Encapsulation of Antifouling Organic Biocides in Poly(lactic acid) Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA Nanoparticles (NPs) and Respective Coating
2.3. Nanoparticles (NPs) Characterization
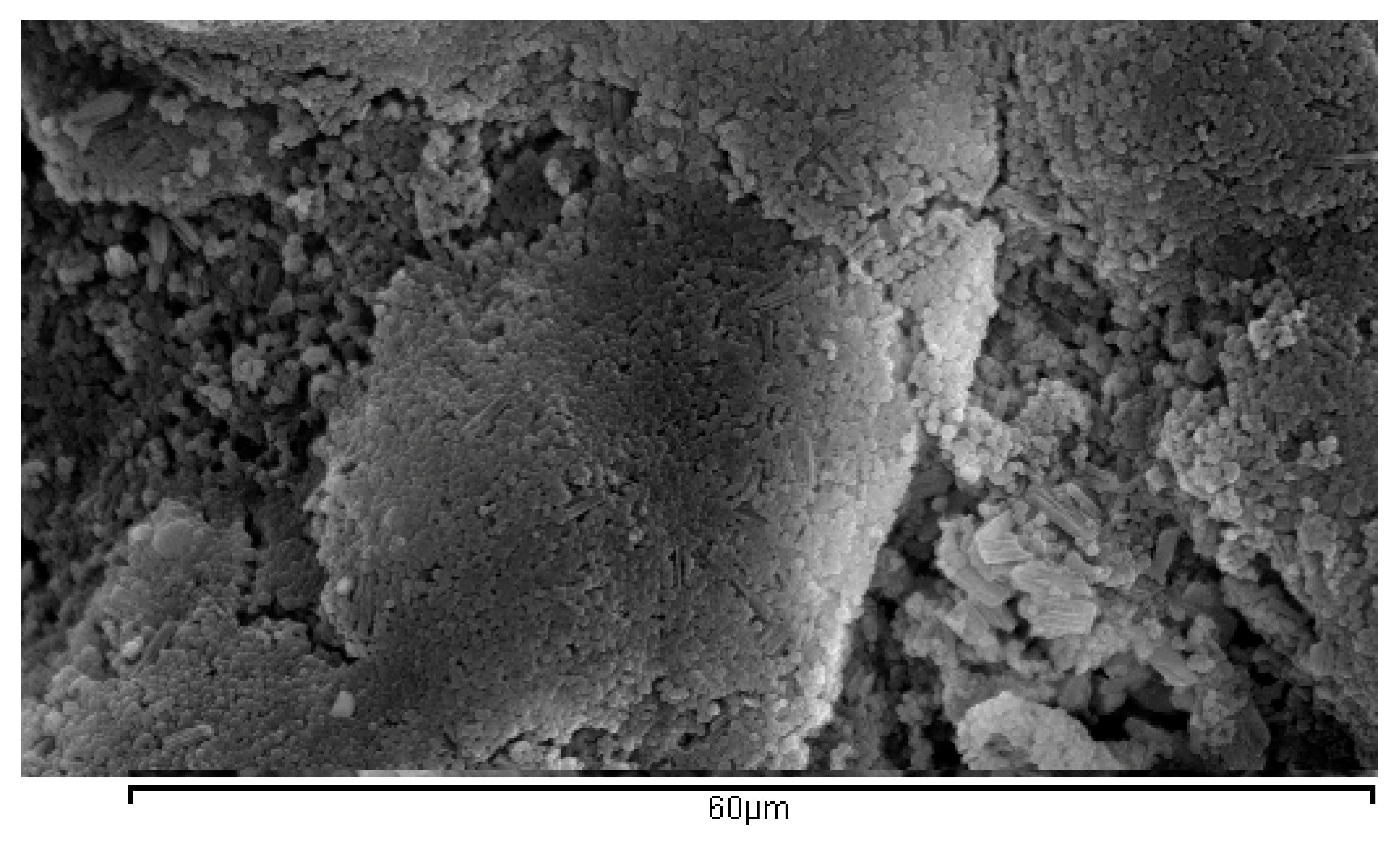
2.3.1. SEM Spectroscopy
2.3.2. Size, Size Distribution and Zeta Potential
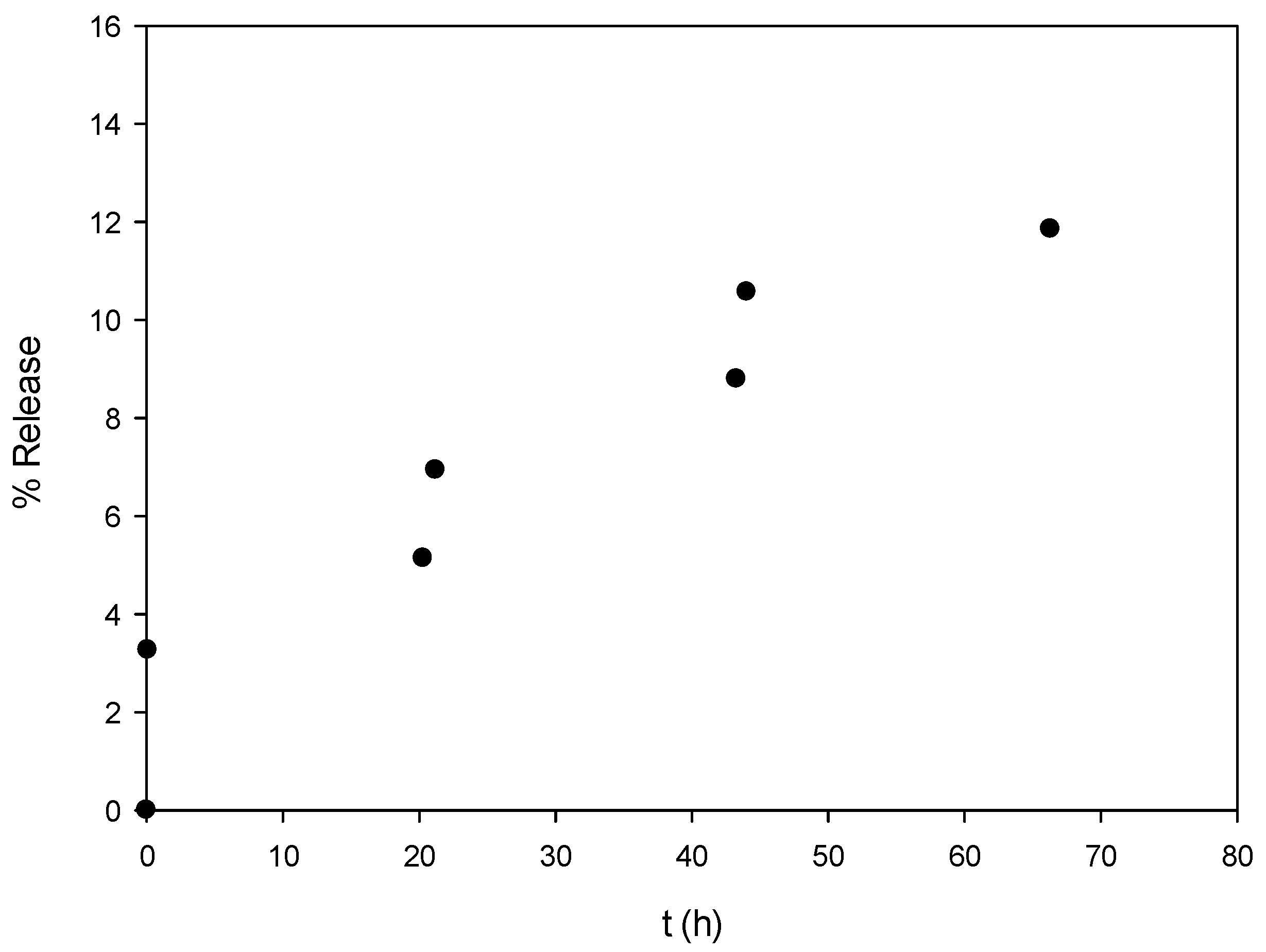
2.3.3. Encapsulation Efficiency (EE) and Preliminary Release Study
2.3.4. FTIR-ATR Spectroscopy
2.3.5. Thermal Properties
3. Results and Discussion
3.1. Preparation of PLA Nanoparticles (NPs)—Process Yield
3.2. Size, Size Distribution and Zeta Potential
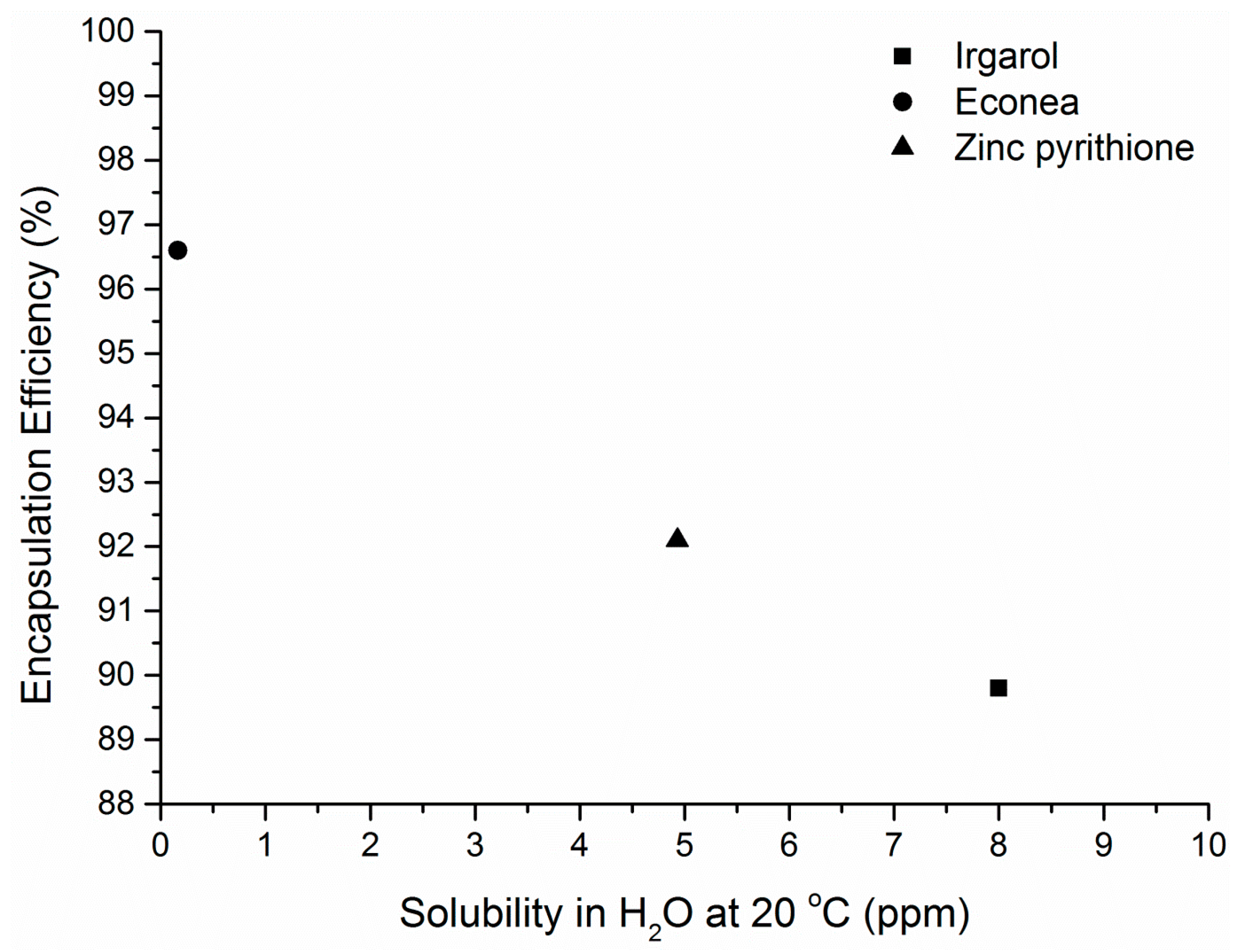
3.3. Encapsualtion Efficiency (ΕΕ) and Preliminary Release Study
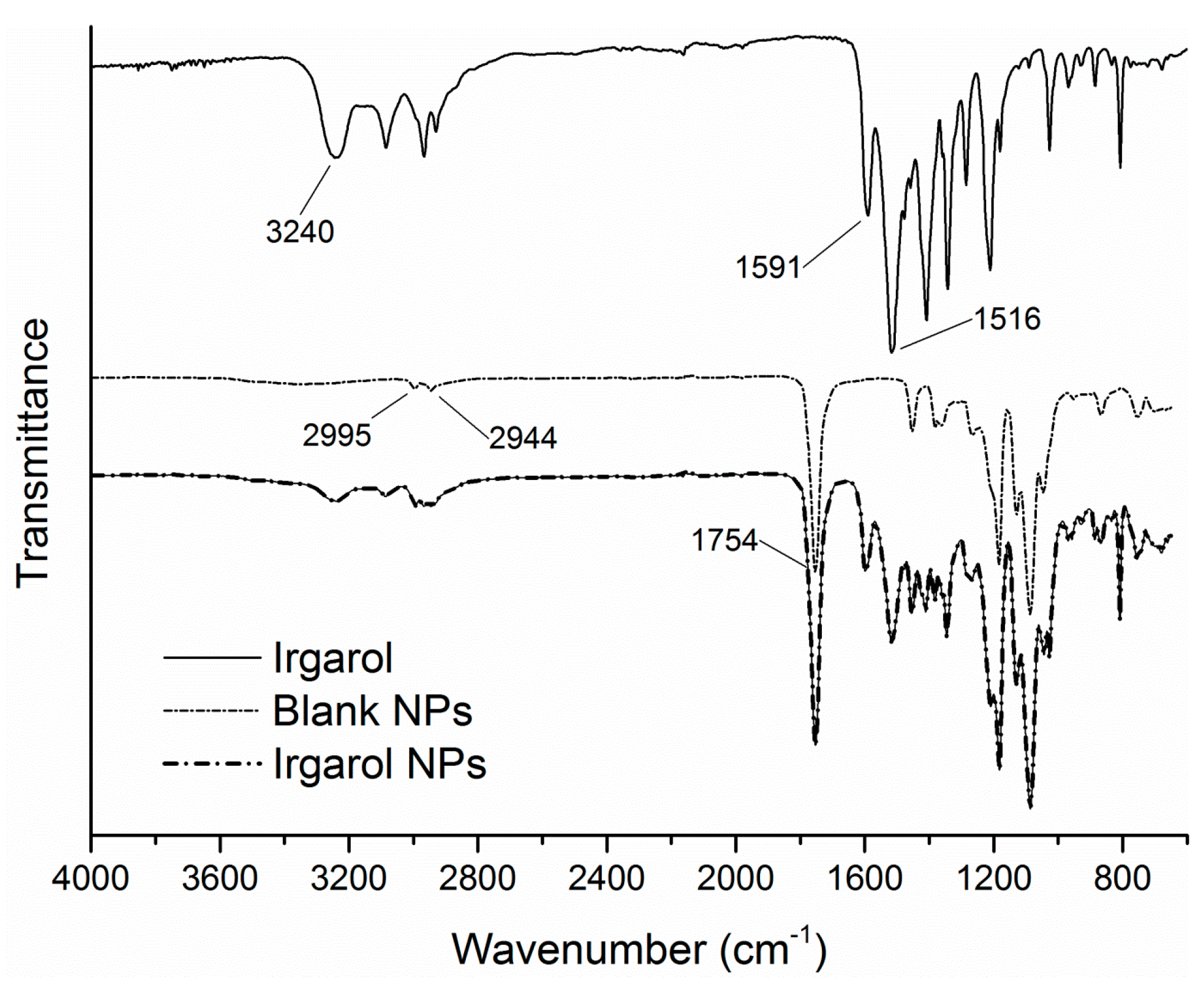
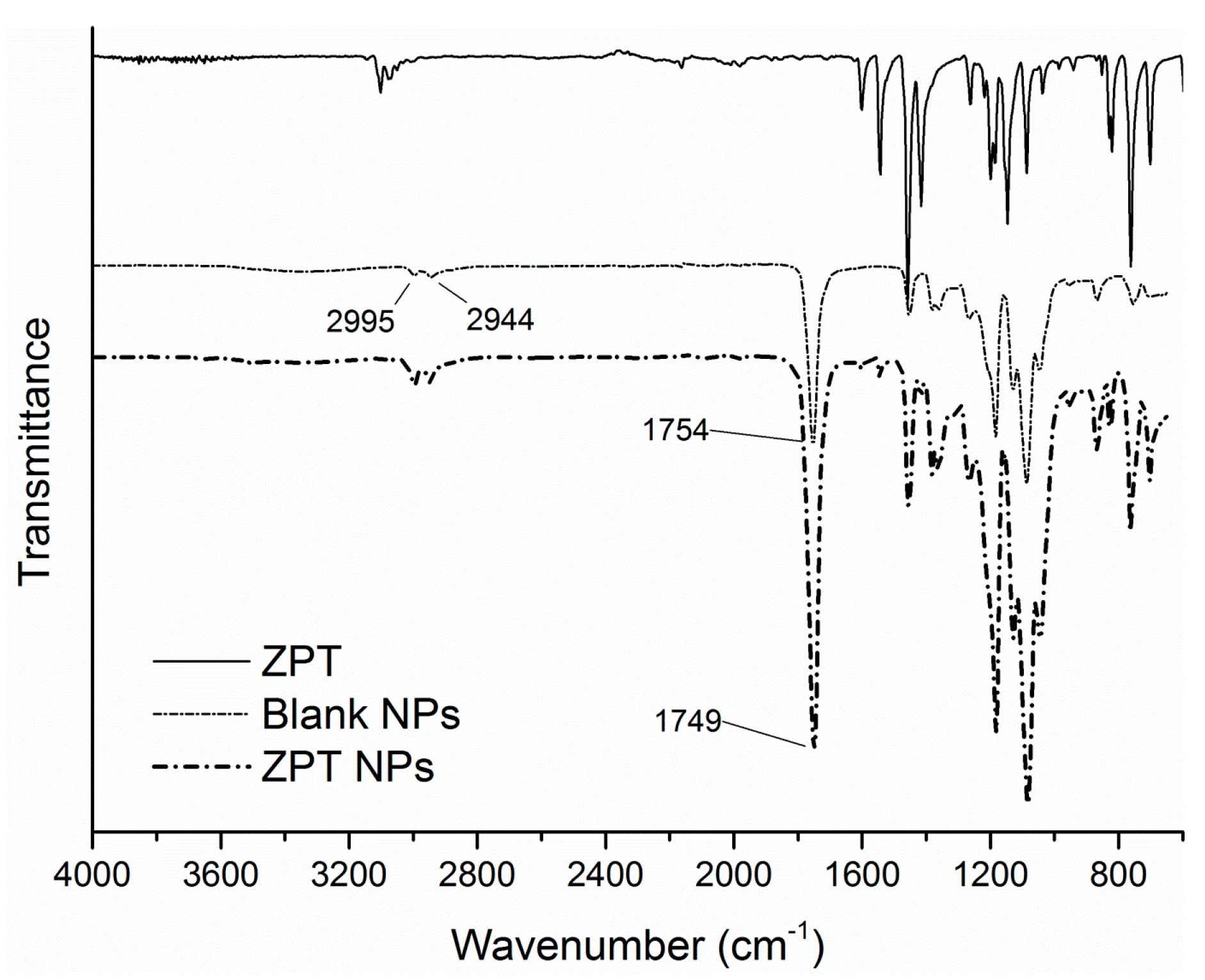
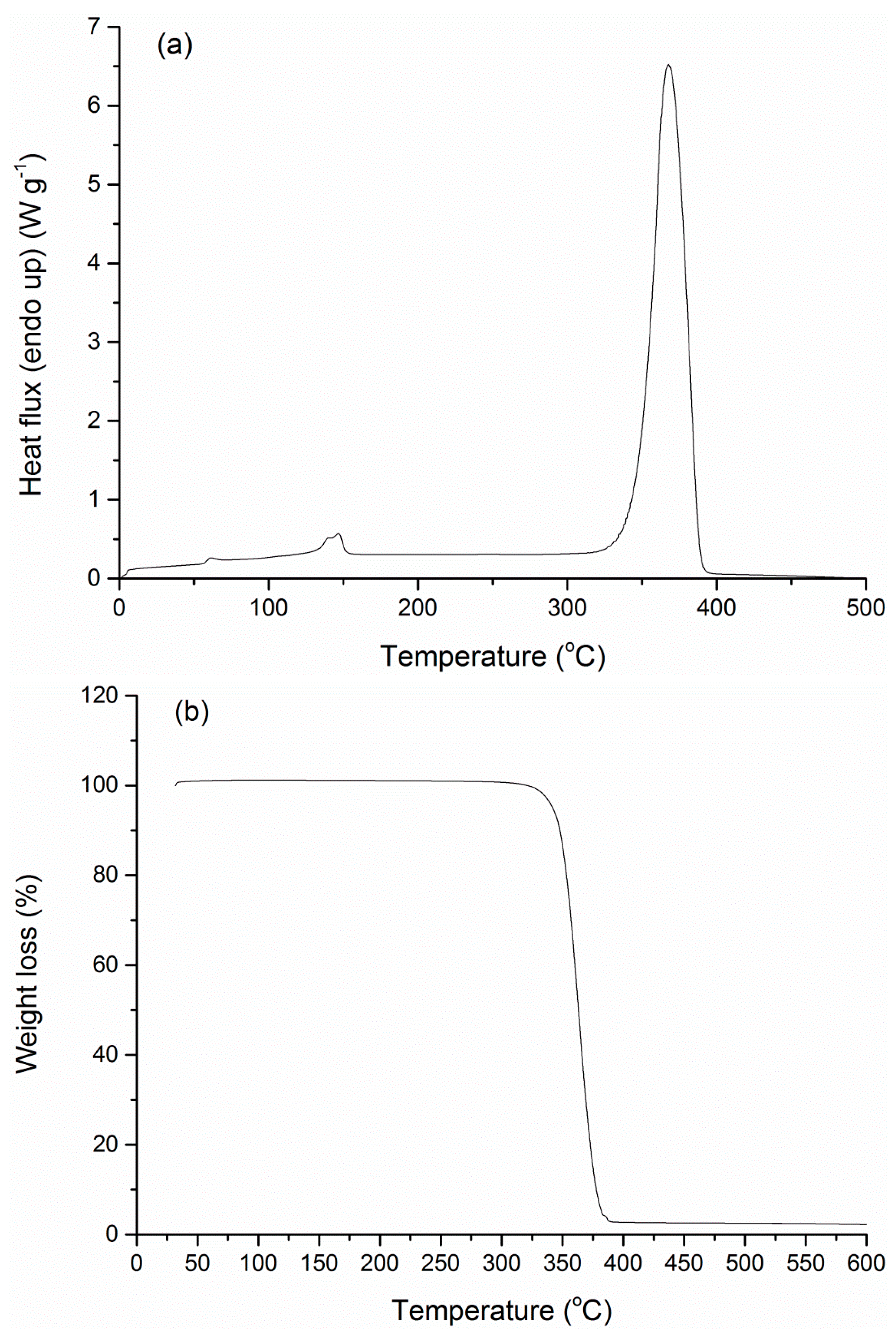
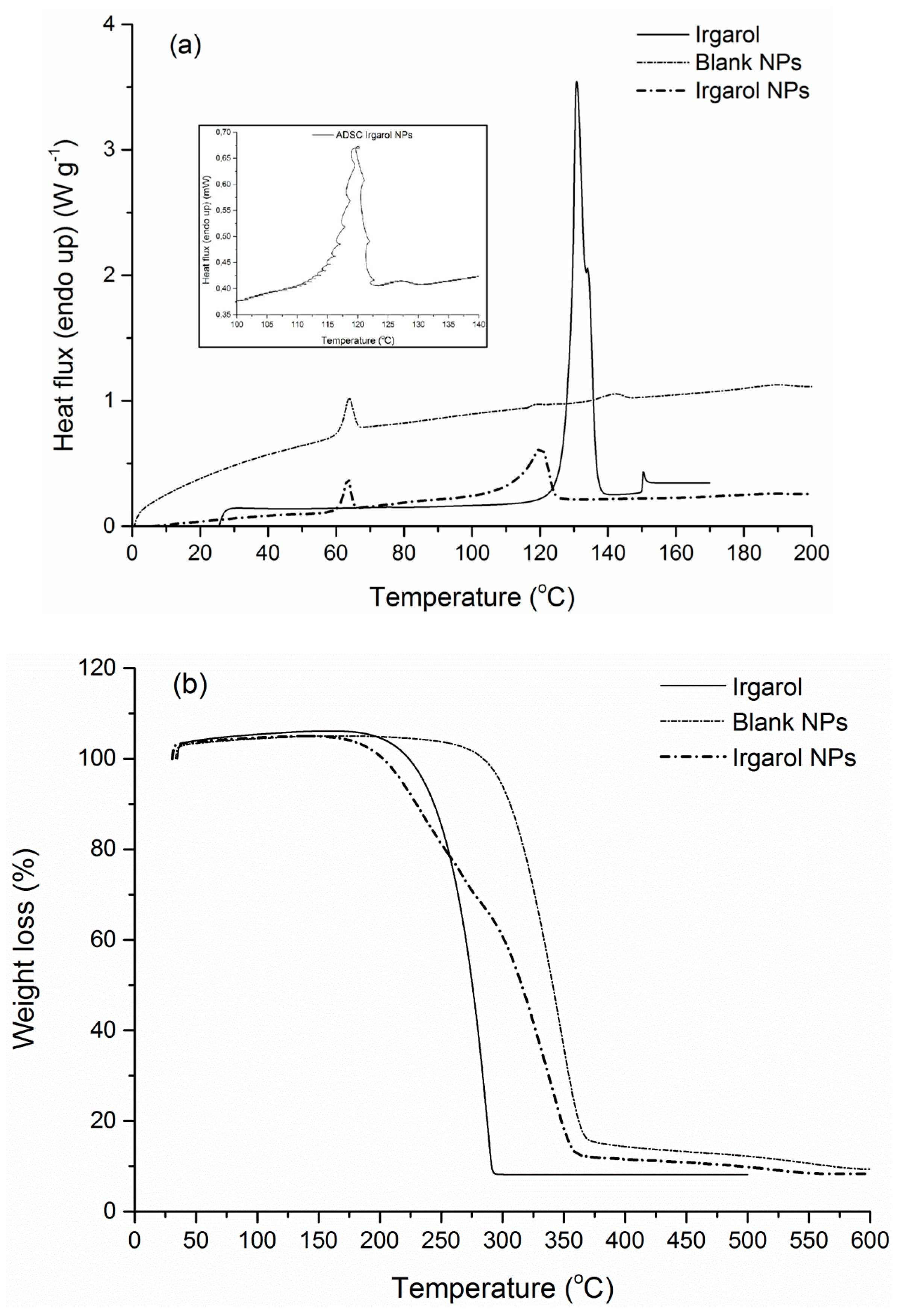
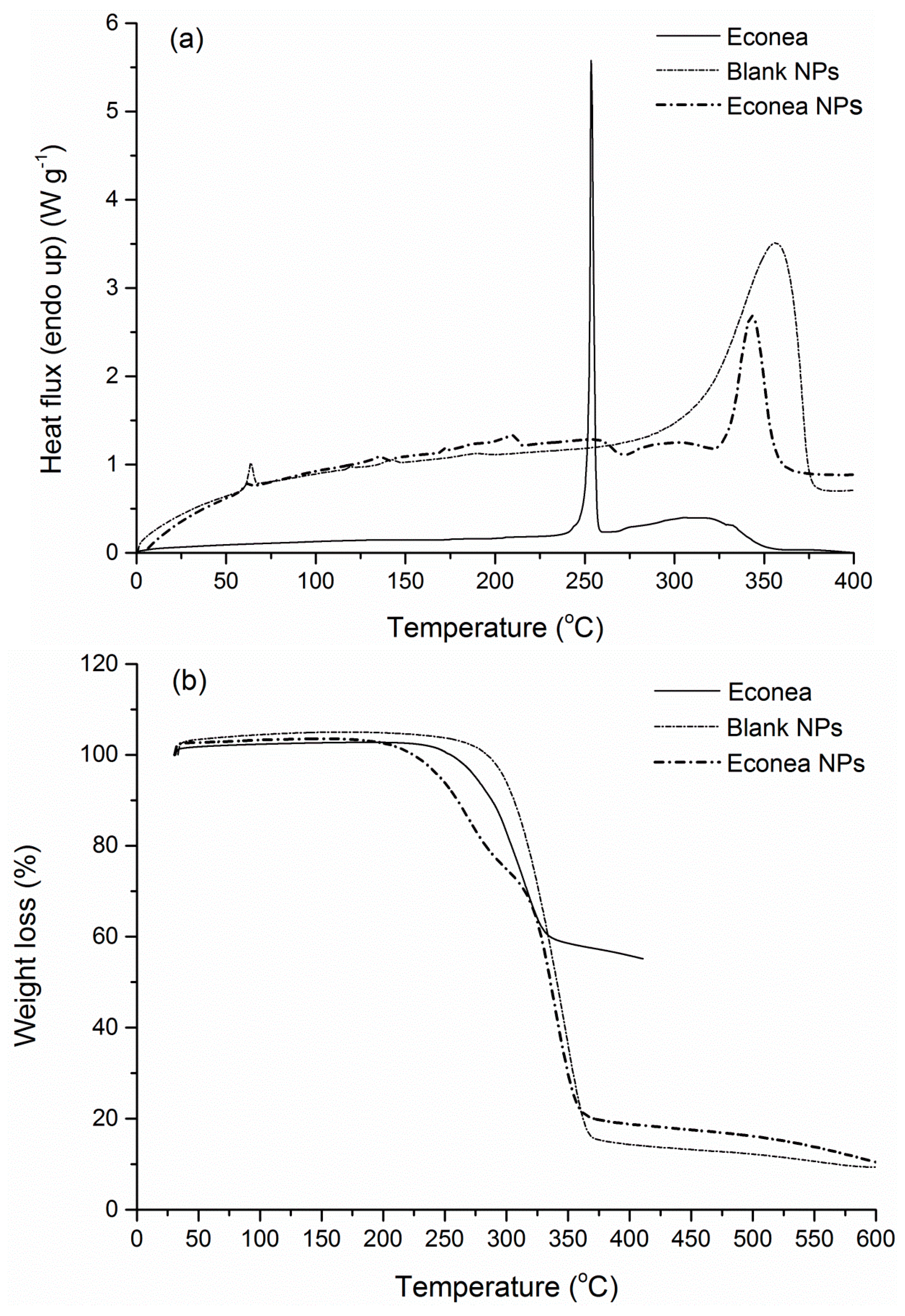
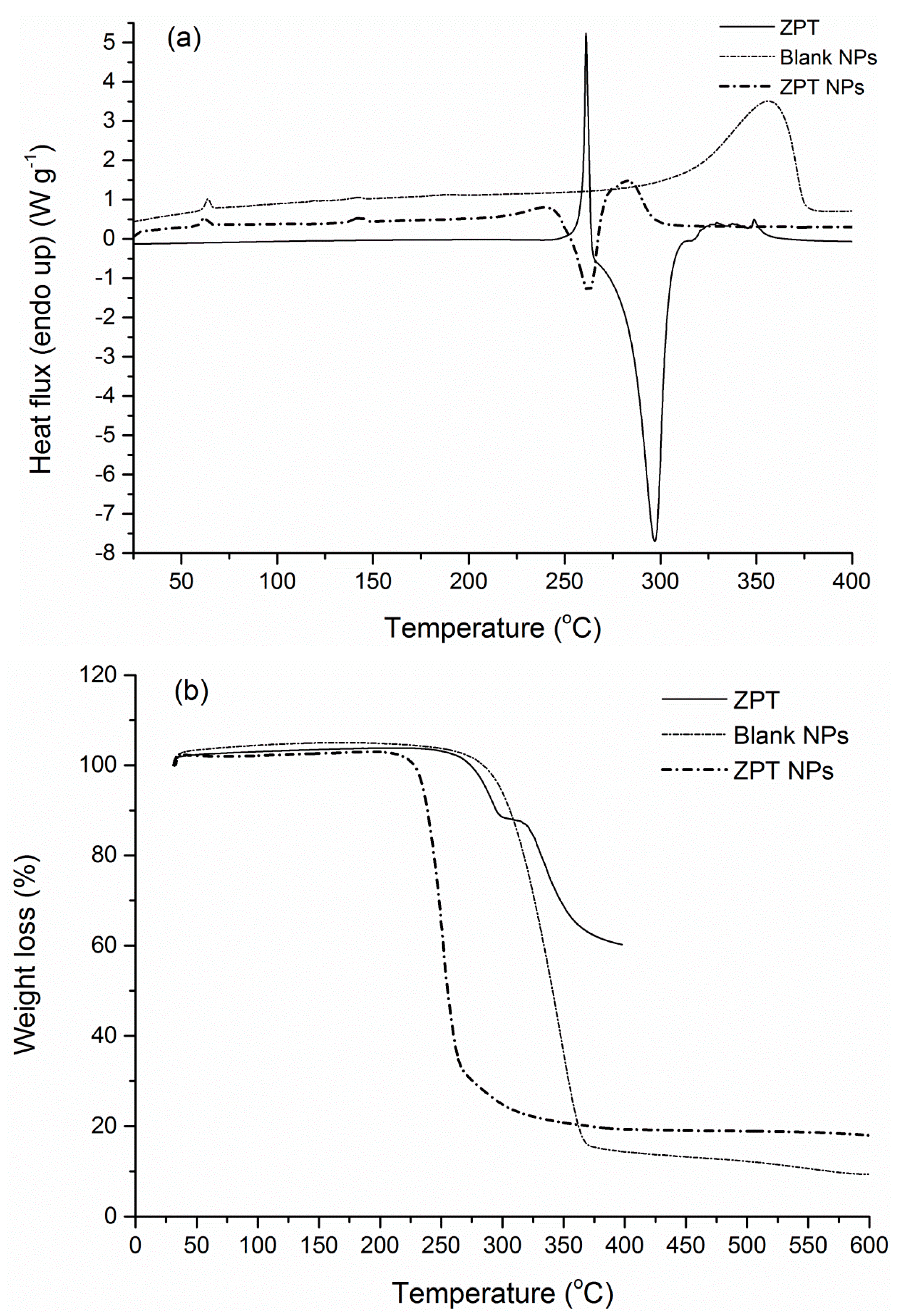
3.4. FTIR-ATR Spectra and Thermal Properties
3.5. Coating Based on Nanoparticles
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yebra, M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Trojer, M.A.; Nordstierna, L.; Bergek, J.; Blanck, H.; Holmberg, K.; Nydén, M. Use of microcapsules as controlled release devices for coatings. Adv. Colloid Interface Sci. 2015, 222, 18–43. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Omae, I. Organotin antifouling paints and their alternatives. Appl. Organomet. Chem. 2003, 17, 81–105. [Google Scholar] [CrossRef]
- Nordstierna, L.; Abdalla, A.A.; Masuda, M.; Skarnemark, G.; Nydén, M. Molecular release from painted surfaces: Free and encapsulated biocides. Prog. Org. Coat. 2010, 69, 45–48. [Google Scholar] [CrossRef]
- Nordstierna, L.; Movahedi, A.; Nydén, M. New Route for Microcapsule Synthesis. J. Dispers. Sci. Technol. 2011, 32, 310–311. [Google Scholar] [CrossRef]
- Bergek, J.; Andersson Trojer, M.; Mok, A.; Nordstierna, L. Controlled release of microencapsulated 2-n-octyl-4-isothiazolin-3-one from coatings: Effect of microscopic and macroscopic pores. Colloids Surf. A Physicochem. Eng. Asp. 2014, 458, 155–167. [Google Scholar] [CrossRef]
- Szabo, T.; Telegdi, J.; Nyikos, L. Linseed oil-filled microcapsules containing drier and corrosion inhibitor—Their effect on self-healing capability of paints. Prog. Org. Coat. 2015, 84, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Telegdi, J.; Szabo, T.; Romanszki, L.; Pavai, M. The Use of Nano-/Microlayers, Self-Healing and Slow-Release Coatings to Prevent Corrosion and Biofouling. In Handbook of Smart Coatings for Materials Protection; Makhlouf, A., Ed.; WP Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Santos, P.D.; Flores, S.; De Oliveira Rios, A.; Campos Chiste, R. Biodegradable polymers as wall materials to the synthesis of bioactive compound nanocapsules. Trends Food Sci. Technol. 2016, 53, 23–33. [Google Scholar] [CrossRef]
- Roussaki, M.; Gaitanarou, A.; Diamanti, P.C.; Vouyiouka, S.; Papaspyrides, C.; Kefalas, P.; Detsi, A. Encapsulation of the natural antioxidant aureusidin in biodegradable PLA nanoparticles. Polym. Degrad. Stab. 2014, 108, 182–187. [Google Scholar] [CrossRef]
- Zhang, M.; Cabane, E.; Claverie, J. Transparent antifouling coatings via nanoencapsulation of a biocide. J. Appl. Polym. Sci. 2007, 105, 3826–3833. [Google Scholar] [CrossRef]
- Nordstierna, L.; Abdalla, A.A.; Nordin, M.; Nydén, M. Comparison of release behaviour from microcapsules and microspheres. Prog. Org. Coat. 2010, 69, 49–51. [Google Scholar] [CrossRef]
- Geiger, T.; Delavy, P.; Hany, R.; Schleuniger, J. Encapsulated Zosteric Acid Embedded in Poly[3-hydroxyalkanoate] Coatings—Protection against Biofouling. Polym. Bull. 2004, 52, 65–72. [Google Scholar] [CrossRef]
- Nikkola, J. Microcapsule-protected actives reduce leaching. Eur. Coat. J. 2014, 4, 36–40. [Google Scholar]
- Pelto, J.M.; Virtanen, S.; Munter, T.; Larismaa, J.; Jämsä, S.; Nikkola, J. Encapsulation of 3-iodo-2-propynyl N-butylcarbamate (IPBC) in polystyrene-polycaprolactone (PS/PCL) blends. J. Microencapsul. 2014, 31, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Troy Corporation. Available online: http://www.troycorp.com/product_view_ashland.asp?cat=Newproducts&ID=222 (accessed on 24 December 2016).
- Price, R.R.; Patchan, M.; Clare, A.; Rittschof, D.; Bonaventura, J. Performance enhancement of natural antifouling compounds and their analogs through microencapsulation and controlled release. Biofouling 1992, 6, 207–216. [Google Scholar] [CrossRef]
- Edge, M.; Allen, N.S.; Turner, D.; Robinson, J.; Seal, K. The enhanced performance of biocidal additives in paints and coatings. Prog. Org. Coat. 2001, 43, 10–17. [Google Scholar] [CrossRef]
- Kim, J.; Delio, R.; Dordick, J.S. Protease-containing silicates as active antifouling materials. Biotechnol. Prog. 2002, 18, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Iconomopoulou, S.M.; Andreopoulou, A.K.; Soto, A.; Kallitsis, J.K.; Voyiatzis, G.A. Incorporation of low molecular weight biocides into polystyrene-divinyl benzene beads with controlled release characteristics. J. Control. Release 2005, 102, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Faÿ, F.; Linossier, I.; Legendre, G.; Vallée-Réhel, K. Micro-encapsulation and antifouling coatings: Development of poly(lactic acid) microspheres containing bioactive molecules. Macromol. Symp. 2008, 272, 45–51. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Meyer, R.L.; Poulsen, C.H.; Kragh, K.M.; Besenbacher, F.; Laursen, B.S. Biomimetic silica encapsulation of enzymes for replacement of biocides in antifouling coatings. Green Chem. 2010, 12, 387–394. [Google Scholar] [CrossRef]
- Sørensen, G.; Nielsen, A.L.; Pedersen, M.M.; Poulsen, S.; Nissen, H.; Poulsen, M.; Nygaard, S.D. Controlled release of biocide from silica microparticles in wood paint. Prog. Org. Coat. 2010, 68, 299–306. [Google Scholar] [CrossRef]
- Hart, R.L.; Virgallito, D.R.; Work, D.E. Microencapsulation of Biocides and Antifouling Agents. US 7,938,897 B2, 10 May 2011. [Google Scholar]
- Szabó, T.; Molnár-Nagy, L.; Bognár, J.; Nyikos, L.; Telegdi, J. Self-healing microcapsules and slow release microspheres in paints. Prog. Org. Coat. 2011, 72, 52–57. [Google Scholar] [CrossRef]
- Wallström, E.; Jespersen, H.T.; Schaumburg, K. A new concept for anti-fouling paint for Yachts. Prog. Org. Coat. 2011, 72, 109–114. [Google Scholar] [CrossRef]
- Xu, F.L.; Lin, C.G.; Zheng, J.Y.; Zhang, J.W.; Wang, L. Preparation of Thermoresponsive Chitosan Polymers and Its Application Controlling Release of Antifouling Agent. Key Eng. Mater. 2013, 562–565, 664–667. [Google Scholar] [CrossRef]
- Jamsa, S.; Mahlberg, R.; Holopainen, U.; Ropponen, J.; Savolainen, A.; Ritschkodd, A. Slow release of a biocidal agent from polymeric microcapsules for preventing biodeteration. Prog. Polym. Sci. 2013, 76, 269–276. [Google Scholar] [CrossRef]
- Borodina, T.N.; Grigoriev, D.O.; Carillo, M.A.; Hartmann, J.; Moehwald, H.; Shchukin, D.G. Preparation of multifunctional polysaccharide microcontainers for lipophilic bioactive agents. ACS Appl. Mater. Interfaces 2014, 6, 6570–6578. [Google Scholar] [CrossRef] [PubMed]
- Szabo, T.; Mihaly, J.; Sajo, I.; Telegdi, J.; Nyikos, L. One-pot synthesis of gelatin-based, slow-release polymer microparticles containing silver nanoparticles and their application in anti-fouling paint. Prog. Org. Coat. 2014, 77, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Maia, F.; Silva, A.; Fernandes, S.; Cunha, A.; Almeida, A.; Tedim, J.; Zheludkevich, M.; Ferreira, M. Incorporation of biocides in nanocapsules for protective coatings used in maritime applications. Chem. Eng. J. 2015, 270, 150–157. [Google Scholar] [CrossRef]
- Yan, X.; Li, S.; Pan, Y.; Xing, B.; Li, R.; Jang, B.; Liu, X. Tunable Ag+ ion release from Ag@C for antibacterial and antifouling performances. RSC Adv. 2015, 5, 39384–39391. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D. Layered double hydroxides as a nanocontainer for encapsulating marine natural product antifoulant: Intercalation and tunable controlled release of cinnamate. Mater. Res. Bull. 2015, 63, 205–210. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Y.; Zhou, L.; Gao, J. Bienzyme system immobilized in biomimetic silica for application in antifouling coatings. Chin. J. Chem. Eng. 2015, 23, 1384–1388. [Google Scholar] [CrossRef]
- Avelelas, F.; Martins, R.; Oliveira, T.; Maia, F.; Malheiro, E.; Soares, A.; Loureiro, S.; Tedim, J. Efficacy and ecotoxicity of novel anti-fouling nanomaterials in target and non-target marine species. Mar. Biotechnol. 2017, 19, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Suo, X.; Wang, Z.; Gong, Y.; Wang, X.; Li, H. Developing polyimide-copper antifouling coatings with capsule structures for sustainable release of copper. Mater. Des. 2017, 130, 285–293. [Google Scholar] [CrossRef]
- Fu, Y.; Gong, C.; Wang, W.; Zhang, L.; Ivanov, E.; Lvov, Y. Antifouling thermoplastic composites with maleimide encapsulated in clay nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 30083–30091. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Auras, R.A.; Lim, L.-T.; Selke, S.E.M.; Tsuji, H. (Eds.) Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Okamura, H.; Aoyama, I.; Liu, D.; Maguire, J.; Pacepavicius, G.J.; Lau, Y.L. Photodegradation of Irgarol 1051 in water. J. Environ. Sci. Health Part B 1999, 34, 225–238. [Google Scholar] [CrossRef]
- Tsang, V.W.-H.; Lei, N.-Y.; Lam, M.H.-W. Determination of Irgarol-1051 and its related s-triazine species in coastal sediments and mussel tissues by HPLC–ESI-MS/MS. Mar. Pollut. Bull. 2009, 58, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Albanis, T.A. Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: A review. Environ. Int. 2004, 30, 235–248. [Google Scholar] [CrossRef]
- Gatidou, G.; Kotrikla, A.; Thomaidis, N.S.; Lekkas, T.D. Determination of two antifouling booster biocides and their degradation products in marine sediments by high performance liquid chromatography-diode array detection. Anal. Chim. Acta 2004, 505, 153–159. [Google Scholar] [CrossRef]
- Cresswell, T.; Richards, J.P.; Glegg, G.A.; Readman, J.W. The impact of legislation on the usage and environmental concentrations of Irgarol 1051 in UK coastal waters. Mar. Pollut. Bull. 2006, 52, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.; Behrends, B. Consequences of Antifouling Coatings—The Chemist’s Perspective. Biofouling 2010, 226–242. [Google Scholar] [CrossRef]
- Ash, M.; Ash, I. Handbook of Preservatives, 1st ed.; Synapse Info Resources: New York, NY, USA, 2004. [Google Scholar]
- Turley, P.A.; Fenn, R.J.; Ritter, J.C.; Callow, M.E. Pyrithiones as antifoulants: Environmental fate and loss of toxicity. Biofouling 2005, 21, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Vouyiouka, S.; Theodoulou, P.; Symeonidou, A.; Papaspyrides, C.D.; Pfaendner, R. Solid state polymerization of poly(lactic acid): Some fundamental parameters. Polym. Degrad. Stab. 2013, 98, 2473–2481. [Google Scholar] [CrossRef]
- Lassalle, V.; Ferreira, M.L. PLA nano- and microparticles for drug delivery: An overview of the methods of preparation. Macromol. Biosci. 2007, 7, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.; Merkle, H.P.; Gander, B. Microencapsulation by solvent extraction/evaporation: Reviewing the state of the art of microsphere preparation process technology. J. Control. Release 2005, 102, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Rouaud, O.; Poncelet, D. Microencapsulation by solvent evaporation: State of the art for process engineering approaches. Int. J. Pharm. 2008, 363, 26–39. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.B.; McGinity, J.W. Preparation of caprolactone microcapsules by the solvent evaporation technique. Adv. Drug Deliv. Rev. 1997, 28, 25–42. [Google Scholar] [CrossRef]
- Wendorf, J.R.; Singh, M.; O’Hagan, D.T. Nanoparticles and Microparticles as Vaccine Adjuvants. In Nanoparticulates as Drug Carriers; Torchilin, V.P., Ed.; Imperial College Press: London, UK, 2006; pp. 675–696. [Google Scholar]
- Kohane, D.S. Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng. 2007, 96, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Judefeind, A.; de Villiers, M.M. Drug Loading into and In Vitro Release from nanosized drug delivery systems. In Nanotechnology in Drug; Springer: New York, NY, USA, 2009; pp. 129–162. [Google Scholar]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Papadimitriou, S.; Papageorgiou, G.Z.; Kanaze, F.I.; Georgarakis, M.; Bikiaris, D.N. Nanoencapsulation of Nimodipine in Novel Biocompatible Poly(propylene-co-butylene succinate) Aliphatic Copolyesters for Sustained Release. J. Nanomater. 2009. [CrossRef]
- Gupta, P.N. Mucosal Vaccine Delivery and M Cell Targeting. In Targeted Drug Delivery: Concepts and Design; Springer International Publishing: Cham, Switzerland, 2015; pp. 313–337. [Google Scholar]
- Chakravarthi, S.; Robinson, D.; De, S. Nanoparticles Prepared Using Natural and Synthetic Polymers. In Nanoparticulate Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2007; pp. 51–60. [Google Scholar]
- Roth, C.B.; Dutcher, J.R. Glass transition and chain mobility in thin polymer films. J. Electroanal. Chem. 2005, 584, 13–22. [Google Scholar] [CrossRef]
- Abe, H.; Takahashi, N.; Ju Kim, K.; Mochizuki, M.; Doi, Y. Thermal degradation processes of end-capped poly(l-lactide acid) in the presence and absence of residual zinc catalyst. Biomacromolecules 2004, 5, 1606–1614. [Google Scholar] [CrossRef] [PubMed]

| Publish Year | Carrier | AF Agent | Encapsulation Technique | Reference |
|---|---|---|---|---|
| 1992 | Metallic (Cu) microtubules | Renilla extract | Introduction of dry microcylinder powder in saturated solution of the AF agent | [19] |
| 2001 | Silica, zeolites | Isothiazolinones (e.g., OIT) | Adsorption of the biocides on the surface of the siliceous frameworks | [20] |
| 2002 | Silicates | α-chymotryspin | Two-step polymerization process | [21] |
| 2004 | Polystyrene | Zosteric acid | Emulsification–solvent evaporation | [15] |
| 2005 | Polystyrene-divinyl benzene beads | Triclosan, phosphonium salts | Dispersion polymerization | [22] |
| 2007 | Poly(methyl methacrylate-co-butyl acrylate) | 4,5-dichloro-2-octyl-4-isothiazolin-3-one (DCOIT) | Two-stage miniemulsion polymerization | [13] |
| 2008 | Poly(lactic acid) | Chlorhexidine | Emulsification–solvent evaporation | [23] |
| 2010 | Polyethylenimine-Silica | Hexose oxidase | Co-precipitation | [24] |
| 2010 | Poly(methyl methacrylate) | Medetomidine | Emulsification–solvent evaporation | [6] |
| 2010 | Poly(methyl methacrylate) | 4-nitroanisole | Emulsification–solvent evaporation | [14] |
| 2010 | Silica | 3-iodoprop-2-ynyl N-butylcarbamate (IPBC) | Emulsification and cross-linking | [25] |
| 2011 | Various polymer layers | DCOIT | Emulsification and cross-linking of the shell | [26] |
| 2011 | Poly(methyl methacrylate) | IPBC | Emulsification–solvent evaporation | [7] |
| 2011 | Natural polymer (N/A) | Ag compound | Emulsion polymerization | [27] |
| 2011 | Silica gel | Zinc pyrithione | Sol-gel technology—Production of aerogels | [28] |
| 2013 | Chitosan | Paeonol | Emulsification and ionic gelation | [29] |
| 2013 | Polyethyleneimine | Sodium benzoate | Interfacial polyaddition | [30] |
| 2014 | Poly(methyl methacrylate) | OIT | Internal phase separation | [8] |
| 2014 | Polysaccharide complex of chitosan and xanthan gum | DCOIT | Simultaneous emulsification and cross-linking via ultrasonication | [31] |
| 2014 | Polystyrene | IPBC | Emulsification–solvent evaporation | [16] |
| 2014 | Polystyrene–polycaprolactone blends | IPBC | Emulsification–solvent evaporation | [17] |
| 2014 | Gelatin-urea-formaldehyde | Ag nanoparticles | Dispersion polymerization | [32] |
| 2015 | Silica | 2-mercaptobenzothiazole (MBT), DCOIT | Emulsification and silica precursor (TEOS) polycondensation | [33] |
| 2015 | Carbon | Ag ions | Hydrothermal treatment | [34] |
| 2015 | Layered double hydroxides | Cinnamate anions | Acid-salt treatment and ion exchange | [35] |
| 2015 | Silica | Bienzyme system | Biomimetic silicification | [36] |
| 2017 | Silica | Copper and zinc pyrithione | Emulsification and TEOS polycondensation | [37] |
| 2017 | Polyimide | Cu nanoparticles | Solution precursor flame spray | [38] |
| 2017 | Halloysite nanotubes | TCPM | Physical entrapment with pressure cycles | [39] |
| Encapsulated Biocide | Collected Mass (mg) | Yield (%) | |
|---|---|---|---|
| Blank NPs | 15.6 | 30 | |
| Irgarol NPs |  | 41.0 | 56 |
| Econea NPs |  | 59.6 | 83 |
| ZPT NPs |  | 48.3 | 72 |
| Samples | PS (nm) | PdI | ζ-P (mV) | Direct EE (%) |
|---|---|---|---|---|
| Blank NPs | 311.9 ± 7.5 | 0.169 ± 0.015 | −11.10 ± 0.46 | |
| Irgarol NPs | 465.0 ± 11.9 | 0.400 ± 0.039 | −9.43 ± 0.43 | 90 |
| Econea NPs | 529.2 ± 3.5 | 0.300 ± 0.009 | −2.17 ± 0.11 | 96 |
| ZPT NPs | 1013.3 ± 53.5 | 0.592 ± 0.008 | −11.33 ± 0.57 | 92 |
| Tg (°C) | Tendo (°C) | ΔHendo (J·g−1) | Td (°C) | Residue (%) | |
|---|---|---|---|---|---|
| PLA | 58.8 | 146.7 | 20.6 | 365.5 | 2.5 |
| Blank NPs | 60.1 | 118.7, 142.0 | 0.32, 1.6 | 348.2 | 9.7 |
| Irgarol | 130.7 | 107.1 | 286.2 | 8.2 | |
| Irgarol NPs | 59.1 | 120.3 | n.d | 232.8, 339.5 | 8.5 |
| Econea | 253.5 | 98.8 | 314.2 | 55.3 | |
| Econea NPs | 57.7 | 135.6, 210.2 | n.d | 270.6, 338.8 | 10.5 |
| ZPT | 289.5, 335.6 | 60.2 | |||
| ZPT NPs | 59.6 | 143.2 | n.d | 251.2 | 17.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamtsikakis, A.; Kavetsou, E.; Chronaki, K.; Kiosidou, E.; Pavlatou, E.; Karana, A.; Papaspyrides, C.; Detsi, A.; Karantonis, A.; Vouyiouka, S. Encapsulation of Antifouling Organic Biocides in Poly(lactic acid) Nanoparticles. Bioengineering 2017, 4, 81. https://doi.org/10.3390/bioengineering4040081
Kamtsikakis A, Kavetsou E, Chronaki K, Kiosidou E, Pavlatou E, Karana A, Papaspyrides C, Detsi A, Karantonis A, Vouyiouka S. Encapsulation of Antifouling Organic Biocides in Poly(lactic acid) Nanoparticles. Bioengineering. 2017; 4(4):81. https://doi.org/10.3390/bioengineering4040081
Chicago/Turabian StyleKamtsikakis, Aristotelis, Eleni Kavetsou, Konstantina Chronaki, Evangelia Kiosidou, Evangelia Pavlatou, Alexandra Karana, Constantine Papaspyrides, Anastasia Detsi, Antonis Karantonis, and Stamatina Vouyiouka. 2017. "Encapsulation of Antifouling Organic Biocides in Poly(lactic acid) Nanoparticles" Bioengineering 4, no. 4: 81. https://doi.org/10.3390/bioengineering4040081









