencing the biomass steam gasification process. Fuel 2003, 82, 835–842. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, S.H.; Kim, S.D. Coal gasification characteristics in a downer reactor. Fuel 2001, 80, 1915–1922. [Google Scholar] [CrossRef]
- Taba, L.E.; Irfan, M.F.; Wan Daud, W.A.M.F.; Chakrabarti, M.H. The effect of temperature on various parameters in coal, biomass and co-gasification: A review. Renew. Sustain. Energy Rev. 2012, 16, 5584–5596. [Google Scholar] [CrossRef]
- Grigiante, M.; Antolini, D. Experimental results of mass and energy yield referred to different torrefaction pathways. Waste Biomass Valor 2014, 5, 11–17. [Google Scholar] [CrossRef]
- Zakri, B. Integration of Torrefaction with Steam Power Plant; Department of Energy Technology, Lappeenranta University of Technology: Lappeenranta, Finland, 2013. [Google Scholar]
- Tumuluru, J.S.; Sokhansanj, S.; Wright, C.T.; Kremer, T. GC Analysis of Volatiles and Other Products from Biomass Torrefaction Process. In Advanced Gas Chromatography-Progress in Agricultural, Biomedical, and Industrial Applications; Mohd, M.A., Ed.; InTech Open Access Publishing Company: Shanghai, China, 2012. [Google Scholar]
- Bergman, P.C.A.; Prins, M.J.; Boersma, A.R.; Ptasinki, K.J.; Kiel, J.H.A.; Janssen, F.J.J.G. Torrefied Biomass for Entrained-Flow Gasification of Biomass. 2005. Report ECN-C-05-026. Available online: http://www.ecn.nl/docs/library/report/2005/c05067 (accessed on 19 May 2015).
- Larsson, A.; Seemann, M.; Thunman, H. Assessment of the Mass and Energy Flows in the Chalmers Gasifier. In Proceedings of the Scandinavian-Nordic Section of the Combustion Institute, Cambridge, UK, 27–28 March 2014; pp. 1–14.
- Brar, J.S.; Singh, K.; Wang, J.; Kumar, S. Co-gasification of coal and biomass: A review. Int. J. For. Res. 2012, 1–10. [Google Scholar] [CrossRef]
- Sarkar, P.; Sahu, S.G.; Chakraborty, N.; Adak, A.K. Studies on potential utilization of rice husk char in blend with lignite for co-combustion application. J. Therm. Anal. Calorim. 2014, 115, 1573–1581. [Google Scholar] [CrossRef]
- Sarkar, P.; Sahu, S.G.; Mukherjee, A.; Kumar, M.; Adak, A.K.; Chakraborty, N.; Biswas, S. Co-combustion studies for potential application of sawdust or its low temperature char as co-fuel with coal. Appl. Therm. Eng. 2014, 63, 616–623. [Google Scholar] [CrossRef]
- Li, J.; Brzdekiewicz, A.; Yang, W.; Blasiak, W. Co-firing based on biomass torrefaction in a pulverized coal boiler with aim of 100% fuel switching. Appl. Energy 2012, 99, 344–354. [Google Scholar] [CrossRef]
- Blasi, C.D.; Branca, C.; D’Errico, G. Degradation characteristics of straw and washed straw. Thermochim. Acta 2000, 364, 133–142. [Google Scholar] [CrossRef]
- Olsson, J.G.; Jäglid, U.; Pettersson, J.B.C. Alkali metal emission during pyrolysis of biomass. Energy Fuels 1997, 11, 779–784. [Google Scholar] [CrossRef]
- Evergreen Renewables LLC. Biomass Torrefaction as a Preprocessing Step for Thermal Conversion: Reducing Costs in the Biomass Supply Chain. White Paper 2009. Available online: http://evergreenrenewable.com/welcome_files/Biomass%20torrefaction.pdf (accessed on 4 June 2012).
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Boardman, R.D.; Wright, C.T.; Richard Hess, J.R. Some chemical compositional changes in miscanthus and white oak sawdust samples during torrefaction. Energies 2012, 5, 3928–3947. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Sokhansanj, S.; Hess, R.J.; Wright, C.T.; Boardman, R.D. A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Hess, R.J.; Boardman, R.D.; Wright, C.T.; Westover, T.L. Formulation, pretreatment, and densification options to improve biomass specifications for co-firing high percentages with coal. Ind. Biotechnol. 2012, 8, 113–132. [Google Scholar] [CrossRef]
- Couhert, C.; Commandre, J.M.; Salvador, S. Is it possible to predict gas yields of any biomass after rapid pyrolysis at high temperature from its composition in cellulose, hemi-cellulose and lignin? Fuel 2009, 88, 408–417. [Google Scholar] [CrossRef]
- Datar, R.P.; Shenkman, R.M.; Cateni, B.G.; Huhnke, R.L.; Lewis, R.S. Fermentation of biomass-generated producer gas to ethanol. Biotechnol. Bioenergy 2004, 86, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Borgwardt, R. Transportation fuel from cellulosic biomass: A comparative assessment of ethanol and methanol options. J. Power Energy 1999, 213, 399–407. [Google Scholar] [CrossRef]
- Pereira, E.G.; da Silva, J.N.; de Oliveira, J.L.; Machado, C.S. Sustainable energy: a review of gasification technologies. Renew. Sustain. Energy Rev. 2012, 16, 4753–4762. [Google Scholar] [CrossRef]
- Nhuchhen, D.R.; Basu, P.; Acharya, B. A comprehensive review on biomass torrefaction. Int. J. Renew. Energy Biofuels 2014, 1–56. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Bakker, R.R.; Wei, J.B. On the properties of washed straw. Biomass Bioenergy 1996, 10, 177–200. [Google Scholar] [CrossRef]
- Chen, W.H.; Kuo, P.C. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 2010, 35, 2580–2586. [Google Scholar] [CrossRef]
- Chen, W.H.; Kuo, P.C. Torrefaction and co-torrefaction characterization of hemicellulose, cellulose and lignin as well as torrefaction of some basic constituents in biomass. Energy 2011, 36, 803–811. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C. Combustion kinetics of secondary biomass chars in the kinetic regime. Energy Fuels 2010, 24, 5741–5750. [Google Scholar] [CrossRef]
- Felsner, M.L.; Cano, C.B.; Matos, J.R.; de Almeida-Muradian, L.B.; Bruns, R.E. Optimization of thermogravimetric analysis of ash content in honey. J. Braz. Chem. Soc. 2004, 15, 797–802. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.H.; Liu, J.Z. Catalysis effect of carbide lime on various coal combustion. J. Fuel Chem. Technol. 2004, 32, 37–42. [Google Scholar]
- Li, X.G.; Lv, Y.; Ma, B.G.; Jian, S.W.; Tan, H.B. Thermogravimetric investigation on co-combustion characteristics of tobacco residue and high-ash anthracite coal. Bioresour. Technol. 2011, 102, 9783–9787. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.G.; Sarkar, P.; Chakraborty, N.; Adak, A.K. Thermogravimetric assessment of combustion characteristics of blends of a coal with different biomass chars. Fuel Process. Technol. 2010, 91, 369–378. [Google Scholar] [CrossRef]
- Ahrenfeldt, J. Characterization of Biomass Producer Gas as Fuel for Stationary Gas Engines in Combined Heat and Power Production. Ph.D. Thesis, Technical University of Denmark, Kongens Lyngby, Denmark, 2007. [Google Scholar]
- Moilanen, A. Thermogravimetric Characterisations of Biomass and Waste for Gasification Processes. Ph.D. Thesis, Abo Akademi University, Turku, Finland, 2006. [Google Scholar]
- Nordin, A. Torrefaction of Biomass Technology and Development. In Proceedings of the Panndagarna Värme–Och Kraftföreningens Conference, Panndagarna, Örnsköldsvik, 1–2 February 2012.
- Eseyin, A.E.; Steele, P.H.; Pittman, C.U., Jr. Current trends in the production and applications of torrefied wood/biomass–A review. Bioresources 2015, 10, 8812–8858. [Google Scholar] [CrossRef]



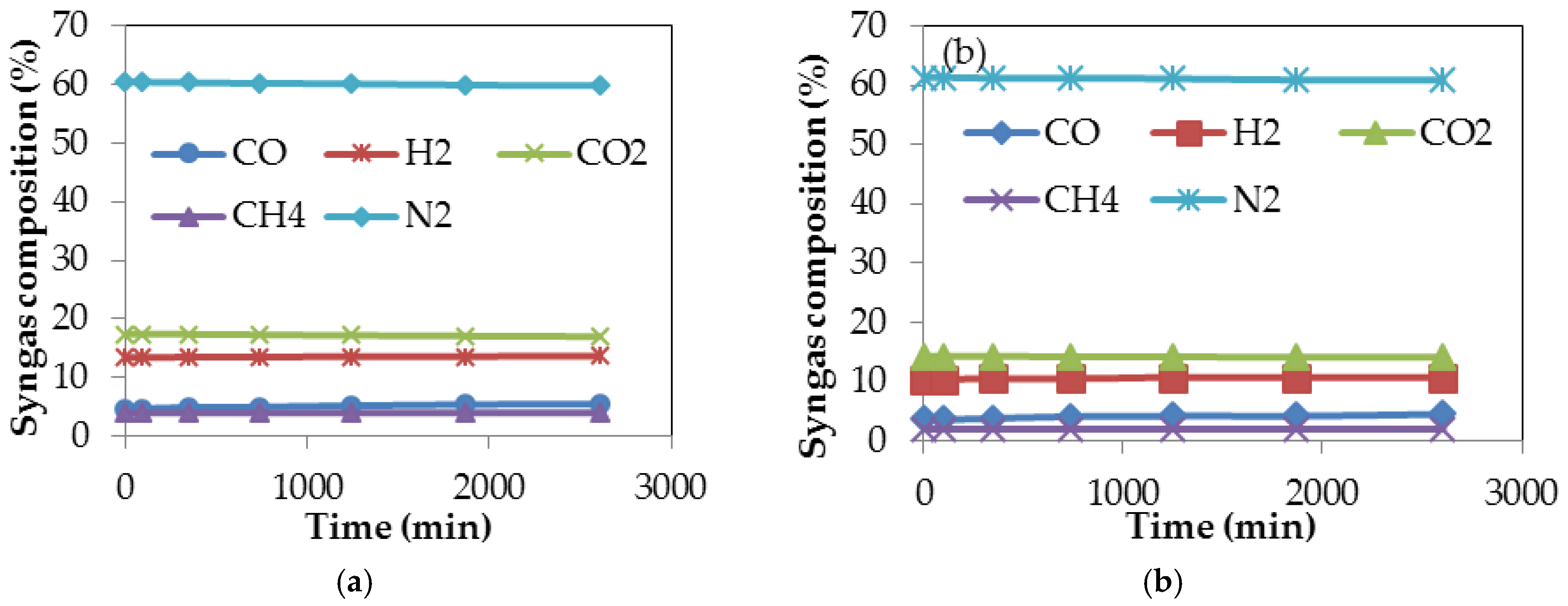
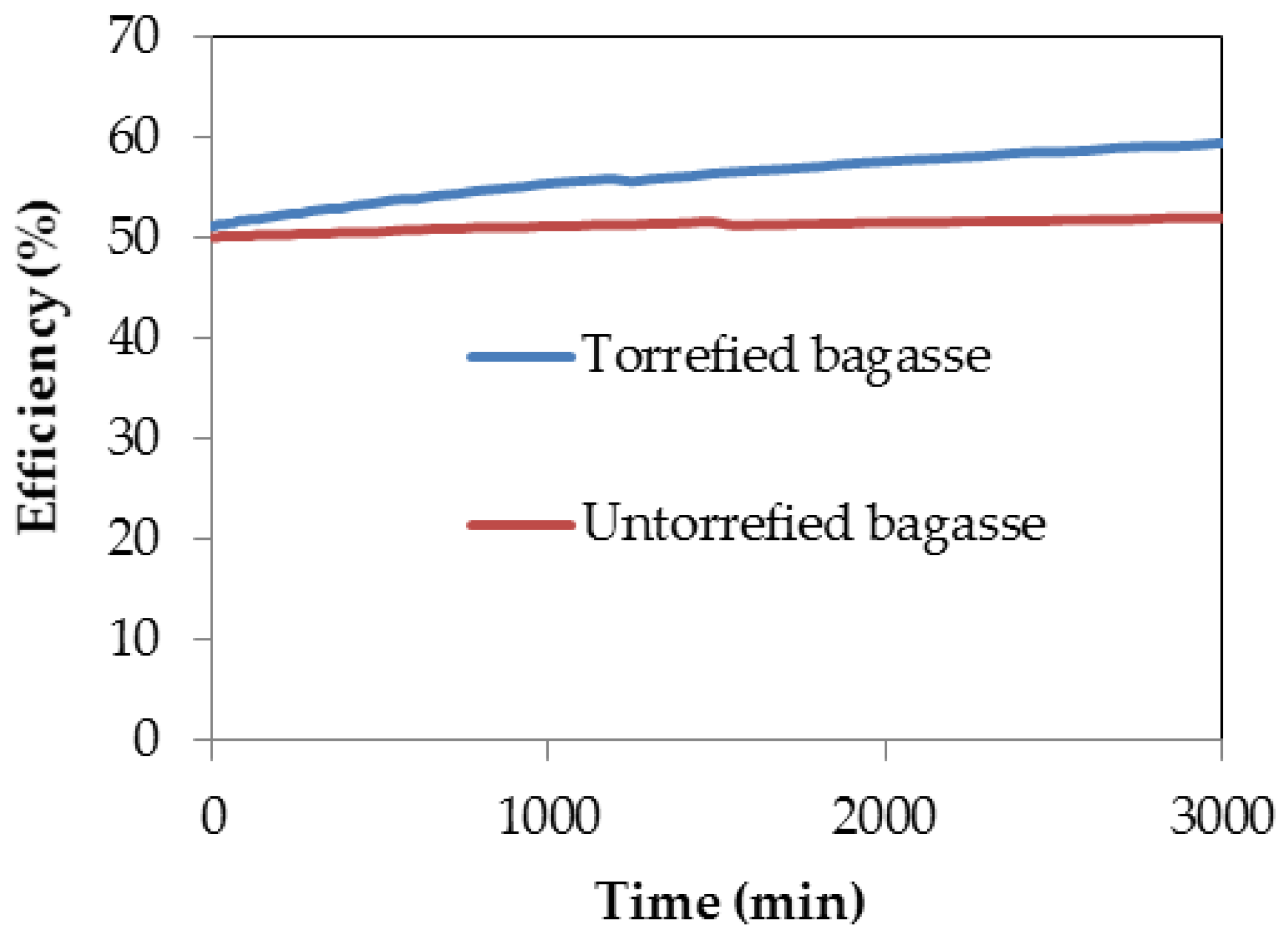
| Standard Gasification Conditions [53] | Untorrefied Sugarcane Bagasse (SCB) | Torrefied SCB | |||
|---|---|---|---|---|---|
| Gasifier operating parameters | Value | Fuel properties | Value | Fuel properties | Value |
| Throat diameter (cm) | 25.5 | C (wt %) | 44.1 | C (wt %) | 56.16 |
| Throat angle (°) | 30 | H (wt %) | 5.7 | H (wt %) | 3.94 |
| Insulation thickness (cm) | 17.5 | O (wt %) | 47.7 | O (wt %) | 37.27 |
| Thermal conductivity (W·cm−1·K) | 2.8 | N (wt %) | 0.20 | N (wt %) | 1.80 |
| Temperature of input air (K) | 300 | Fixed carbon (wt %) | 18.19 | Fixed carbon (wt %) | 28.45 |
| Air input (kg·h−1) | 44.5 | Bulk density (g·cm−3) | 0.178 | Bulk density (g·cm−3) | 1.70 |
| Feed input (kg·h−1) | 40 | Diameter of particle (cm) | 14.3 | Diameter of particle (cm) | 14.3 |
| Heat loss (%) | 12.8 | Moisture content (%) | 1.14 | Moisture content (%) | 0.87 |
| PropertiesCaption | Torrefied SCB | Untorrefied SCB |
|---|---|---|
| Proximate Analysis | (%) ± SD * | (%) ± SD * |
| Moisture content | 0.87 ± 0.01 | 1.14 ± 0.01 |
| Volatile matter content | 30.07 ± 0.01 | 71.73 ± 0.01 |
| Fixed carbon | 28.45 ± 0.01 | 18.19 ± 0.01 |
| Ash | 16.61 ± 0.01 | 1.32 ± 0.01 |
| Ultimate Analysis | ||
| C (%) | 56.16 ± 0.22 | 44.1 ± 0.06 |
| H (%) | 3.94 ± 0.05 | 5.7 ± 0.06 |
| O (%) | 37.27 ± 0.01 | 47.7 ± 0.06 |
| N (%) | 1.80 ± 0.01 | 0.20 ± 0.01 |
| O-C molar ratio | 0.50 | 0.82 |
| H-C molar ratio | 0.84 | 1.55 |
| Other Properties | ||
| Calorific value (MJ·kg−1) | 20.19 ± 0.01 | 17.86 ± 0.02 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anukam, A.; Mamphweli, S.; Okoh, O.; Reddy, P. Influence of Torrefaction on the Conversion Efficiency of the Gasification Process of Sugarcane Bagasse. Bioengineering 2017, 4, 22. https://doi.org/10.3390/bioengineering4010022
Anukam A, Mamphweli S, Okoh O, Reddy P. Influence of Torrefaction on the Conversion Efficiency of the Gasification Process of Sugarcane Bagasse. Bioengineering. 2017; 4(1):22. https://doi.org/10.3390/bioengineering4010022
Chicago/Turabian StyleAnukam, Anthony, Sampson Mamphweli, Omobola Okoh, and Prashant Reddy. 2017. "Influence of Torrefaction on the Conversion Efficiency of the Gasification Process of Sugarcane Bagasse" Bioengineering 4, no. 1: 22. https://doi.org/10.3390/bioengineering4010022