An Approach to In Vitro Manufacturing of Hypertrophic Cartilage Matrix for Bone Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Devitalization and Decellularization
2.3. Histological Staining and Immunostaining
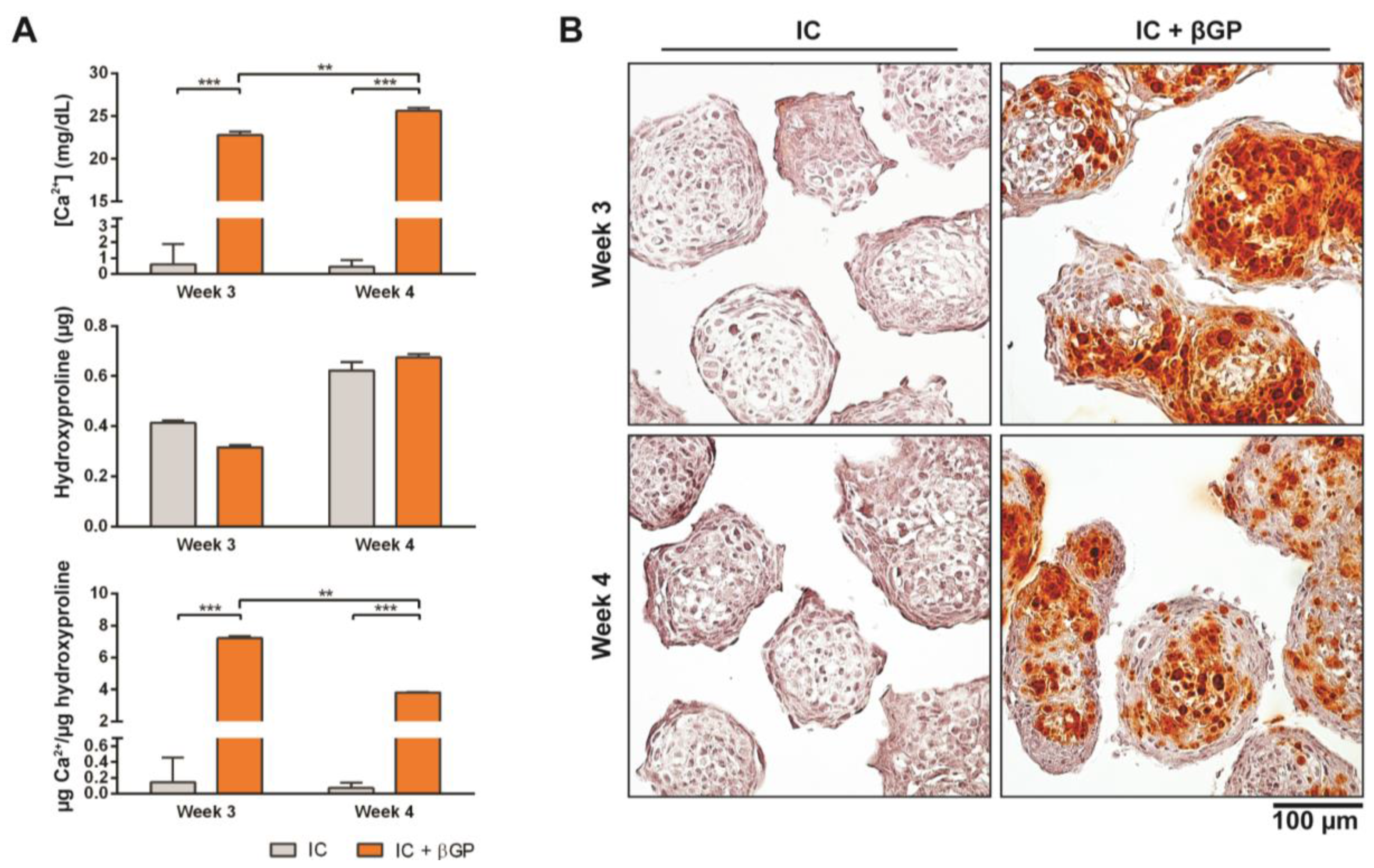
2.4. Mineralization, Calcium Assay and Hydroxyproline Assay
2.5. Gene Expression Analysis
2.6. ELISA
2.7. Statistics
3. Results
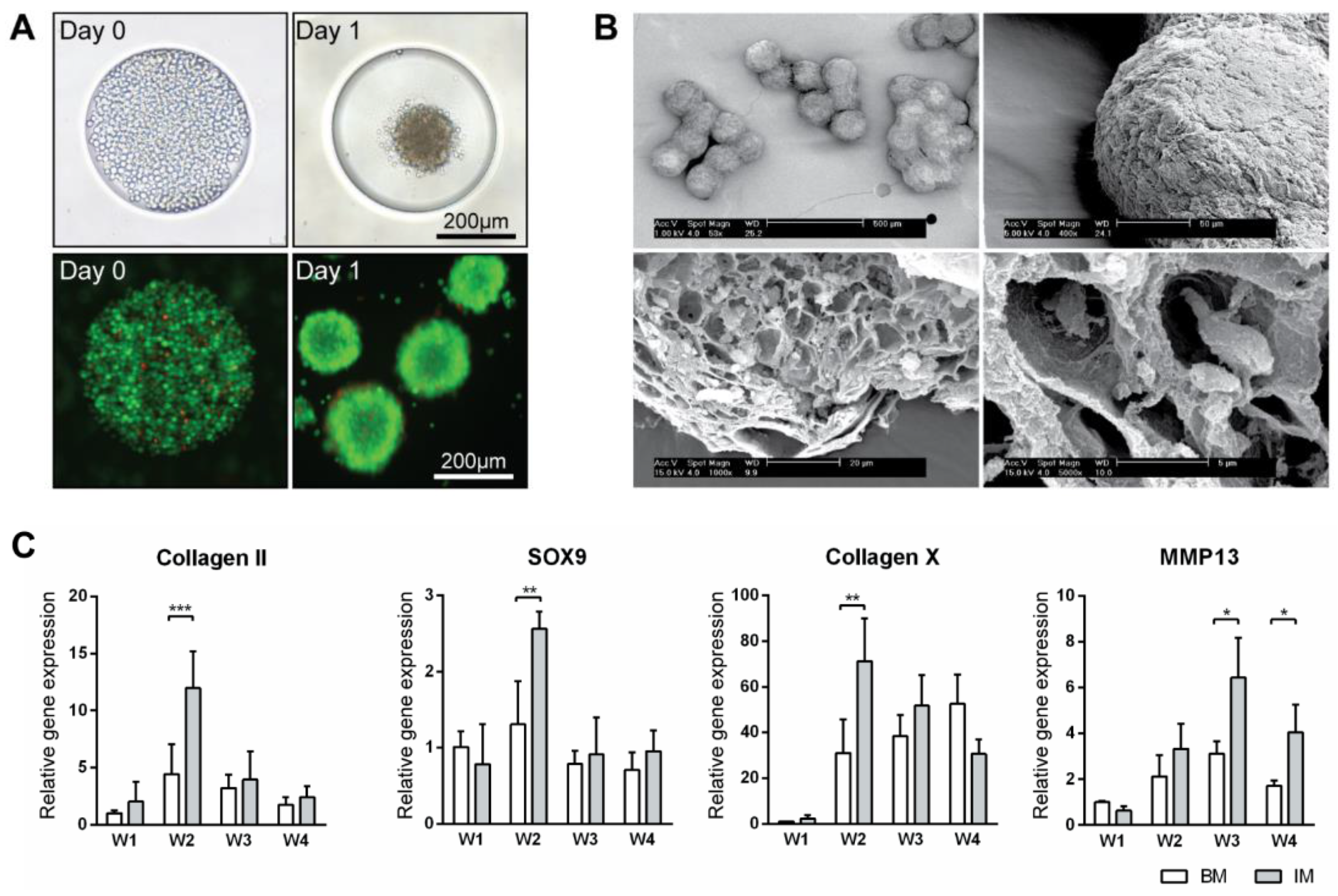
3.1. Bulk Production of Micro-Tissue Engineered Cartilage (MiTEC)
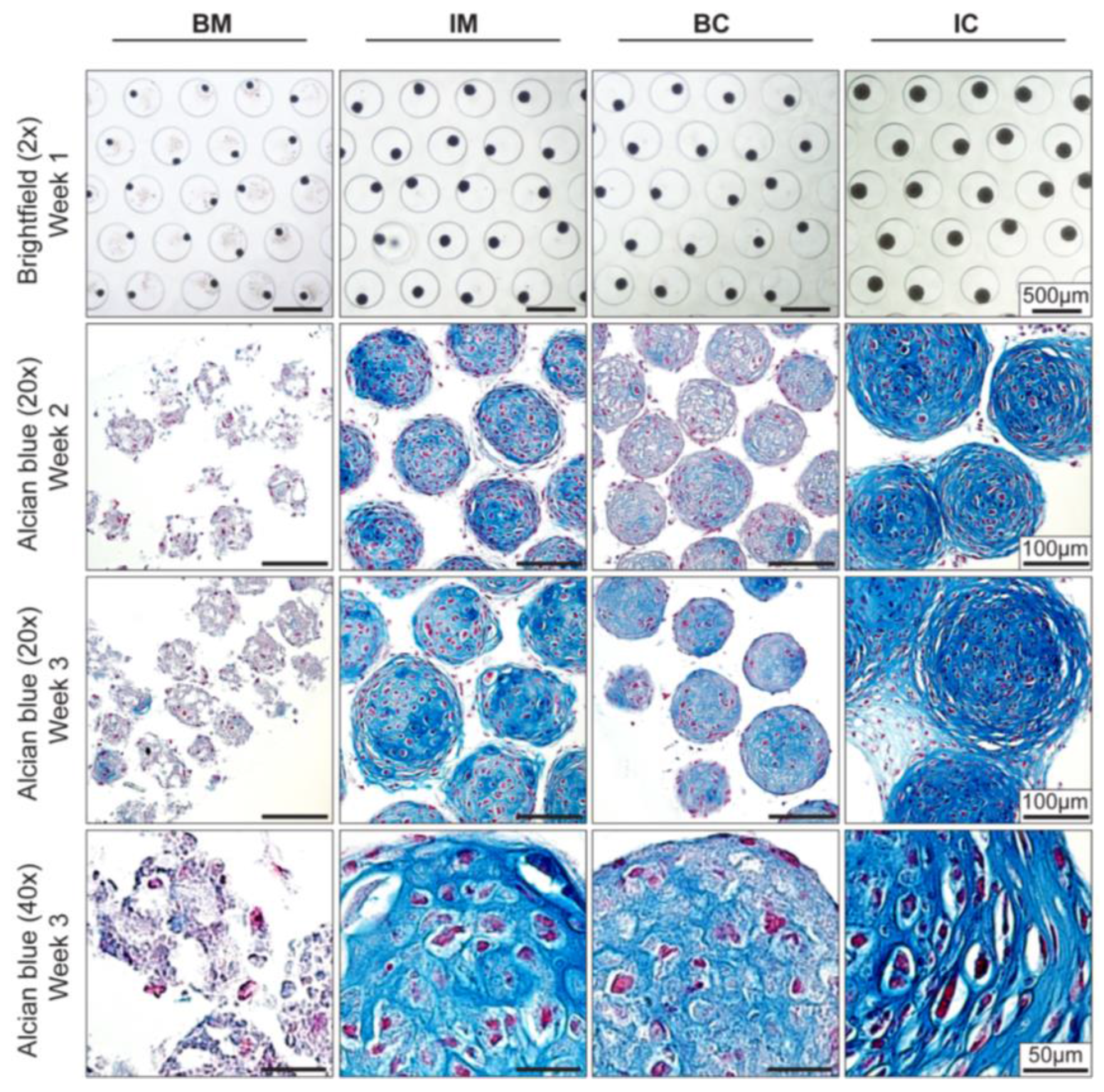
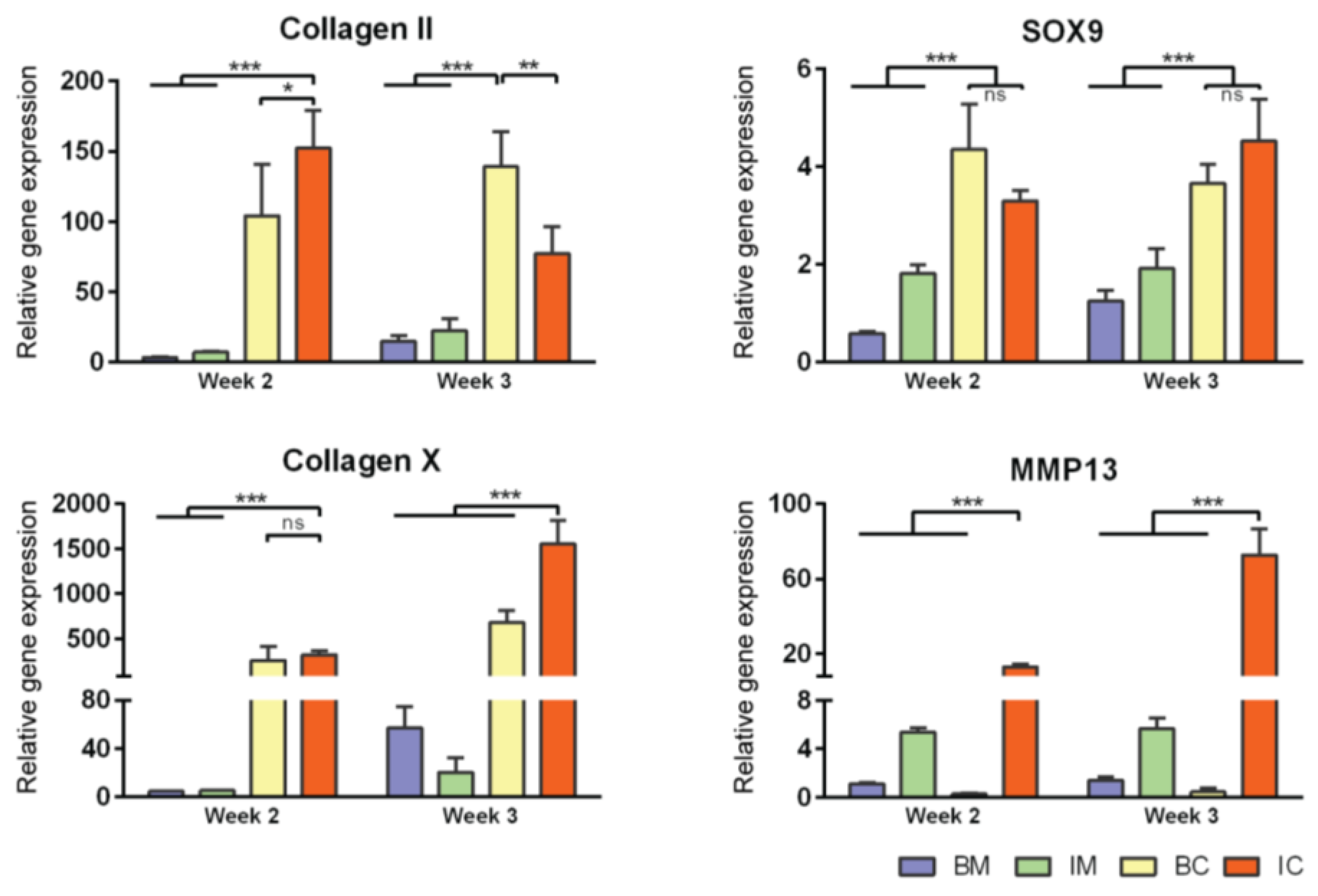
3.2. Optimization of Chondrogenic Differentiation of MiTEC
3.3. Induction of MiTEC Mineralization with Beta-Glycerophosphate
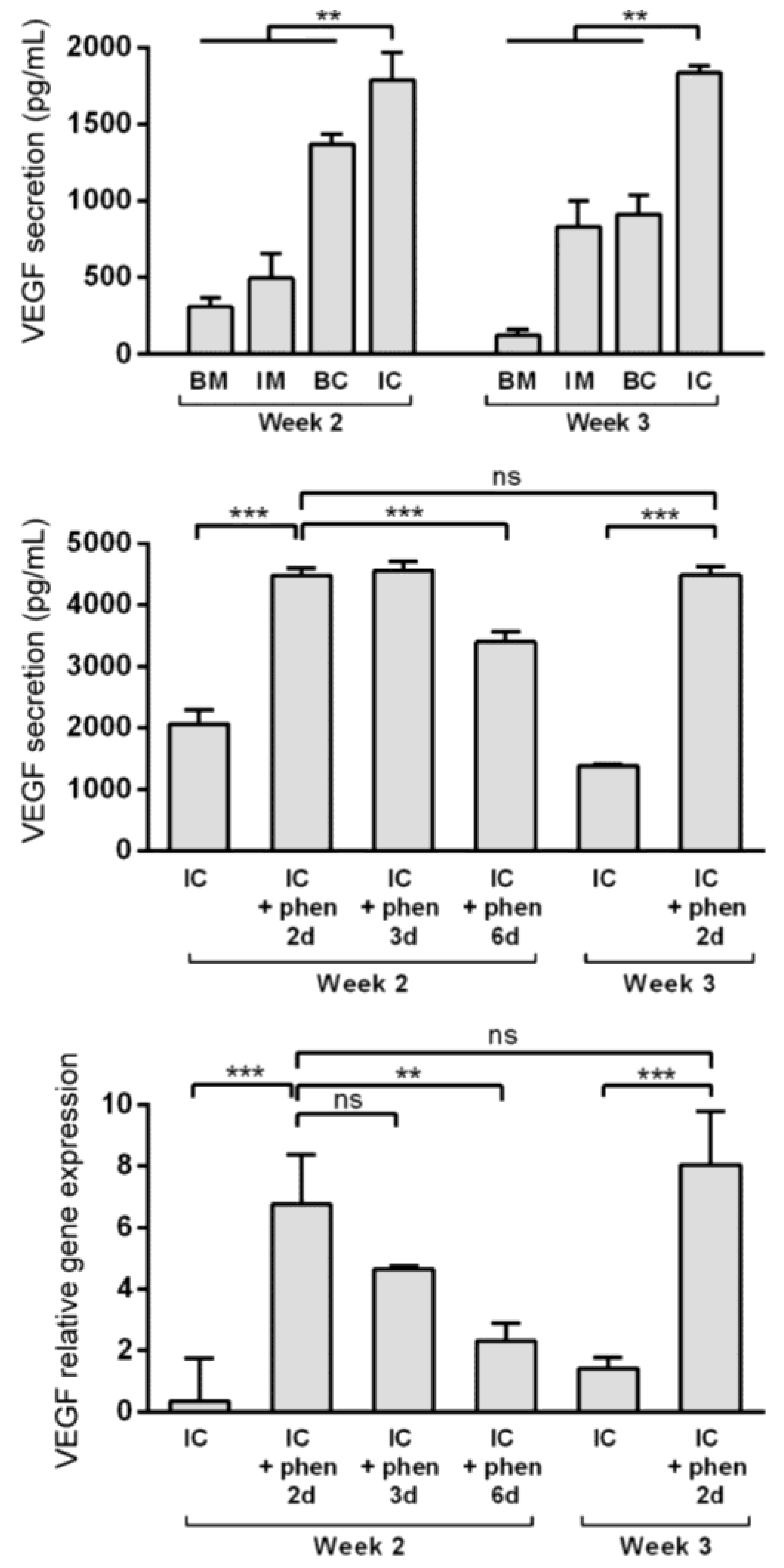
3.4. Boosting Vascular Endothelial Growth Factor (VEGF) Secretion from MiTEC Using the Hypoxia Mimetic Phenanthroline
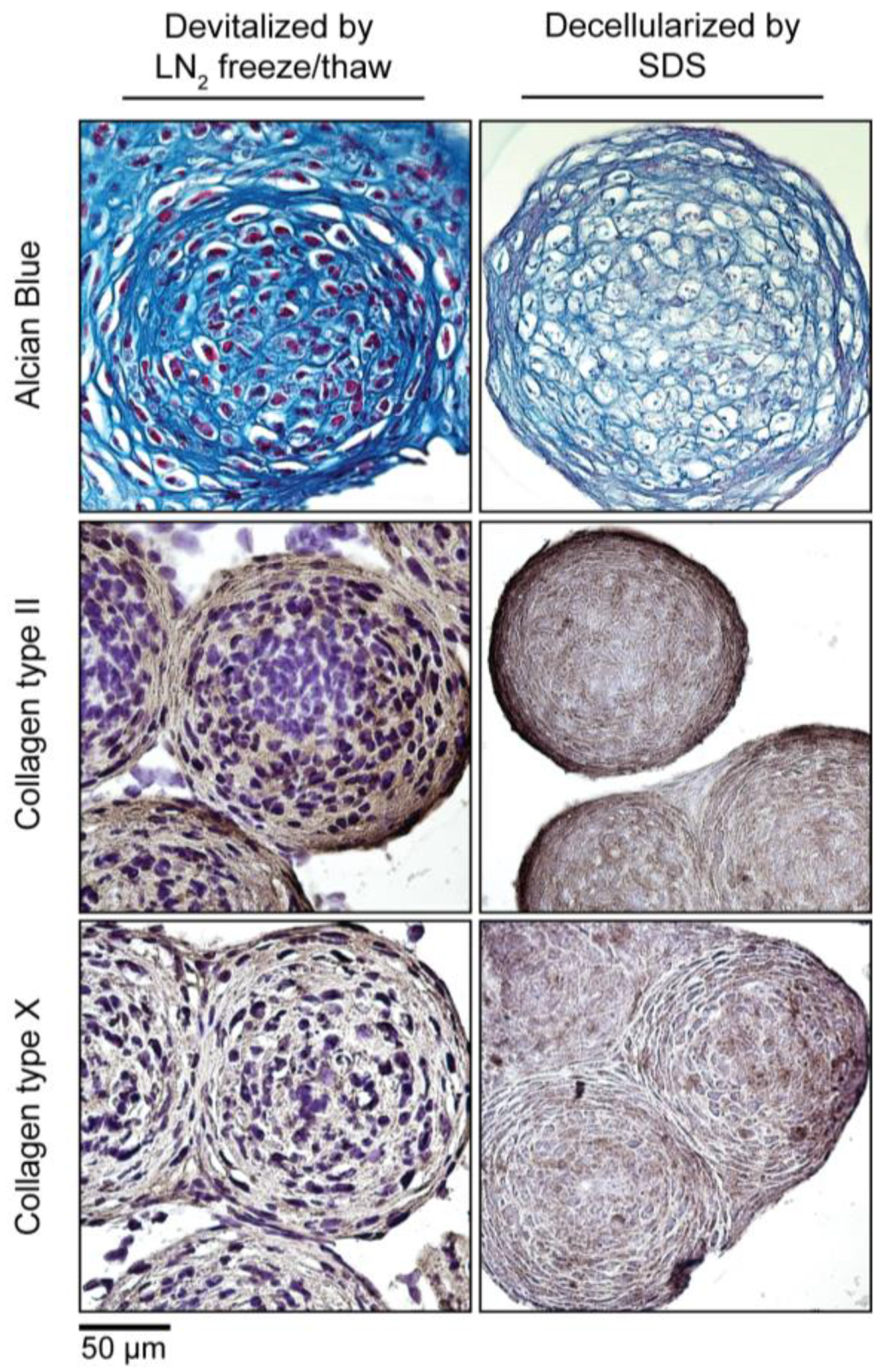
3.5. Devitalization and Decellularization of MiTEC
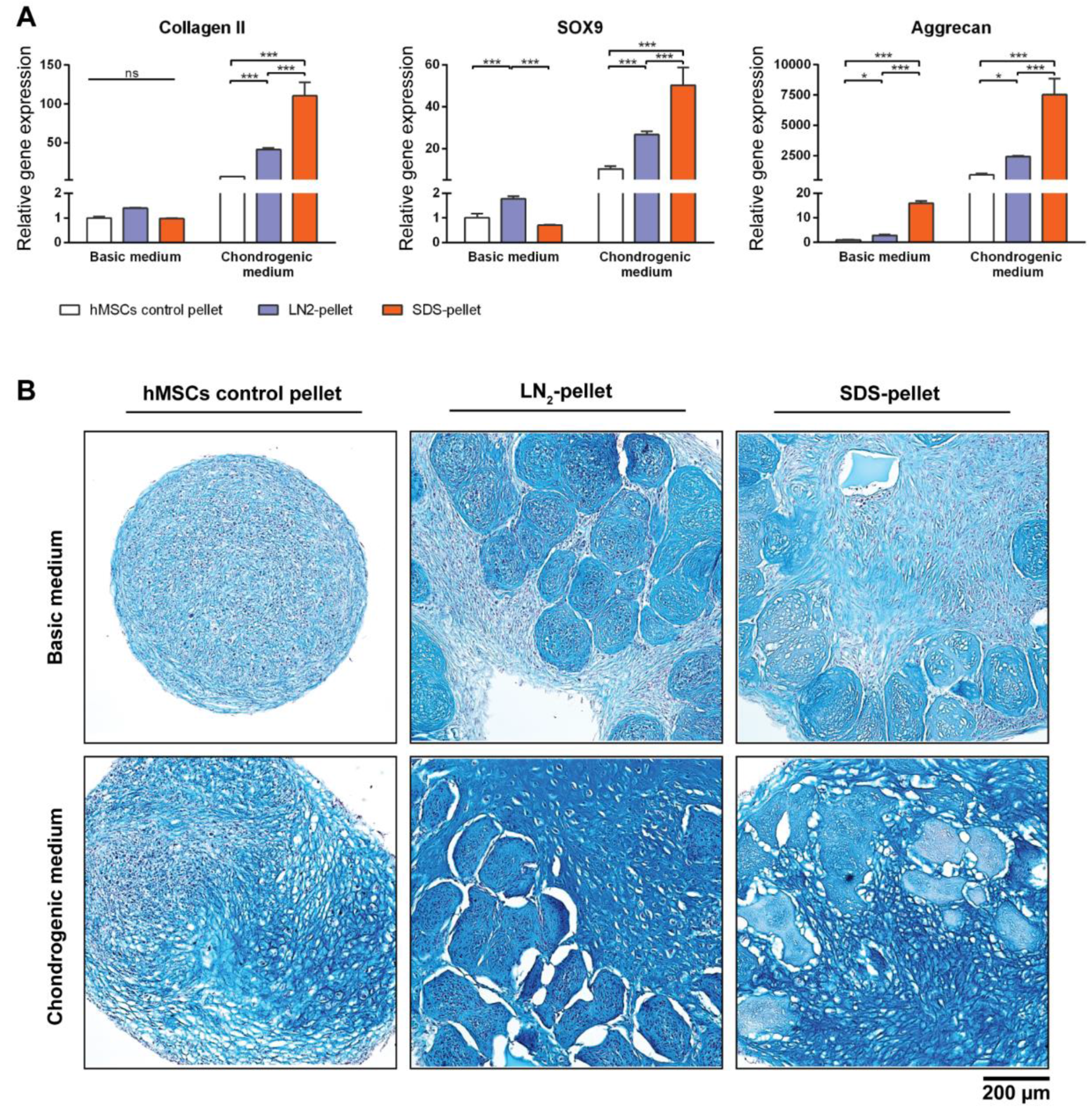
3.6. DCM Influences Chondrogenic Differentiation of hMSCs
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferguson, C.; Alpern, E.; Miclau, T.; Helms, J.A. Does adult fracture repair recapitulate embryonic skeletal formation? Mech. Dev. 1999, 87, 57–66. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Cullinane, D.M.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 2003, 88, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Durao, S.F.; Gomes, P.S.; Silva-Marques, J.M.; Fonseca, H.R.; Carvalho, J.F.; Duarte, J.A.; Fernandes, M.H. Bone regeneration in osteoporotic conditions: Healing of subcritical-size calvarial defects in the ovariectomized rat. Int. J. Oral Maxillofac. Implants 2012, 27, 1400–1408. [Google Scholar] [PubMed]
- Dinopoulos, H.; Dimitriou, R.; Giannoudis, P.V. Bone graft substitutes: What are the options? Surg. J. R. Coll. Surg. Edinb. Irel. 2012, 10, 230–239. [Google Scholar]
- Jukes, J.M.; Both, S.K.; Leusink, A.; Sterk, L.M.; van Blitterswijk, C.A.; de Boer, J. Endochondral bone tissue engineering using embryonic stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 6840–6845. [Google Scholar] [CrossRef] [PubMed]
- Scotti, C.; Tonnarelli, B.; Papadimitropoulos, A.; Scherberich, A.; Schaeren, S.; Schauerte, A.; Lopez-Rios, J.; Zeller, R.; Barbero, A.; Martin, I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 7251–7256. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.E.; Roberts, S.J.; Schrooten, J.; Luyten, F.P. A semi-autonomous model of endochondral ossification for developmental tissue engineering. Tissue Eng. Part A 2012, 18, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Farrell, E.; Both, S.K.; Odorfer, K.I.; Koevoet, W.; Kops, N.; O’Brien, F.J.; Baatenburg de Jong, R.J.; Verhaar, J.A.; Cuijpers, V.; Jansen, J.; et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet. Disord. 2011, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Gawlitta, D.; Farrell, E.; Malda, J.; Creemers, L.B.; Alblas, J.; Dhert, W.J. Modulating endochondral ossification of multipotent stromal cells for bone regeneration. Tissue Eng. Part B Rev. 2010, 16, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Scotti, C.; Piccinini, E.; Takizawa, H.; Todorov, A.; Bourgine, P.; Papadimitropoulos, A.; Barbero, A.; Manz, M.G.; Martin, I. Engineering of a functional bone organ through endochondral ossification. Proc. Natl. Acad. Sci. USA 2013, 110, 3997–4002. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Hu, D.P.; Taylor, A.J.; Ferro, F.; Britz, H.M.; Hallgrimsson, B.; Johnstone, B.; Miclau, T.; Marcucio, R.S. Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation. J. Bone Miner. Res. 2014, 29, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Bridges, J.B.; Pritchard, J.J. Bone and cartilage induction in the rabbit. J. Anat. 1958, 92, 28–38. [Google Scholar] [PubMed]
- Urist, M.R.; Mc, L.F. Osteogenetic potency and new-bone formation by induction in transplants to the anterior chamber of the eye. J. Bone Jt. Surg. Am. 1952, 34-A, 443–476. [Google Scholar] [CrossRef]
- Urist, M.R.; Wallace, T.H.; Adams, T. The function of fibrocartilaginous fracture callus. Observations on transplants labelled with tritiated thymidine. J. Bone Jt. Surg. Br. 1965, 47, 304–318. [Google Scholar]
- Urist, M.R.; Adams, T. Cartilage or bone induction by articular cartilage. Observations with radioisotope labelling techniques. J. Bone Jt. Surg. Br. 1968, 50, 198–215. [Google Scholar]
- Bourgine, P.E.; Scotti, C.; Pigeot, S.; Tchang, L.A.; Todorov, A.; Martin, I. Osteoinductivity of engineered cartilaginous templates devitalized by inducible apoptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 17426–17431. [Google Scholar] [CrossRef] [PubMed]
- Cunniffe, G.M.; Vinardell, T.; Murphy, J.M.; Thompson, E.M.; Matsiko, A.; O’Brien, F.J.; Kelly, D.J. Porous decellularized tissue engineered hypertrophic cartilage as a scaffold for large bone defect healing. Acta Biomater. 2015, 23, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Jukes, J.M.; Moroni, L.; van Blitterswijk, C.A.; de Boer, J. Critical steps toward a tissue-engineered cartilage implant using embryonic stem cells. Tissue Eng. Part A 2008, 14, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Siddappa, R.; Licht, R.; van Blitterswijk, C.; de Boer, J. Donor variation and loss of multipotency during in vivo expansion of human mesenchymal stem cells for bone tissue engineering. J. Orthop. Res. 2007, 25, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, Y. Atdc5: An excellent in vivo model cell line for skeletal development. J. Cell. Biochem. 2013, 114, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Altaf, F.M.; Hering, T.M.; Kazmi, N.H.; Yoo, J.U.; Johnstone, B. Ascorbate-enhanced chondrogenesis of atdc5 cells. Eur. Cells Mater. 2006, 12, 64–69; discussion 69–70. [Google Scholar] [CrossRef]
- Caron, M.M.; Emans, P.J.; Cremers, A.; Surtel, D.A.; Coolsen, M.M.; van Rhijn, L.W.; Welting, T.J. Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by bmp-2 but suppressed by bmp-7. Osteoarthr. Cartil. 2013, 21, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Rivron, N.C.; Vrij, E.J.; Rouwkema, J.; Le Gac, S.; van den Berg, A.; Truckenmuller, R.K.; van Blitterswijk, C.A. Tissue deformation spatially modulates vegf signaling and angiogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 6886–6891. [Google Scholar] [CrossRef] [PubMed]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid culture as a tool for creating 3d complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.; Mentink, A.; Le, B.; van Blitterswijk, C.A.; de Boer, J. Effect of antioxidant supplementation on the total yield, oxidative stress levels, and multipotency of bone marrow-derived human mesenchymal stromal cells. Tissue Eng. Part A 2013, 19, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.M.; Beck, S.C.; Murphy, J.M.; Barry, F.P.; Chichester, C.O.; Pittenger, M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998, 4, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Kheir, E.; Stapleton, T.; Shaw, D.; Jin, Z.; Fisher, J.; Ingham, E. Development and characterization of an acellular porcine cartilage bone matrix for use in tissue engineering. J. Biomed. Mater. Res. Part A 2011, 99, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Doorn, J.; Fernandes, H.A.; Le, B.Q.; van de Peppel, J.; van Leeuwen, J.P.; De Vries, M.R.; Aref, Z.; Quax, P.H.; Myklebost, O.; Saris, D.B.; et al. A small molecule approach to engineering vascularized tissue. Biomaterials 2013, 34, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Rabie, A.B. Vegf: An essential mediator of both angiogenesis and endochondral ossification. J. Dent. Res. 2007, 86, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Tan, Y.Y.; Wong, R.; Wenden, A.; Zhang, L.K.; Rabie, A.B. The role of vascular endothelial growth factor in ossification. Int. J. Oral Sci. 2012, 4, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous bone graft: Properties and techniques. J. Orthop. Trauma 2010, 24 (Suppl. 1), S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.N. Bone graft substitutes: Past, present, future. J. Postgrad. Med. 2002, 48, 142–148. [Google Scholar] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cells Mater. 2008, 15, 53–76. [Google Scholar] [CrossRef]
- Einhorn, T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S7–S21. [Google Scholar] [CrossRef]
- Hu, D.P.; Ferro, F.; Yang, F.; Taylor, A.J.; Chang, W.; Miclau, T.; Marcucio, R.S.; Bahney, C.S. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development 2017, 144, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Guidance for Industry: Source animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans; Food and Drug Administration: Rockville, MD, USA, 2011.
- Lu, H.; Hoshiba, T.; Kawazoe, N.; Chen, G. Comparison of decellularization techniques for preparation of extracellular matrix scaffolds derived from three-dimensional cell culture. J. Biomed. Mater. Res. Part A 2012, 100, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Mo, X.; Li, Y.; Chen, D. [recent research progress of decellularization of native tissues]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2012, 29, 1007–1013. [Google Scholar] [PubMed]
- Aryal, R.; Chen, X.P.; Fang, C.; Hu, Y.C. Bone morphogenetic protein-2 and vascular endothelial growth factor in bone tissue regeneration: New insight and perspectives. Orthop. Surg. 2014, 6, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, L.; Willett, N.J.; Guldberg, R.E. Vascularization strategies for bone regeneration. Ann. Biomed. Eng. 2014, 42, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, X.; Barbieri, D.; Barradas, A.M.; de Bruijn, J.D.; van Blitterswijk, C.A.; Yuan, H. The size of surface microstructures as an osteogenic factor in calcium phosphate ceramics. Acta Biomater. 2014, 10, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Barradas, A.M.; Fernandes, H.A.; Groen, N.; Chai, Y.C.; Schrooten, J.; van de Peppel, J.; van Leeuwen, J.P.; van Blitterswijk, C.A.; de Boer, J. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 2012, 33, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Hoganson, D.M.; Meppelink, A.M.; Hinkel, C.J.; Goldman, S.M.; Liu, X.H.; Nunley, R.M.; Gaut, J.P.; Vacanti, J.P. Differentiation of human bone marrow mesenchymal stem cells on decellularized extracellular matrix materials. J. Biomed. Mater. Res. Part A 2014, 102, 2875–2883. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zeng, Q.; Yan, Y.; Shen, L.; Liu, L.; Li, R.; Zhang, X.; Wu, J.; Guan, J.; Huang, S. Proliferative effect and osteoinductive potential of extracellular matrix coated on cell culture plates. SpringerPlus 2013, 2, 303. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Powers, R.M., Jr.; Wolfinbarger, L., Jr. A quantitative assessment of osteoinductivity of human demineralized bone matrix. J. Periodontol. 1997, 68, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Aghdasi, B.; Montgomery, S.R.; Daubs, M.D.; Wang, J.C. A review of demineralized bone matrices for spinal fusion: The evidence for efficacy. Surg. J. R. Coll. Surg. Edinb. Irel. 2013, 11, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.W.; Zhao, L.; Kanim, L.E.; Wong, P.; Delamarter, R.B.; Dawson, E.G. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine 2006, 31, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, A.S.; Boden, S.D.; Goldberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N. Bone-graft substitutes: Facts, fictions, and applications. J. Bone Jt. Surg. Am. 2001, 83-A (Suppl. 2 Pt 2), 98–103. [Google Scholar] [CrossRef]
| Name | Primer Sequence |
|---|---|
| Mouse beta-2 microglobulin | 5′-CATGGCTCGCTCGGTGACC-3′ |
| 5′- AATGTGAGGCGGGTGGAACTG-3′ | |
| Mouse collagen 2 alpha | 5′-CAAGGCCCCCGAGGTGACAAA-3′ |
| 5′-GGGGCCAGGGATTCCATTAGAGC-3′ | |
| Mouse collagen 10 alpha | 5′-CATAAAGGGCCCACTTGCTA-3′ |
| 5′-TGGCTGATATTCCTGGTGGT-3′ | |
| Mouse aggrecan | 5′-AGAACCTTCGCTCCAATGACTC-3′ |
| 5′-AGGGTGTAGCGTGTGGAAATAG-3′ | |
| Mouse Sry-related HMG box 9 (SOX9) | 5′-CCACGGAACAGACTCACATCTCTC-3′ |
| 5′-CTGCTCAGTTCACCGATGTCCACG-3′ | |
| Mouse hypoxia-inducible factors 1 alpha (HIF1α) | 5′-TGCTCATCAGTTGCCACTTC-3′ |
| 5′-TGGGCCATTTCTGTGTGTAA-3′ | |
| Mouse hypoxia-inducible factors 2 alpha (HIF2α) | 5′-TGAGTTGGCTCATGAGTTGC-3′ |
| 5′-CTCACGGATCTCCTCATGGT-3′ | |
| Mouse alkaline phosphatase (ALP) | 5′-AACCCAGACACAAGCATTCC-3′ |
| 5′-GAGACATTTTCCCGTTCACC-3′ | |
| Mouse matrix metalloproteinases 13 (MMP13) | 5′-AGGCCTTCAGAAAAGCCTTC-3′ |
| 5′-TCCTTGGAGTGATCCAGACC-3′ | |
| Human B2M | 5′-GACTTGTCTTTCAGCAAGGA-3′ |
| 5′-ACAAAGTCACATGGTTCACA-3′ | |
| Human collagen 2 alpha | 5′-CGTCCAGATGACCTTCCTACG-3′ |
| 5′-TGAGCAGGGCCTTCTTGAG-3′ | |
| Human aggrecan | 5′-AGAATCCACCACCACCAG-3′ |
| 5′-ATGCTGGTGCTGATGACA-3′ | |
| Human SOX9 | 5′-TGGGCAAGCTCTGGAGACTTC-3′ |
| 5′-ATCCGGGTGGTCCTTCTTGTG-3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quang Le, B.; Van Blitterswijk, C.; De Boer, J. An Approach to In Vitro Manufacturing of Hypertrophic Cartilage Matrix for Bone Repair. Bioengineering 2017, 4, 35. https://doi.org/10.3390/bioengineering4020035
Quang Le B, Van Blitterswijk C, De Boer J. An Approach to In Vitro Manufacturing of Hypertrophic Cartilage Matrix for Bone Repair. Bioengineering. 2017; 4(2):35. https://doi.org/10.3390/bioengineering4020035
Chicago/Turabian StyleQuang Le, Bach, Clemens Van Blitterswijk, and Jan De Boer. 2017. "An Approach to In Vitro Manufacturing of Hypertrophic Cartilage Matrix for Bone Repair" Bioengineering 4, no. 2: 35. https://doi.org/10.3390/bioengineering4020035





