Encapsulation of Oregano (Origanum onites L.) Essential Oil in β-Cyclodextrin (β-CD): Synthesis and Characterization of the Inclusion Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of β-CD—Oregano EO Inclusion Complexes (ICs)
2.3. Characterization of the β-CD—Oregano EO ICs
2.3.1. Dynamic Light Scattering (DLS)
2.3.2. Fourier Transform Infrared Spectroscopy (FT-IR (ATR) Spectroscopy)
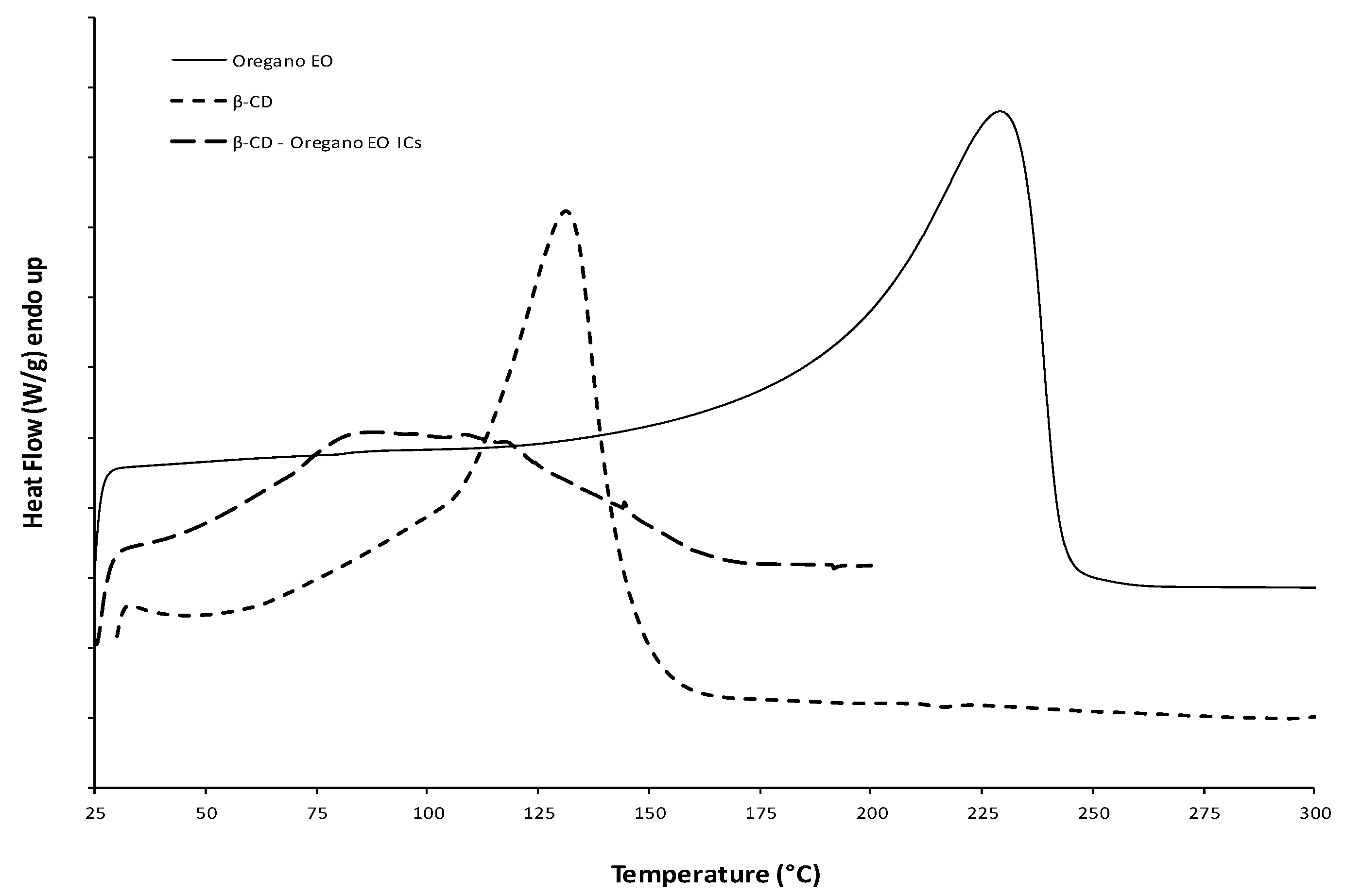
2.3.3. Differential Scanning Calorimetry (DSC)
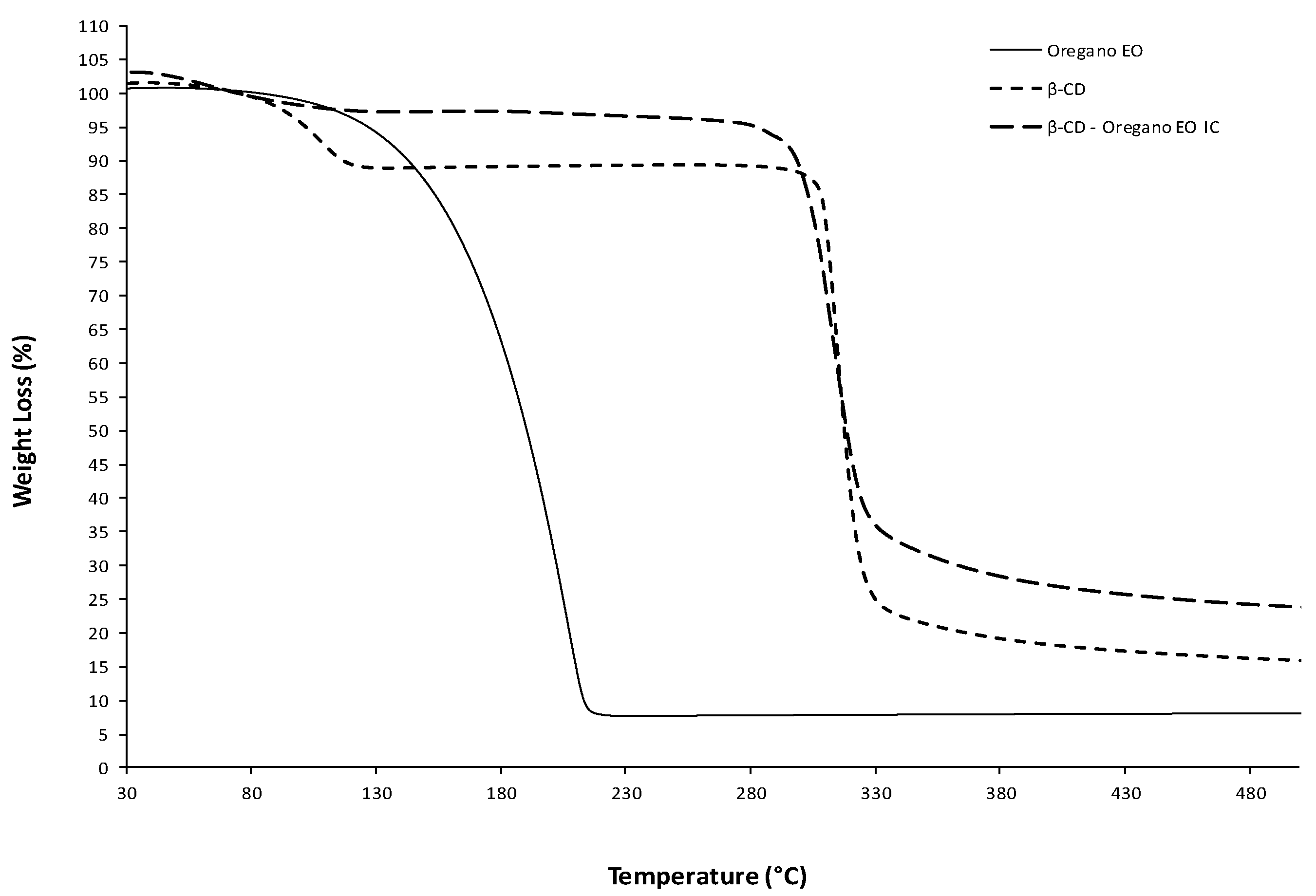
2.3.4. Thermogravimetric Analysis (TGA)
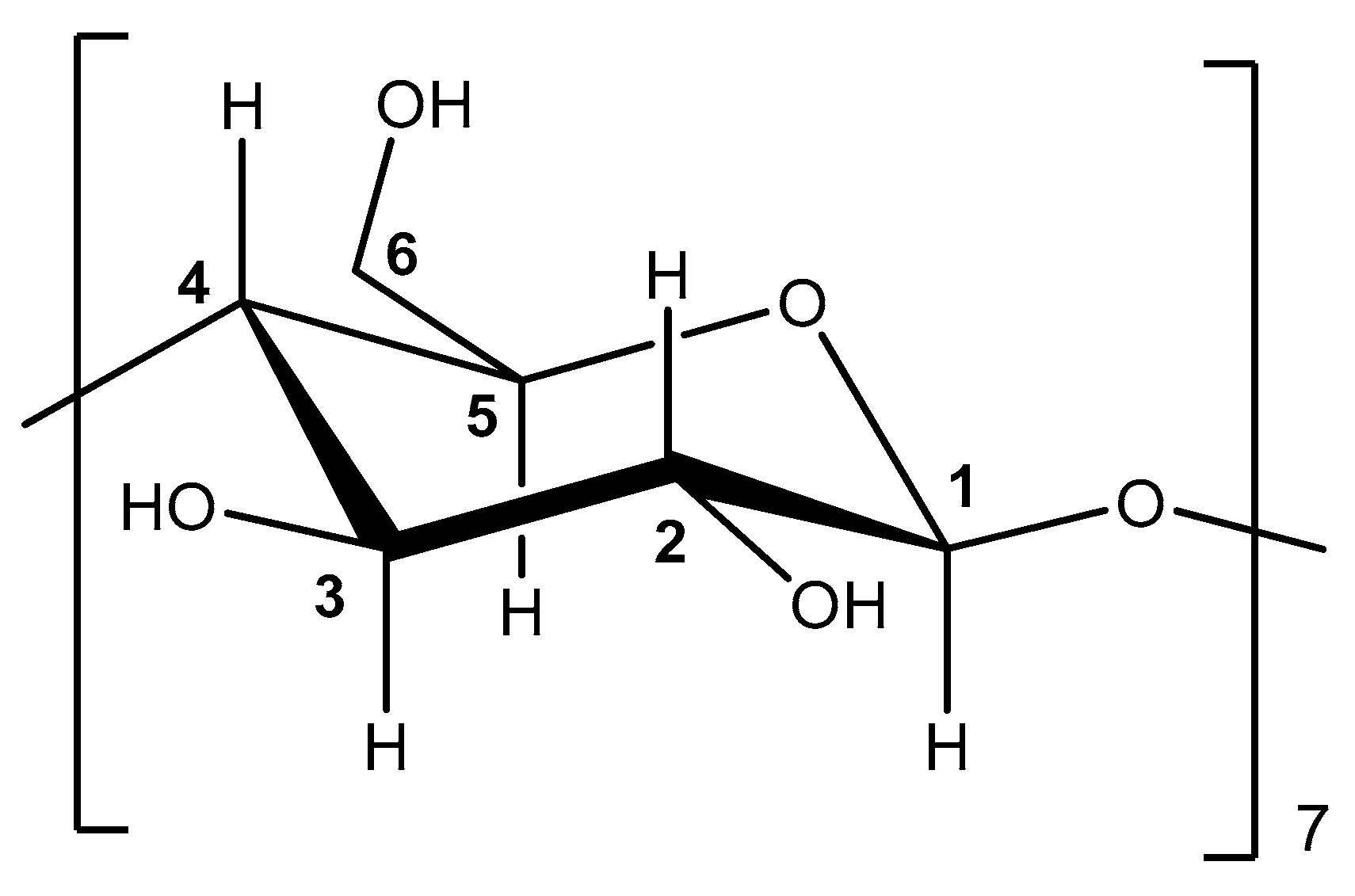
2.3.5. Nuclear Magnetic Resonance Spectroscopy (NMR Spectroscopy)
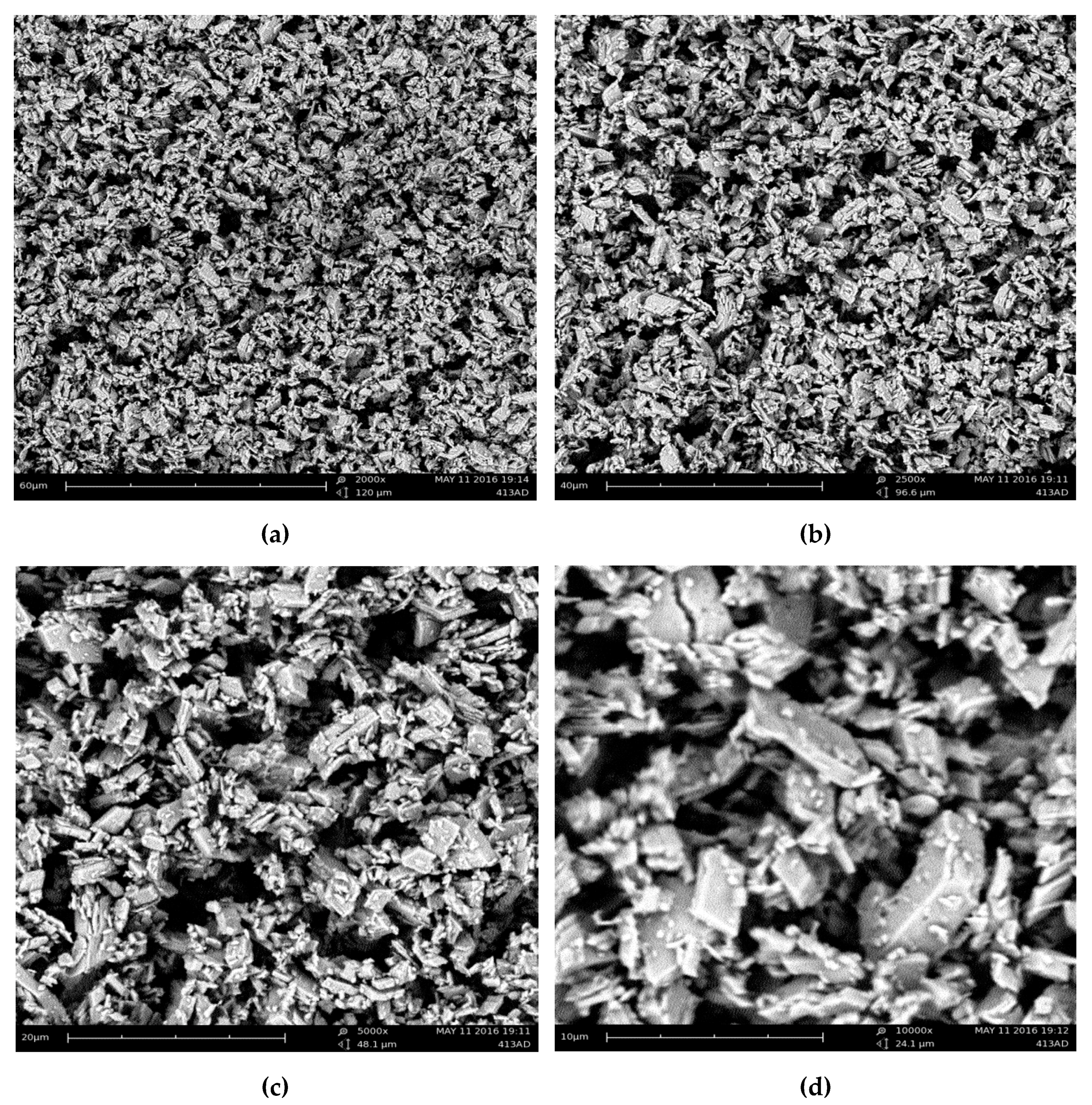
2.3.6. Scanning Electron Microscopy (SEM)
2.4. Inclusion Efficiency of the β-CD—Oregano EO ICs
2.5. In Vitro Release Studies of the Oregano EO from the β-CD—Oregano EO ICs
3. Results and Discussion
3.1. Characterization of the β-CD—Oregano EO ICs
3.1.1. Size, Size Distribution and Zeta-Potential (ζ-Potential)
3.1.2. FT-IR Analysis
3.1.3. Thermal Analysis by Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
3.1.4. Nuclear Magnetic Resonance (NMR) Analysis
3.1.5. Morphology
3.2. Inclusion Efficiency of the β-CD—Oregano EO ICs
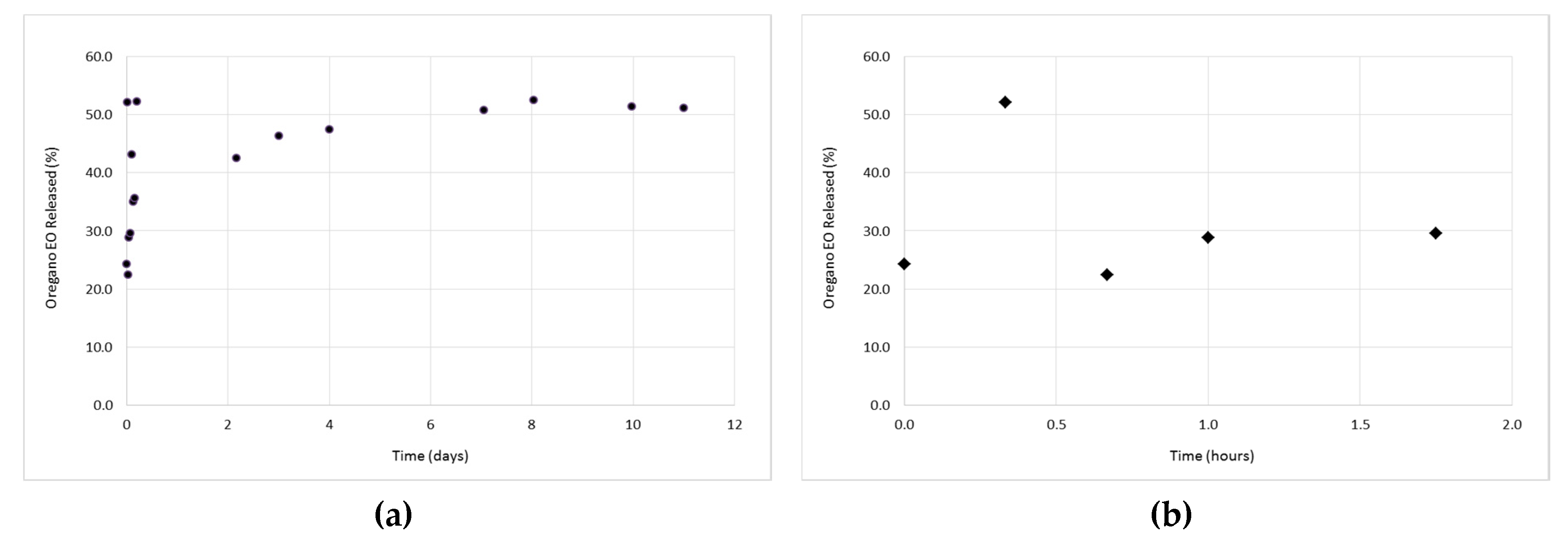
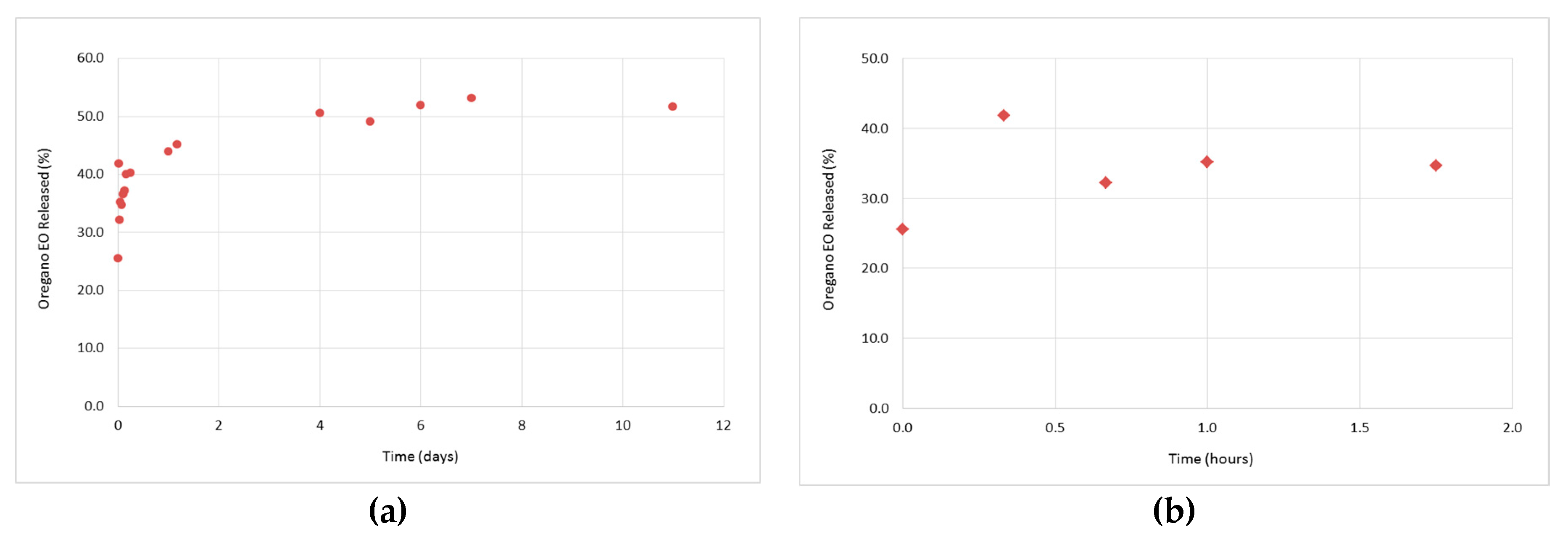
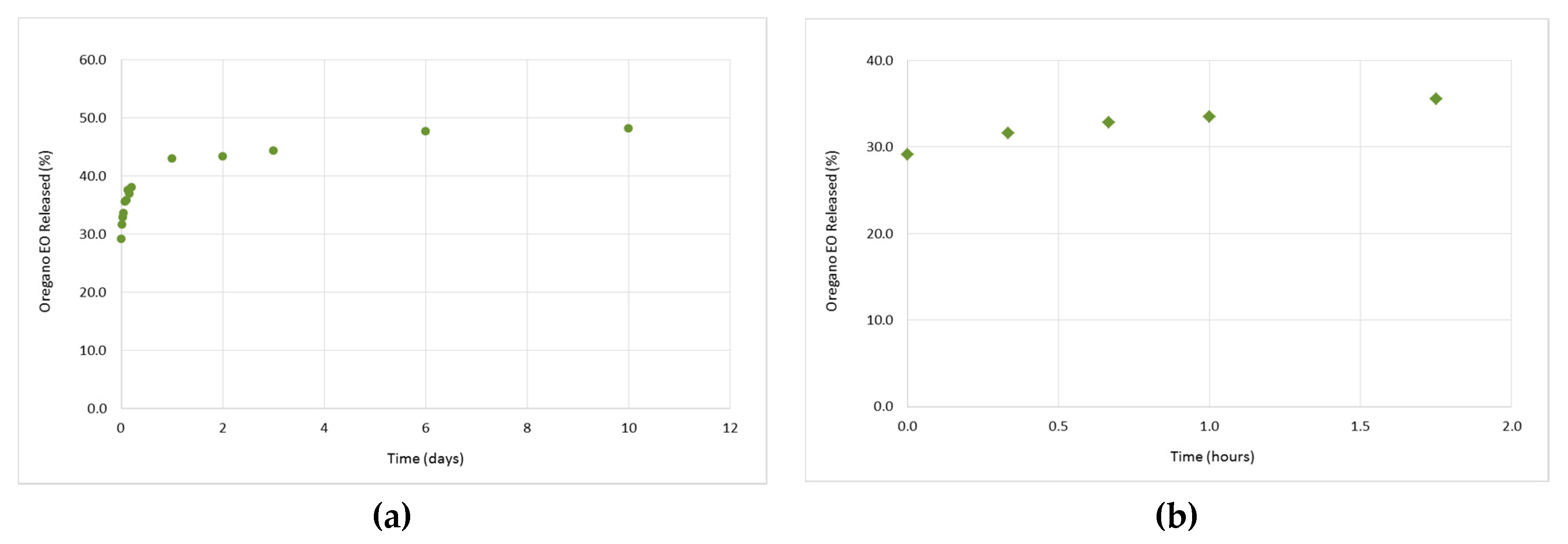
3.3. In Vitro Release Studies of the Oregano EO from the β-CD—Oregano EO ICs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Almeida, A.P.; Rodríguez-Rojo, S.; Serra, A.T.; Vila-Real, H.; Simplicio, A.L.; Delgadilho, I.; Beirão da Costa, S.; Beirão da Costa, L.; Nogueira, I.D.; Duarte, C.M.M. Microencapsulation of oregano essential oil in starch-based materials using supercritical fluid technology. Innov. Food Sci. Emerg. Technol. 2013, 20, 140–145. [Google Scholar] [CrossRef]
- Beirão da Costa, S.; Duarte, C.; Bourbon, A.I.; Pinheiro, A.C.; Serra, A.T.; Moldão Martins, M.; Nunes Januário, M.I.; Vicente, A.A.; Delgadillo, I.; et al.; Beirão da Costa, M.L. Effect of the matrix system in the delivery and in vitro bioactivity of microencapsulated Oregano essential oil. J. Food Eng. 2012, 110, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Stefanaki, A.; Cook, C.M.; Lanaras, T.; Kokkini, S. The Oregano plants of Chios Island (Greece): Essential oils of Origanum onites L. growing wild in different habitats. Ind. Crops Prod. 2016, 82, 107–113. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Lavoine, N.; Givord, C.; Tabary, N.; Desloges, I.; Martel, B.; Bras, J. Elaboration of a new antibacterial bio-nano-material for food-packaging by synergistic action of cyclodextrin and microfibrillated cellulose. Innov. Food Sci. Emerg. Technol. 2014, 26, 330–340. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Promising applications of cyclodextrins in food: Improvement of essential oils retention, controlled release and antiradical activity. Carbohydr. Polym. 2015, 131, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Fathi, M.; Mozafari, M.R.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Marques, C.H.M. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Fathi, M.; Martín, A.; McClements, D.J. Nanoencapsulation of Food Ingredients using Carbohydrate Based Delivery Systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Costa, P.; Medronho, B.; Gonçalves, S.; Romano, A. Cyclodextrins enhance the antioxidant activity of essential oils from three Lamiaceae species. Ind. Crops Prod. 2015, 70, 341–346. [Google Scholar] [CrossRef]
- Song, L.X.; Wang, H.M.; Xu, P.; Yang, Y.; Zhang, Z.Q. Experimental and Theoretical Studies on the Inclusion Complexation of Syringic Acid with α-, β-, γ- and Heptakis(2,6-di-O-methyl)-β- cyclodextrin. Chem. Pharmaceut. Bull. 2008, 56, 468–474. [Google Scholar] [CrossRef]
- Sun, X.; Sui, S.; Ference, C.; Zhang, Y.; Sun, S.; Zhou, N.; Zhu, W.; Zhou, K. Antimicrobial and Mechanical Properties of β-Cyclodextrin Inclusion with Essential Oils in Chitosan Films. J. Agricult. Food Chem. 2014, 62, 8914–8918. [Google Scholar] [CrossRef] [PubMed]
- Voncina, B.; Vivod, V. Cyclodextrins in Textile Finishing. In Eco-Friendly Textile Dyeing and Finishing; Günay, M., Ed.; INTECH Open Access Publisher: Rijeka, Croatia, 2013; pp. 53–76. [Google Scholar]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physicochemical Properties and Antimicrobial Efficacy of Carvacrol Nanoemulsions Formed by Spontaneous Emulsification. J. Agricult. Food Chem. 2013, 61, 8906–8913. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Luo, Y.; Wang, Q. Antioxidant and antimicrobial properties of essential oils encapsulated in zein nanoparticles prepared by liquid-liquid dispersion method. LWT Food Sci. Technol. 2012, 48, 283–290. [Google Scholar] [CrossRef]
- Parris, N.; Cooke, P.H.; Hicks, K.B. Encapsulation of Essential Oils in Zein Nanospherical Particles. J. Agric. Food Chem. 2005, 53, 4788–4792. [Google Scholar] [CrossRef] [PubMed]
- Beirão-da-Costa, S.; Duarte, C.; Bourbon, A.I.; Pinheiro, A.C.; Januário, M.I.N.; Vicente, A.A.; Beirão-da-Costa, M.L.; Delgadillo, I. Inulin potential for encapsulation and controlled delivery of Oregano essential oil. Food Hydrocoll. 2013, 33, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, K.; Conti, D.S.; Da Rocha, S.R.P.; Zhang, Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippia graveolens HBK) with different composition when microencapsulated in β-cyclodextrin. Lett. Appl. Microbiol. 2010, 50, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Judefeind, A.; de Villiers, M.M. Drug Loading into and In Vitro Release from Nanosized Drug Delivery Systems. In Nanotechnology in Drug Delivery; De Villiers, M.M., Aramwit, P., Kwon, G.S., Eds.; Springer: New York, NY, USA, 2009; Volume X, pp. 129–162. [Google Scholar]
- Kohane, D.S. Microparticles and Nanoparticles for Drug Delivery. Biotechnol. Bioeng. 2007, 96, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Wendorf, J.R.; Singh, M.; O'Hagan, D.T. Nanoparticles and Microparticles as Vaccine Adjuvants. In Nanoparticulates as Drug Carriers; Torchilin, V.P., Ed.; Imperial College Press: London, UK, 2006; pp. 675–696. [Google Scholar]
- Mohanraj, V.J.; Chen, Y. Nanoparticles—A Review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Wiechers, J.W.; Musee, N. Engineered inorganic nanoparticles and cosmetics: Facts, issues, knowledge gaps and challenges. J. Biomed. Nanotechnol. 2010, 6, 408–431. [Google Scholar] [CrossRef] [PubMed]
- Roussaki, M.; Gaitanarou, A.; Diamanti, P.C.; Vouyiouka, S.; Papaspyrides, C.; Kefalas, P.; Detsi, A. Encapsulation of the natural antioxidant aureusidin in biodegradable PLA nanoparticles. Polym. Degrad. Stab. 2014, 108, 182–187. [Google Scholar] [CrossRef]
- Rakmaia, J.; Cheirsilp, B.; Torrado-Agrasar, A.; Simal-Gándara, J.; Mejuto, J.C. Encapsulation of yarrow essential oil in hydroxypropyl-beta-cyclodextrin: Physiochemical characterization and evaluation of bio-efficacies. CyTA J. Food 2017, 1–9. [Google Scholar] [CrossRef]
- Sancho, M.I.; Andujar, S.; Porasso, R.D.; Enriz, R.D. Theoretical and Experimental Study of Inclusion Complexes of β-Cyclodextrins with Chalcone and 2′,4′-Dihydroxychalcone. J. Phys. Chem. B 2016, 120, 3000–3011. [Google Scholar] [CrossRef] [PubMed]
- Cannava, C.; Crupi, V.; Guardo, M.; Majolino, D.; Stancanelli, R.; Tommasini, S.; Ventura, C.A.; Venuti, V. Phase solubility and FTIR-ATR studies of idebenone/sulfobutyl ether b-cyclodextrin inclusion complex. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 255–262. [Google Scholar] [CrossRef]
- Karathanos, V.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007, 101, 652–658. [Google Scholar] [CrossRef]
- Gomes, C.; Moreira, R.; Castell-Perez, E. Microencapsulated antimicrobial compounds as a means to enhance electron beam irradiation treatment for inactivation of pathogens on fresh spinach leaves. J. Food Sci. 2011, 76, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Min, S.; Choi, M. Release characteristics of freeze-dried eugenol encapsulated with β-cyclodextrin by molecular inclusion method. J Microencapsul. 2010, 27, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Pessine, F.B.T.; Calderini, A.; Alexandrino, G.L. Review: Cyclodextrin Inclusion Complexes Probed by NMR Techniques. In Magnetic Resonance Spectroscopy; Kim, D.H., Ed.; InTech: Rijeka, Croatia, 2012; pp. 238–264. [Google Scholar]
- Locci, E.; Lai, S.; Piras, A.; Marongiu, B.; Lai, A. 13C-CPMAS and 1H-NMR Study of the Inclusion Complexes of β-Cyclodextrin with Carvacrol, Thymol, and Eugenol Prepared in Supercritical Carbon Dioxide. Chem. Biodivers. 2004, 1, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Sugiyama, H.; Shimosegawa, H.; Nakane, R.; Ishida, Y.; Uekaji, Y.; Nakata, D.; Pallauf, K.; Rimbach, G.; et al.; Matsugo, S. Analysis of the Enhanced Stability of R(+)-Alpha Lipoic Acid by the Complex Formation with Cyclodextrins. Int. J. Molecul. Sci. 2013, 14, 3639–3655. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Cotarlet, M.; Tiberius, B.; Bahrim, G.; Alexe, P.; Dima, S. Encapsulation of Coriander Essential Oil in Beta-Cyclodextrin: Antioxidant and Antimicrobial Properties Evaluation. Rom. Biotechnol. Lett. 2014, 19, 9128–9140. [Google Scholar]
- Kamimura, J.A.; Santos, E.H.; Hill, L.E.; Gomes, C.L. Antimicrobial and antioxidant activities of carvacrol microencapsulated in hydroxypropyl-beta-cyclodextrin. LWT Food Sci. Technol. 2014, 57, 701–709. [Google Scholar] [CrossRef]
- Pagington, J.S. β-cyclodextrin and its uses in the flavour industry. In Developments in Food Flavors; Birch, G.G., Linley, M.G., Eds.; Elsevier Applied Science: London, UK, 1986; pp. 131–150. [Google Scholar]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Comprehens. Rev. Food Sci. Food Safet. 2016, 15, 143–182. [Google Scholar] [CrossRef]

| Size (nm) | Polydispersity Index (PDI) | ζ-potential (mV) | |
|---|---|---|---|
| ICs (1) | 531.8 ± 7.7 | 0.308 ± 0.062 | −21.5 ± 1.2 |
| ICs (2) | 450.3 ± 11.5 | 0.484 ± 0.029 | −30.7 ± 1.8 |
| Proton | Chemical Shifts (δ1) of β-CD Protons (ppm) | Chemical Shifts (δ2) of β-CD Protons in β-CD—oregano EO ICs (ppm) | Δδ = δ2 − δ1 (ppm) |
|---|---|---|---|
| Η-1 | 5.089 | 5.082 | −0.007 |
| Η-2 | 3.675 | 3.670 | −0.005 |
| Η-3 | 3.991 | 3.961 | −0.030 |
| Η-4 | 3.610 | 3.610 | 0 |
| Η-5 | 3.875 | 3.836 | −0.039 |
| Η-6 | 3.905 | 3.882 | −0.023 |
| β-CD—Oregano EO ICs (Exp. 1) | β-CD—Oregano EO ICs (Exp. 2) | |
|---|---|---|
| Initial Oregano EO Mass (mg) | 124.60 | 124.70 |
| Encapsulated Oregano EO Mass (mg) | 28.16 | 31.84 |
| IE (%) | 22.60 | 25.53 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotronia, M.; Kavetsou, E.; Loupassaki, S.; Kikionis, S.; Vouyiouka, S.; Detsi, A. Encapsulation of Oregano (Origanum onites L.) Essential Oil in β-Cyclodextrin (β-CD): Synthesis and Characterization of the Inclusion Complexes. Bioengineering 2017, 4, 74. https://doi.org/10.3390/bioengineering4030074
Kotronia M, Kavetsou E, Loupassaki S, Kikionis S, Vouyiouka S, Detsi A. Encapsulation of Oregano (Origanum onites L.) Essential Oil in β-Cyclodextrin (β-CD): Synthesis and Characterization of the Inclusion Complexes. Bioengineering. 2017; 4(3):74. https://doi.org/10.3390/bioengineering4030074
Chicago/Turabian StyleKotronia, Margarita, Eleni Kavetsou, Sofia Loupassaki, Stefanos Kikionis, Stamatina Vouyiouka, and Anastasia Detsi. 2017. "Encapsulation of Oregano (Origanum onites L.) Essential Oil in β-Cyclodextrin (β-CD): Synthesis and Characterization of the Inclusion Complexes" Bioengineering 4, no. 3: 74. https://doi.org/10.3390/bioengineering4030074







