A Novel Multifunctional β-N-Acetylhexosaminidase Revealed through Metagenomics of an Oil-Spilled Mangrove
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metagenomic Fosmid Library Construction
2.2. Screening for Cellulase Activity
2.3. Fosmid DNA Extraction and Sequencing
2.4. Sequence Assembly and Analysis
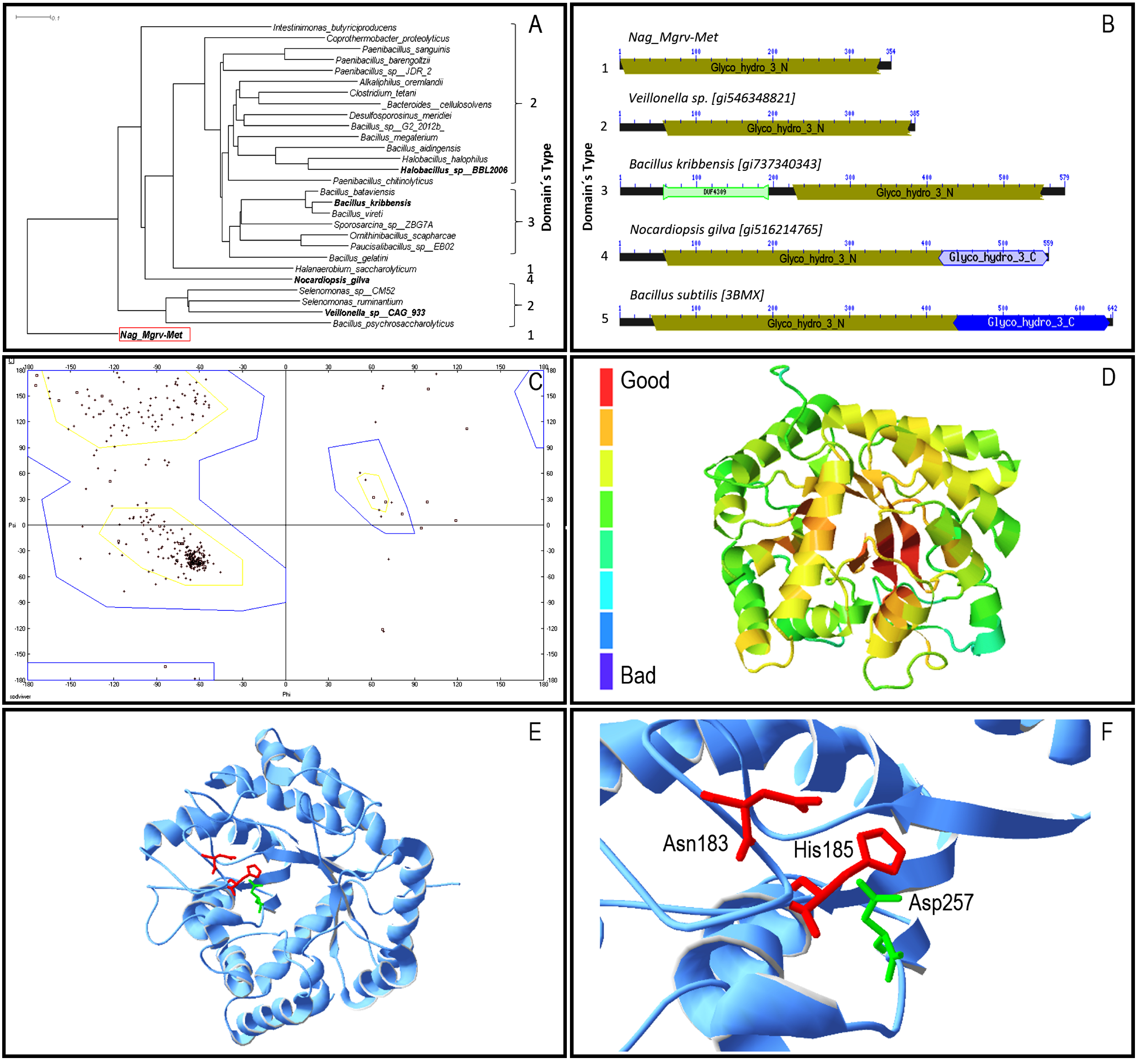
2.5. Inferences on the Structure of the Enzyme
2.6. Sub-Cloning of the β-N-Acetylhexosaminidase-Related ORF
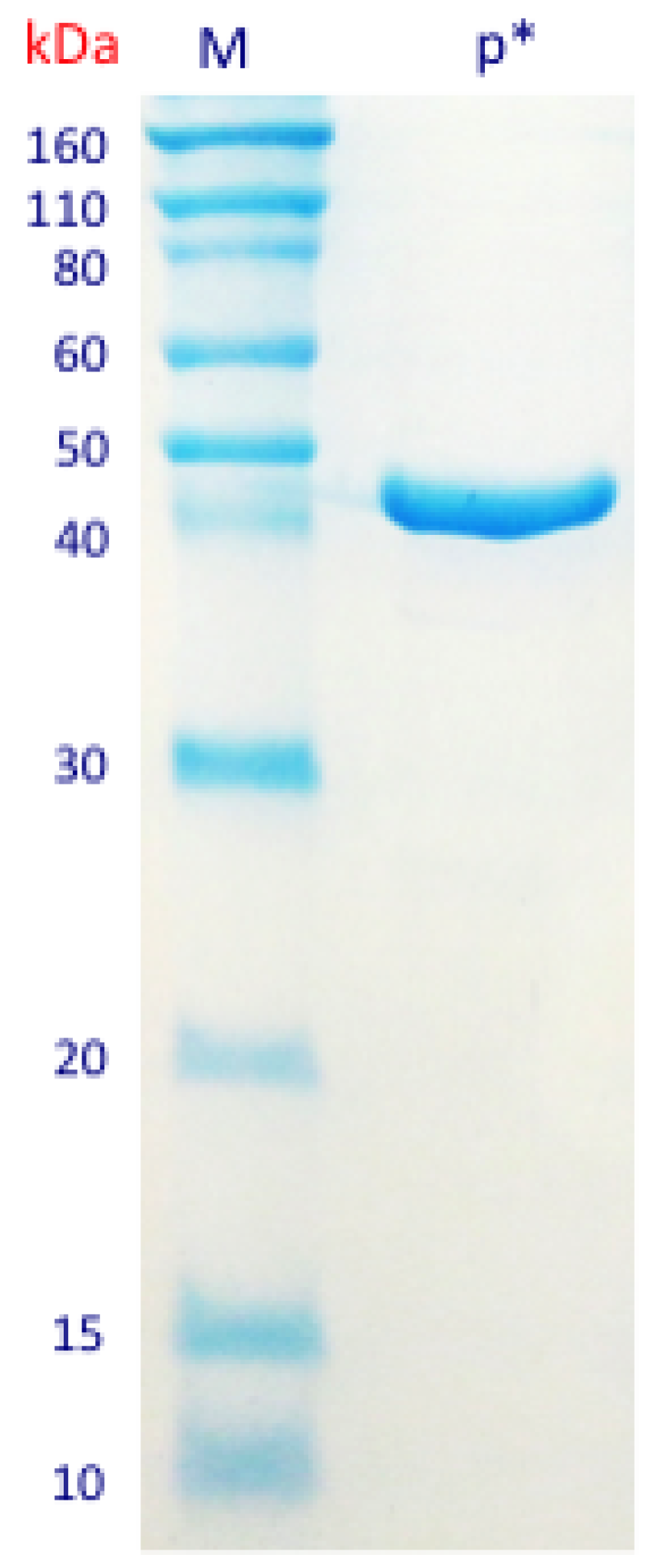
2.7. Purification of the 6xHis-Tagged β-N-Acetylhexosaminidase
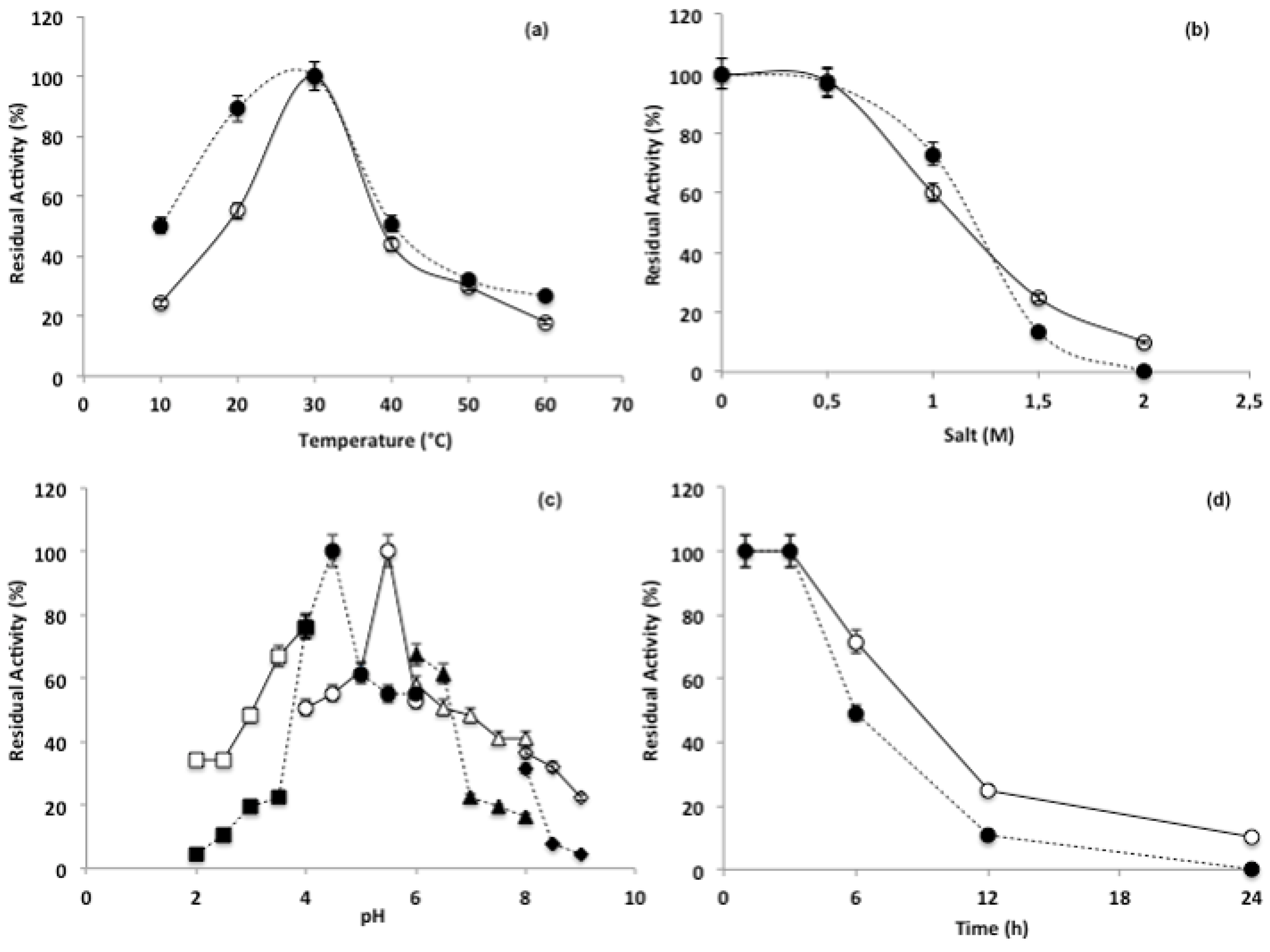
2.8. Assays for Characterization of the Enzymatic Activity
3. Results
3.1. Detection and Sequencing of the Active Clone
3.2. Prediction of the Enzyme Structure
3.3. Purification and Characterization of the β-N-Acetylhexosaminidase
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holguin, G.; Zamorano, P.G.; De-Bashan, L.E.; Mendoza, R.; Amador, E.; Bashan, Y. Mangrove health in an arid environment encroached by urban development—A case study. Sci. Tot. Environ. 2006, 363, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Andreote, F.D.; Jiménez, D.J.; Chaves, D.; Dias, A.C.F.; Luvizotto, D.M.; Dini-Andreote, F.; Fasanella, C.C.L.; Baena, M.V.; Taketani, R.G.; Melo, I.S. The microbiome of Brazilian mangroves sediments as revealed by metagenomics. PLoS ONE 2012, 7, e38600. [Google Scholar] [CrossRef] [PubMed]
- Leresche, J.; Meyer, H.P. Chemocatalysis and biocatalysis (Biotrasformation): Some thoughts of chemist and of a biothecnologist. Org. Process Res. Dev. 2006, 10, 572–580. [Google Scholar] [CrossRef]
- Soares, F.L., Jr.; Dias, A.C.F.; Fasanella, C.C.; Taketani, R.G.; Lima, A.O.D.S.; Melo, I.S.; Andreote, F.D. Endo-and exoglucanase activities in bacteria from mangrove sediment. Braz. J. Microbiol. 2013, 44, 969–976. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguin, G. Plant growth-promoting bacteria: A potential tool for arid mangrove reforestation. Trees Struct. Funct. 2002, 16, 159–166. [Google Scholar] [CrossRef]
- Dias, A.C.F.; Pereira e Silva, M.C.; Cotta, S.R.; Dini-Andreote, F.; Soares, F.L., Jr.; Salles, J.F.; Azevedo, J.L.; van Elsas, J.D.; Andreote, F. Abundance and genetic diversity of nifH gene sequences in anthropogenically affected Brazilian mangrove sediments. Appl. Environ. Microbiol. 2012, 78, 7960–7967. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.C.; Cleary, D.F.; Almeida, A.; Cunha, Â.; Dealtry, S.; Mendonça-Hagler, L.C.; Gomes, N.C. Denaturing gradient gel electrophoresis and barcoded pyrosequencing reveal unprecedented archaeal diversity in mangrove sediment and rhizosphere samples. Appl. Environ. Microbiol. 2012, 78, 5520–5528. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Winans, S.C.; Holguin, G. Molecular characterization of diazotrophic and denitrifying bacteria associated with mangrove roots. Appl. Environ. Microbiol. 2007, 73, 7308–7321. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.C.F.; Andreote, F.D.; Dini-Andreote, F.; Lacava, P.T.; Sá, A.L.B.; Melo, I.S.; Azevedo, J.L.; Araújo, W.L. Diversity and biotechnological potential of culturable bacteria from Brazilian mangrove sediment. World J. Microbiol. Biotechnol. 2009, 25, 1305–1311. [Google Scholar] [CrossRef]
- Souza, D.S.; Grossi-de-Sa, M.F.; Silva, L.P.; Franco, O.L.; Gomes-Junior, J.E.; Oliveira, G.R.; Romano, E. Identification of a novel β-N-acetylhexosaminidase (Pcb-NAHA1) from marine zoanthid Palythoa caribaeorum (Cnidaria, Anthozoa, Zoanthidea). Protein Exp. Purif. 2008, 58, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B. A classification of glycosyl hydrolases based on amino-acid sequence similarities. Biochem. J. 1991, 280, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Vocadlo, D.J.; Mah, M.; Rupitz, K.; Stoll, D.; Warren, R.A.; Withers, S.G. Characterization of a β-N-acetylhexosaminidase and a β-Nacetylglucosaminidase/beta-glucosidase from Cellulomonas fimi. FEBS J. 2006, 273, 2929–2941. [Google Scholar] [CrossRef] [PubMed]
- Slámová, K.; Bojarová, P.; Petrásková, L.; Kren, V. β-N-acetylhexosaminidase: What’s in a name? Biotechnol. Adv. 2010, 28, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Scigelova, M.; Crout, D.H.G. Microbial β-N-acetylglucosaminidases and their biotechnological applications. Enzyme Microb. Technol. 1999, 25, 3–14. [Google Scholar] [CrossRef]
- Marcon, J.; Taketani, R.G.; Dini-Andreote, F.; Mazzero, G.I.; Soares, F.L., Jr.; Melo, I.S.; Azevedo, J.L.; Andreote, F.D. Draft genome sequence of Bacillus thurigiensis strain Brmgv02-JM63, a chitinolytic bacterium isolated from oil-contamined mangrove soil in Brazil. Genome Announc. 2014, 2, e01264-13. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, J.; Rondon, M.R.; Brady, S.F.; Clardy, J.; Goodman, R.M. Molecular biological access to the chemistry of unknown soil microbes: A new frontier for natural products. Chem. Biol. 1998, 5, 245–249. [Google Scholar] [CrossRef]
- Balbas, P.; Bolivas, F. Design and construction of expression plasmid vectors in Escherichia coli. Meth. Enzymol. 1990, 185, 3–40. [Google Scholar]
- Bunterngsook, B.; Kanokratana, P.; Thongaran, T.; Tanapongpipat, S.; Uengwetwanit, T.; Rachdawiong, S.; Vichitsoonthonklut, T.; Eurwilaichitr, L. Identification and characterization of lipolityc enzymes froma a Peat-swamp forest soil metagenome. Biosci. Biotechnol. Biochem. 2010, 74, 1848–1854. [Google Scholar] [CrossRef] [PubMed]
- King, J.W.; Snyder, J.M.; Frykman, H.; Neese, A. Sterol ester production using lipase-catalyzed reactions in supercritical carbon dioxide. Eur. Food Res. Technol. 2001, 212, 566–569. [Google Scholar] [CrossRef]
- Osorio, N.M.; Ferreira Dias, S.; Gusmão, J.H.; Fonseca, M.M.R. Response surface modelling of the production of w3 polyunsaturated fatty acids enriched fats by a commercial immobilized lipase. J. Mol. Catal. B 2001, 11, 677–686. [Google Scholar] [CrossRef]
- Fasanella, C.C.; Dias, A.C.F.; Rigonato, J.; Fiore, M.F.; Soares, F.L., Jr.; Melo, I.S.; Pizzirani-Kleiner, A.A.; van Elsas, J.D.; Andreote, F. The selection exerted by oil contamination on mangrove fungal communities. Water Air Soil Pollut. 2012, 223, 4233–4243. [Google Scholar] [CrossRef]
- Varon-Lopez, M.; Dias, A.C.F.; Fasanella, C.C.; Durrer, A.; Melo, I.S.; Kuramae, E.E.; Andreote, F.D. Sulphur-oxidizing and sulphate-reducing communities in Brazilian mangrove sediments. Environ. Microbiol. 2014, 16, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, S.P.; Angolini, C.F.F.; García, I.N.S.; Dallagnezze, B.; Silva, C.C.; Marsaioli, A.J.; Neto, E.V.S.; Oliveira, V.M. Screening for hydrocarbon biodegraders in a metagenomic clone library derived from Brazilian petroleum reservoirs. Org. Geochem. 2010, 41, 675–681. [Google Scholar] [CrossRef]
- Kasana, R.C.; Salwan, R.; Dhar, H.; Dutt, S.; Gulati, A.A. A rapid and easy method for the detection of microbial cellulases on agar plates using gram’s iodine. Curr. Microbiol. 2008, 57, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.; Gish, W.; Miller, W.; Myers, E.; Lipman, D. Basic local alignment search tool. J. Mol. Biol. 1990, 3, 403–410. [Google Scholar] [CrossRef]
- Altschul, S.F.; Wootton, J.C.; Gertz, E.M.; Agarwala, R.; Morgulis, A.; Schaffer, A.A.; Yu, Y.-K. Protein database searches using compositionally adjusted substitution matrices. FEBS J. 2005, 272, 5101–5109. [Google Scholar] [CrossRef] [PubMed]
- Grishin, N. Estimation of evolutionary distances from protein spatial structures. J. Mol. Evol. 1997, 45, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Ac. Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [PubMed]
- Geer, L.Y.; Domrachev, M.; Lipman, D.J.; Bryant, S.H. CDART: Protein homology by domain architecture. Genome Res. 2002, 12, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 40: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, w252–w258. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Sternberg, M.J. Protein structure prediction on the Web: A case study using the Phyre server. Nat. Protoc. 2009, 4, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Bacik, J.P.; Whitworth, G.E.; Stubbs, K.A.; Vocadlo, D.J.; Mark, B.L. Active site plasticity within the glycoside hydrolase NagZ underlies a dynamic mechanism of substrate distortion. Chem. Biol. 2012, 19, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Lindahl, E.; Wallner, B. Improved model quality assessment using ProQ2. BMC Bioinf. 2012, 13, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Beiko, R.G. Identifying biologically relevant differences between metagenomic communities. Bioinformatics 2010, 26, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Lyimo, T.J.; Pol, A.; Jetten, M.S.; den Camp, H.J.O. Diversity of methanogenic archaea in a mangrove sediment and isolation of a new Methanococcoides strain. FEMS Microbiol. Lett. 2009, 291, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Hong, K.; Yu, Z.N. Archaeal communities in mangrove soil characterized by 16S rRNA gene clones. J. Microbiol. 2006, 44, 566. [Google Scholar] [PubMed]
- Gomes, N.C.M.; Borges, L.R.; Paranhos, R.; Pinto, F.N.; Mendonça-Hagler, L.C.S.; Smalla, K. Exporing the diversity of bacterial communities in sediments of urban mangrove forests. FEMS Microbiol. Ecol. 2008, 3, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Keuskamp, J.A.; Feller, I.C.; Laanbroek, H.J.; Verhoeven, J.T.; Hefting, M.M. Short-and long-term effects of nutrient enrichment on microbial exoenzyme activity in mangrove peat. Soil Biol. Biochem. 2015, 81, 38–47. [Google Scholar] [CrossRef]
- Chambers, L.G.; Guevara, R.; Boyer, J.N.; Troxler, T.G.; Davis, S.E. Effects of salinity and inundation on microbial community structure and function in a mangrove peat soil. Wetlands 2016, 36, 361–371. [Google Scholar] [CrossRef]
- Dinesh, B.; Lau, N.S.; Furusawa, G.; Kim, S.W.; Taylor, T.D.; Foong, S.Y.; Shu-Chien, A.C. Comparative genome analyses of novel Mangrovimonas-like strains isolated from estuarine mangrove sediments reveal xylan and arabinan utilization genes. Mar. Genom. 2016, 25, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.J.; Hrmova, M.; de Gori, R.; Varguese, J.N.; Fincher, G.B. Comparative modeling of the three-dimensional structures of family 3 glycoside hydrolases. Proteins 2000, 41, 257–269. [Google Scholar] [CrossRef]
- Li, J.L.; Cheng, Y.Q.; Wang, P.; Zhao, W.T.; Yin, L.J.; Saito, M.A. Novel improvement in whey protein isolated emulsion stability: Generation of an enzymatically cross-linked pectin using horseradish peroxidase. Food Hydrocoll. 2012, 26, 448–455. [Google Scholar] [CrossRef]
- Patel, A.B.; Patel, A.K.; Shah, M.P.; Parikh, I.K.; Joshi, C.G. Isolation and characterization of novel multifunctional recombinant family 26 glycoside hydrolase from mehsani buffalo rumen metagenome. Biotechnol. Appl. Biochem. 2015, 63, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, J.; Wang, Q.; Fang, W.; Peng, H.; Zhang, X.; Xiao, Y. A novel esterase from a marine metagenomic library exhibiting salt tolerance ability. J. Microbiol. Biotechnol. 2014, 28, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Sae-Lee, R.; Boonmee, A. Newly derived GH43 gene from compost metagenome showing dual xylanase and cellulase activities. Folia Microbiol. 2014, 59, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hayn, M.; Esterbauer, H. Purification and characterization of two extracellular β-glucosidases from Trichoderma reesei. Biochim. Biophys. Acta —Protein Struct. Mol. Enzymol. 1992, 1121, 54–60. [Google Scholar] [CrossRef]
- Varghese, J.N.; Hrmova, M.; Fincher, G.B. Three-dimensional structure of a barley beta-d-glucan exohydrolase, a family 3 glycosyl hydrolase. Structure 1999, 7, 179–190. [Google Scholar] [CrossRef]
- Litzinger, S.; Fischer, S.; Polzer, P.; Diederichs, K.; Welte, W.; Mayer, C. Structural and kinetic analysis of Bacillus subtilis N-acetylglucosaminidase reveals a unique Asp-His dyad mechanism. J. Biol. Chem. 2010, 285, 35675–35684. [Google Scholar] [CrossRef] [PubMed]
- Vocadlo, D.J.; Mayer, C.; He, S.; Withers, S.G. Mechanism of action and identification of Asp242 as the catalytic nucleophile of Vibrio furnisii N-acetyl-beta-Dglucosaminidase using 2-acetamido-2-deoxy-5-fluoro-alpha-L-idopyranosyl fluoride. Biochemistry 2000, 39, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Vocadlo, D.J.; Withers, S.G. Detailed comparative analysis of the catalytic mechanisms of β-N-acetylglucosaminidases from families 3 and 20 of glycoside hydrolases. Biochemistry 2005, 44, 12809–12818. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Kitagawa, M.; Tanaka, H.; Ueda, K.; Watsuji, T.-O.; Beppu, T.; Kondo, A.; Kawachi, R.; Oku, T.; Nishio, T. A β-N-acetylhexosaminidase from Symbiobacterium thermophilum; gene, cloning, overexpression, purification and characterization. Enzyme Microb. Technol. 2006, 38, 457–464. [Google Scholar] [CrossRef]
- Chen, C.L.; Chung, Y.-C.; Chang, Y.-M.; Chang, C.-T. Characterization of β-N-acetylhexosaminidase from a comercial papaya látex preparation. Food Chem. 2011, 124, 1404–1410. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Chung, Y.-C.; Hsu, C.-C.; Chen, L.-C.; Chiang, C.-L.; Chang, C.-T.; Sung, H.-Y. Biochemical characterization of a β-N-acetylhexosaminidase from fig latex. Bot. Stud. 2011, 52, 23–34. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Sinsabaugh, R.L.; Repert, D.A.; Parkhurst, D.F. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 2000, 81, 2359–2365. [Google Scholar] [CrossRef]
- Hreggvidsson, G.O.; Kaiste, E.; Holst, O.; Eggertsson, G.; Palsdottir, A.; Kristjansson, J.K. An extremely thermostable celulase form the thermophilic eubacterium Rhodothermus marinus. Appl. Environ. Microbiol. 1996, 62, 3047–3049. [Google Scholar]
| Carbon Source | Activity (mmol/min/mL) | Relative Activity (%) |
|---|---|---|
| pNP-GlcNac | 74 | 100 |
| pNP-GalNac | 60 | 81 |
| pNP-Glc | 15 | 20 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, F.L.; Marcon, J.; Pereira e Silva, M.D.C.; Khakhum, N.; Cerdeira, L.T.; Ottoni, J.R.; Domingos, D.F.; Taketani, R.G.; De Oliveira, V.M.; Lima, A.O.d.S.; et al. A Novel Multifunctional β-N-Acetylhexosaminidase Revealed through Metagenomics of an Oil-Spilled Mangrove. Bioengineering 2017, 4, 62. https://doi.org/10.3390/bioengineering4030062
Soares FL, Marcon J, Pereira e Silva MDC, Khakhum N, Cerdeira LT, Ottoni JR, Domingos DF, Taketani RG, De Oliveira VM, Lima AOdS, et al. A Novel Multifunctional β-N-Acetylhexosaminidase Revealed through Metagenomics of an Oil-Spilled Mangrove. Bioengineering. 2017; 4(3):62. https://doi.org/10.3390/bioengineering4030062
Chicago/Turabian StyleSoares, Fábio Lino, Joelma Marcon, Michele De Cássia Pereira e Silva, Nittaya Khakhum, Louise Teixeira Cerdeira, Júlia Ronzella Ottoni, Daniela Ferreira Domingos, Rodrigo Gouvea Taketani, Valéria Maia De Oliveira, André Oliveira de Souza Lima, and et al. 2017. "A Novel Multifunctional β-N-Acetylhexosaminidase Revealed through Metagenomics of an Oil-Spilled Mangrove" Bioengineering 4, no. 3: 62. https://doi.org/10.3390/bioengineering4030062






