Production of Polyhydroxyalkanoates Using Hydrolyzates of Spruce Sawdust: Comparison of Hydrolyzates Detoxification by Application of Overliming, Active Carbon, and Lignite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wood Hydrolyzate (WH) Preparation
2.2. Microorganisms and Cultivation
2.3. Detoxification of Hydrolyzates
2.4. Analytical Methods
2.5. PHA Extraction and Content Analysis
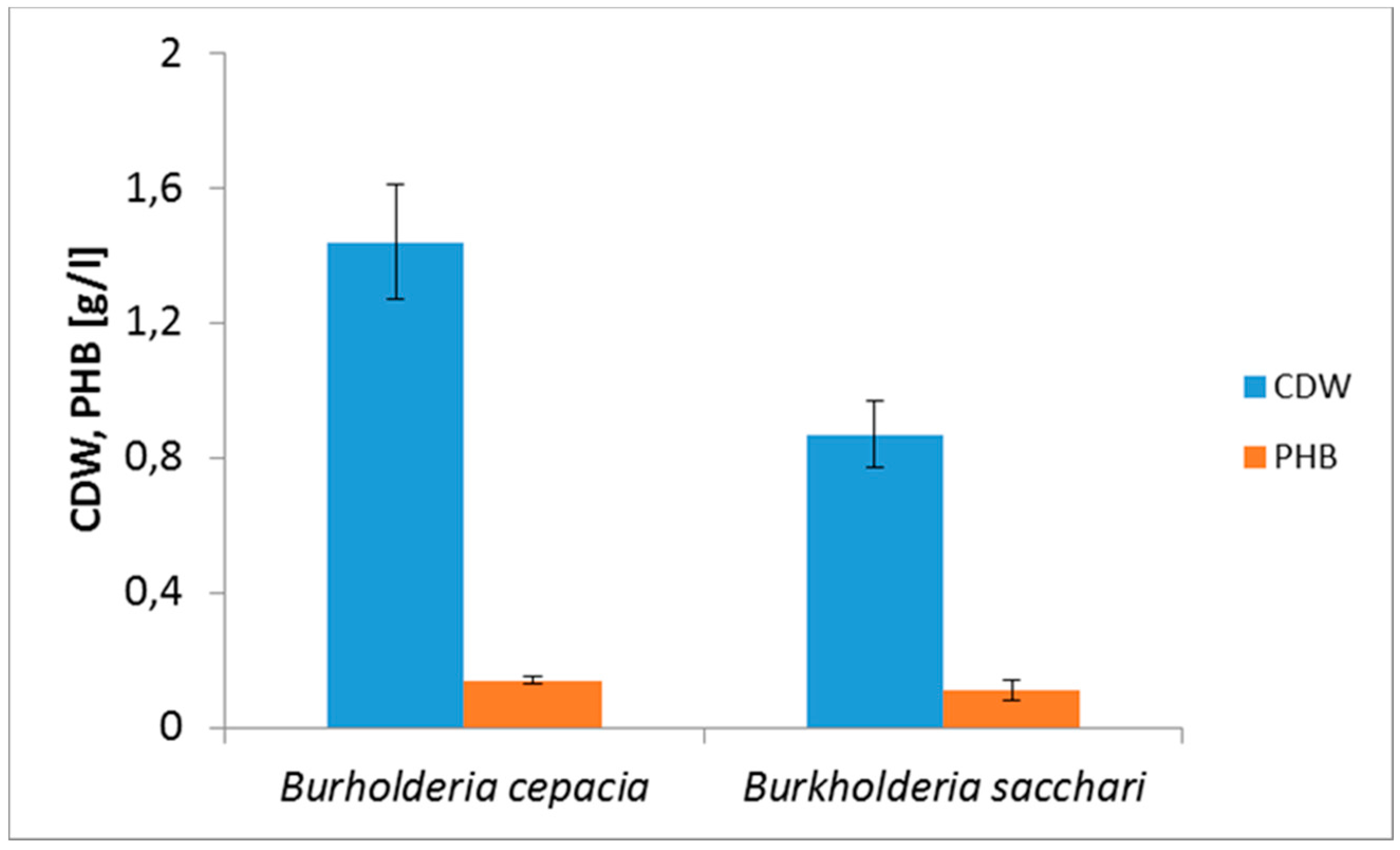
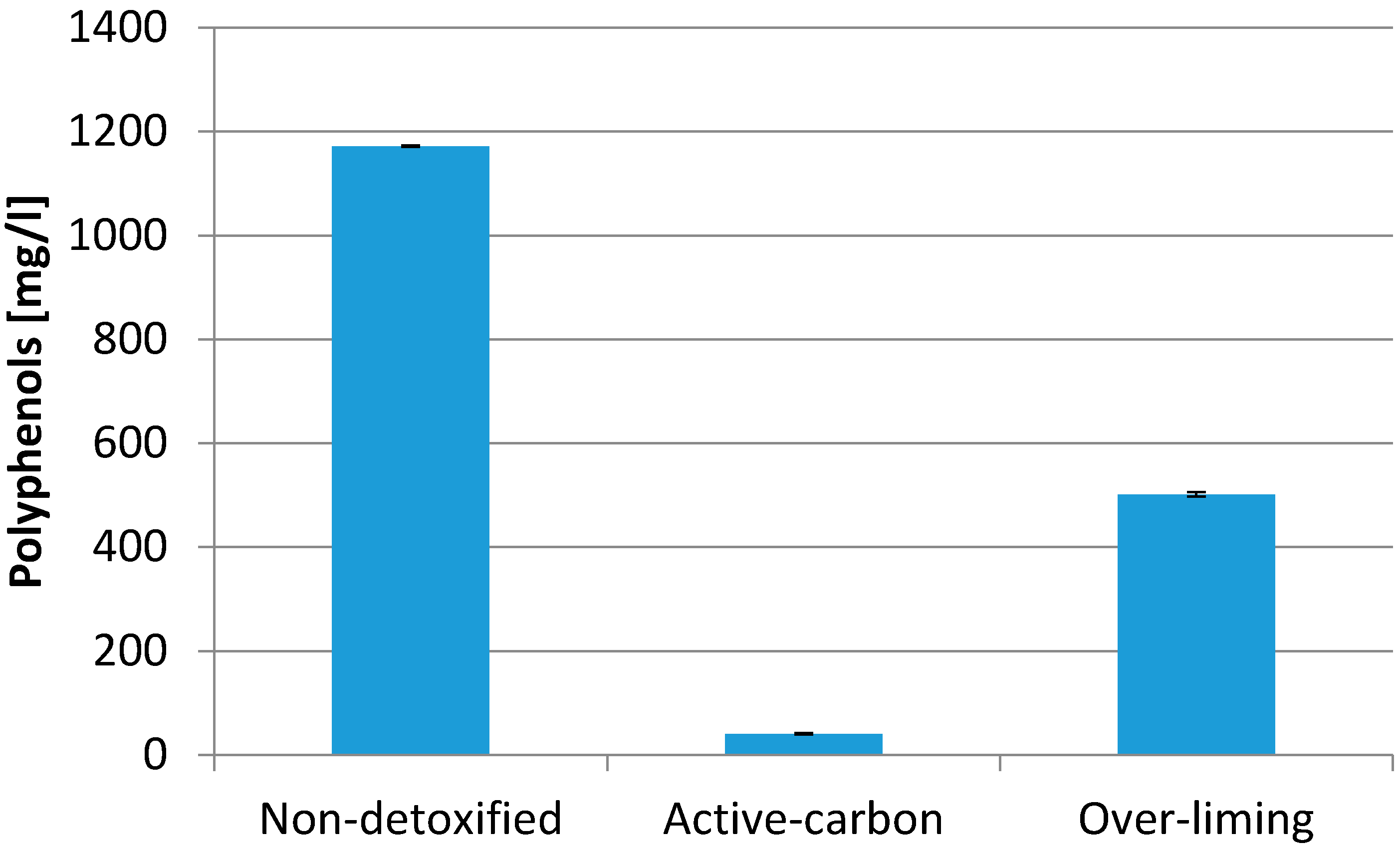
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steinbüchel, A. Perspectives for Biotechnological Production and Utilization of Biopolymers: Metabolic Engineering of Polyhydroxyalkanoate Biosynthesis Pathways as a Successful Example. Macromol. Biosci. 2001, 1, 1–24. [Google Scholar] [CrossRef]
- Ivanov, V.; Stabnikov, V.; Ahmed, Z.; Dobrenko, S.; Saliuk, A. Production and applications of crude polyhydroxyalkanoate-containing bioplastic from the organic fraction of municipal solid waste. Int. J. Environ. Sci. Technol. 2015, 12, 725–738. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.Y. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl. Microbiol. Biotechnol. 1999, 51, 13–21. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Kucera, D.; Petrik, S.; Marova, I. Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates and carotenoids. New Biotechnol. 2015, 32, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Amidon, T.E.; Wood, C.D.; Shupe, A.M.; Wang, Y.; Graves, M.; Liu, S. Biorefinery: Conversion of Woody Biomass to Chemicals, Energy and Materials. J. Biobased Mater. Bioenergy 2008, 2, 100–120. [Google Scholar] [CrossRef]
- Canilha, L.; Kumar Chandel, A.; dos Santos Milessi, T.S.; Fernandes Antunes, F.A.; da Costa Freitas, W.L.; das Graças Almeida Fellipe, M.; da Silva, S.S. Bioconversion of Sugarcane Biomass into Ethanol: An Overview about Composition, Pretreatment Methods, Detoxification of Hydrolysates, Enzymatic Saccharification, and Ethanol Fermentation. J. Biomed. Biotechnol. 2012, 2012, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Doskočil, L.; Grasset, L.; Enev, V.; Kalina, L.; Pekař, M. Study of water-extractable fractions from South Moravian lignite. Environ. Earth Sci. 2015, 73, 3873–3885. [Google Scholar] [CrossRef]
- Pan, W.; Perrotta, J.A.; Stipanovic, A.J.; Nomura, C.T.; Nakas, J.P. Production of polyhydroxyalkanoates by Burkholderia cepacia ATCC 17759 using a detoxified sugar maple hemicellulosic hydrolysate. J. Ind. Microbiol. Biotechnol. 2012, 39, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Bowers, T.; Vaidya, A.; Smith, D.A.; Lloyd-Jones, G. Softwood hydrolysate as a carbon source for polyhydroxyalkanoate production. J. Chem. Technol. Biotechnol. 2014, 89, 1030–1037. [Google Scholar] [CrossRef]
- Silva, J.A.; Tobella, L.M.; Becerra, J.; Godoy, F.; Martínez, M.A. Biosynthesis of poly-β-hydroxyalkanoate by Brevundimonas vesicularis LMG P-23615 and Sphingopyxis macrogoltabida LMG 17324 using acid-hydrolyzed sawdust as carbon source. J. Biosci. Bioeng. 2007, 103, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.M.; Tanenbaum, S.W.; Stipanovic, A.J.; Nakas, J.P. Production and Characterization of Poly-β-hydroxyalkanoate Copolymers from Burkholderia cepacia Utilizing Xylose and Levulinic Acid. Biotechnol. Prog. 2004, 20, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S. Production of (R)-3-hydroxybutyric acid by Burkholderia cepacia from wood extract hydrolysates. AMB Express 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Ranatunga, T.D.; Jervis, J.; Helm, R.F.; McMillan, J.D.; Wooley, R.J. The effect of overliming on the toxicity of dilute acid pretreated lignocellulosics: The role of inorganics, uronic acids and ether-soluble organics. Enzyme Microb. Technol. 2000, 27, 240–247. [Google Scholar] [CrossRef]
- Li, H.-B.; Wong, C.-C.; Cheng, K.-W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Obruca, S.; Marova, I.; Melusova, S.; Mravcova, L. Production of polyhydroxyalkanoates from cheese whey employing Bacillus megaterium CCM 2037. Ann. Microbiol. 2011, 61, 947–953. [Google Scholar] [CrossRef]
- Brandl, H.; Gross, R.A.; Lenz, R.W.; Fuller, R.C. Pseudomonas oleovorans as a source of poly(beta-hydroxyalkanoates) for potential application as a biodegradable polyester. Appl. Environ. Microbiol. 1988, 54, 1977–1982. [Google Scholar] [PubMed]
- Peters, D. Raw Materials. In White Biotechnology; Ulber, R., Sell, D., Eds.; Springer: Berlin, Germany, 2007; pp. 1–30. [Google Scholar]
- Obruca, S.; Benešová, P.; Maršálek, L.; Márová, I. Use of Lignocellulosic Materials for PHA Production. Chem. Biochem. Eng. Q. 2015, 29, 135–144. [Google Scholar] [CrossRef]
- Wyman, C.E.; Decker, S.R.; Himmel, M.E.; Brady, J.W.; Skopec, C.E.; Viikari, L. Hydrolysis of Cellulose and Hemicellulose; Dumitriu, S., Ed.; CRC Press: New York, NY, USA, 2004; pp. 995–1034. [Google Scholar]
- Obruca, S.; Marova, I.; Svoboda, Z.; Mikulikova, R. Use of controlled exogenous stress for improvement of poly (3-hydroxybutyrate) production in Cupriavidus necator. Folia Microbiol. 2010, 55, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Passanha, P.; Kedia, G.; Dinsdale, R.M.; Guwy, A.J.; Esteves, S.R. The use of NaCl addition for the improvement of polyhydroxyalkanoate production by Cupriavidus necator. Bioresour. Technol. 2014, 163, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; da Silva, S.S.; Singh, O.V. Detoxification of Lignocellulose Hydrolysates: Biochemical and Metabolic Engineering Toward White Biotechnology. Bioenergy Res. 2013, 6, 388–401. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Evaluation of nutrient supplementation to charcoal-treated and untreated rice straw hydrolysate for xylitol production by Candida guilliermondii. Braz. Arch. Biol. Technol. 2005, 48, 497–502. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Lignite. Available online: https://www.alibaba.com (accessed on 26 April 2017).
- Obruca, S.; Benesova, P.; Kucera, D.; Marova, I. Novel Inexpensive Feedstocks from Agriculture and Industry for Microbial Polyester Production. In Recent Advances in Biotechnology; Microbial Biopolyester Production, Performance and Processing Microbiology, Feedstocks, and Metabolism; Koller, M., Ed.; Bentham Science: Berlin, Germany, 2016; pp. 3–99. [Google Scholar]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Mudhoo, A.; Lofrano, G.; Chattopadhyaya, M.C. Biomass-derived biosorbents for metal ions sequestration: Adsorbent modification and activation methods and adsorbent regeneration. J. Environ. Chem. Eng. 2014, 2, 239–259. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Polat, H.; Molva, M.; Polat, M. Capacity and mechanism of phenol adsorption on lignite. Int. J. Miner. Process. 2006, 79, 264–273. [Google Scholar] [CrossRef]
- Klucakova, M.; Pavlikova, M. Lignitic Humic Acids as Environmentally-Friendly Adsorbent for Heavy Metals. J. Chem. 2017, 2017, 1–5. [Google Scholar] [CrossRef]
- Robles, I.; Bustos, E.; Lakatos, J. Adsorption study of mercury on lignite in the presence of different anions. Sustain. Environ. Res. 2016, 26, 136–141. [Google Scholar] [CrossRef]
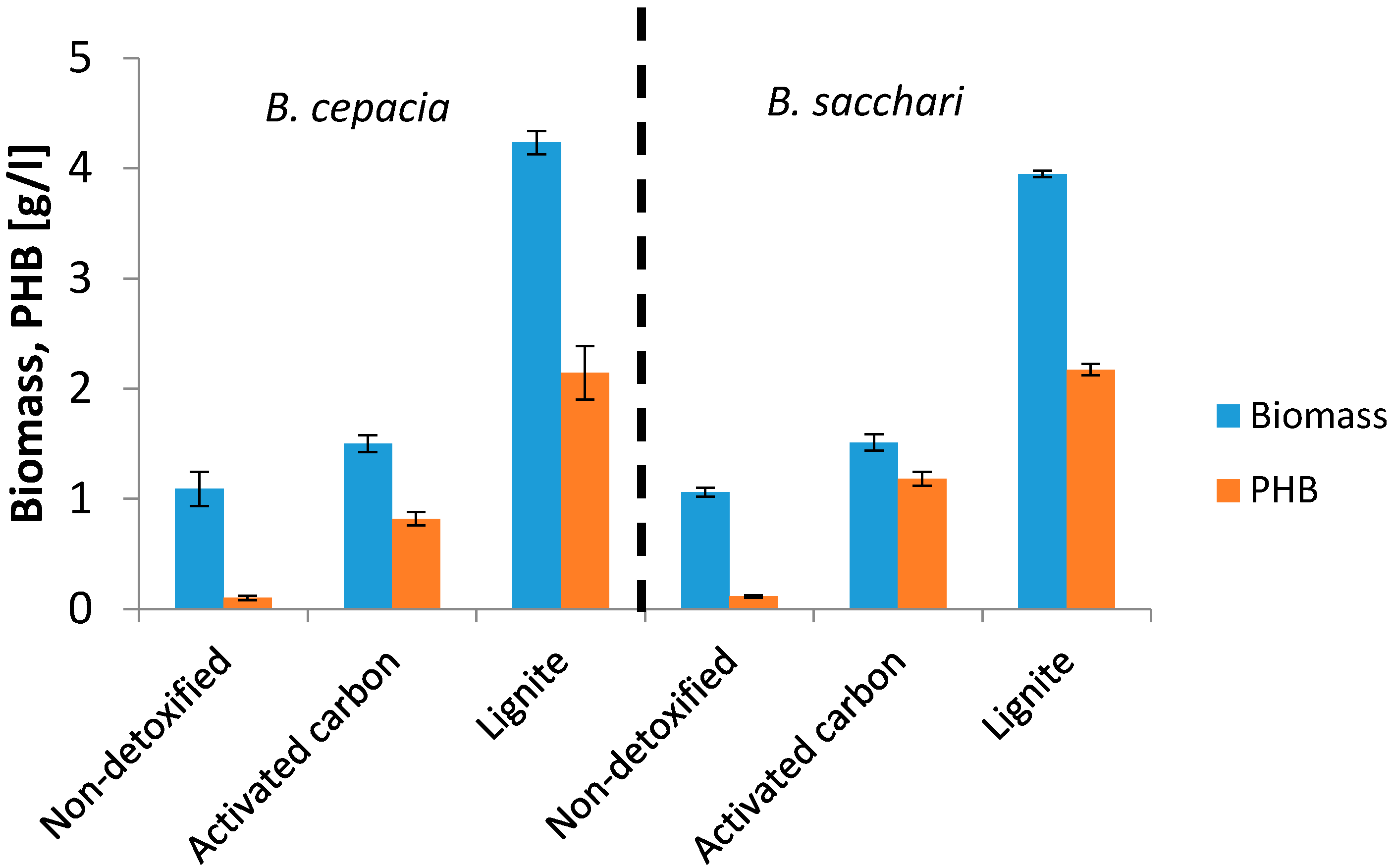
| Concentration | |
|---|---|
| Glucose | 4.5 g/L |
| Xylose | 10.4 g/L |
| Ash | 52,6 g/L |
| Polyphenols | 1205 mg/L |
| Furfural | 52.0 mg/L |
| Acetic acid | 0.53 g/L |
| Levulinic acid | 9.9 mg/L |
| 5-HMF | not detected |
| Detoxification | Biomass (g/L) | PHB (%) | PHB (g/L) | |
|---|---|---|---|---|
| Burkholderia sacchari | Non-detoxified | 0.87 | 12.2 | 0.11 |
| Over-liming | 1.57 | 88.7 | 1.39 | |
| Activated carbon | 1.01 | 87.6 | 0.89 | |
| Burkholderia cepacia | Non-detoxified | 1.44 | 9.8 | 0.14 |
| Over-liming | 2.86 | 30.0 | 0.86 | |
| Activated carbon | 1.40 | 74.7 | 1.05 |
| Glucose (g/L) | Xylose (g/L) | Polyphenols (mg/L) | Furfural (mg/L) | Levulinic Acid (mg/L) | Acetic Acid (g/L) | |
|---|---|---|---|---|---|---|
| Non-detoxified | 4.4 | 10.0 | 998.8 | 41.4 | 10.0 | 0.5 |
| Lignite | 4.6 | 10.3 | 772.4 | 35.7 | 7.9 | 0.4 |
| Activated carbon | 4.5 | 10.1 | 23.8 | 3.8 | 3.6 | 0.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucera, D.; Benesova, P.; Ladicky, P.; Pekar, M.; Sedlacek, P.; Obruca, S. Production of Polyhydroxyalkanoates Using Hydrolyzates of Spruce Sawdust: Comparison of Hydrolyzates Detoxification by Application of Overliming, Active Carbon, and Lignite. Bioengineering 2017, 4, 53. https://doi.org/10.3390/bioengineering4020053
Kucera D, Benesova P, Ladicky P, Pekar M, Sedlacek P, Obruca S. Production of Polyhydroxyalkanoates Using Hydrolyzates of Spruce Sawdust: Comparison of Hydrolyzates Detoxification by Application of Overliming, Active Carbon, and Lignite. Bioengineering. 2017; 4(2):53. https://doi.org/10.3390/bioengineering4020053
Chicago/Turabian StyleKucera, Dan, Pavla Benesova, Peter Ladicky, Miloslav Pekar, Petr Sedlacek, and Stanislav Obruca. 2017. "Production of Polyhydroxyalkanoates Using Hydrolyzates of Spruce Sawdust: Comparison of Hydrolyzates Detoxification by Application of Overliming, Active Carbon, and Lignite" Bioengineering 4, no. 2: 53. https://doi.org/10.3390/bioengineering4020053






