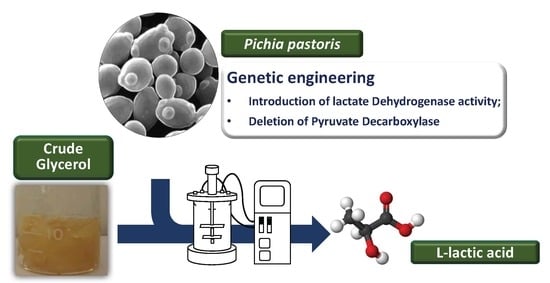
Effect of Pyruvate Decarboxylase Knockout on Product Distribution Using Pichia pastoris (Komagataella phaffii) Engineered for Lactic Acid Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Plasmids
2.2. Identification of Putative Genes Encoding Pyruvate Decarboxylase
2.3. Construction of a PDC Knockout Cassette
2.4. PDC Knockout in P. pastoris Strains
2.5. Ploidy Determination in P. pastoris Strains
2.6. LDH Enzyme Activity
2.7. Fermentation Parameters
2.8. Substrate Consumption and Cellular Products Quantification
3. Results and Discussion
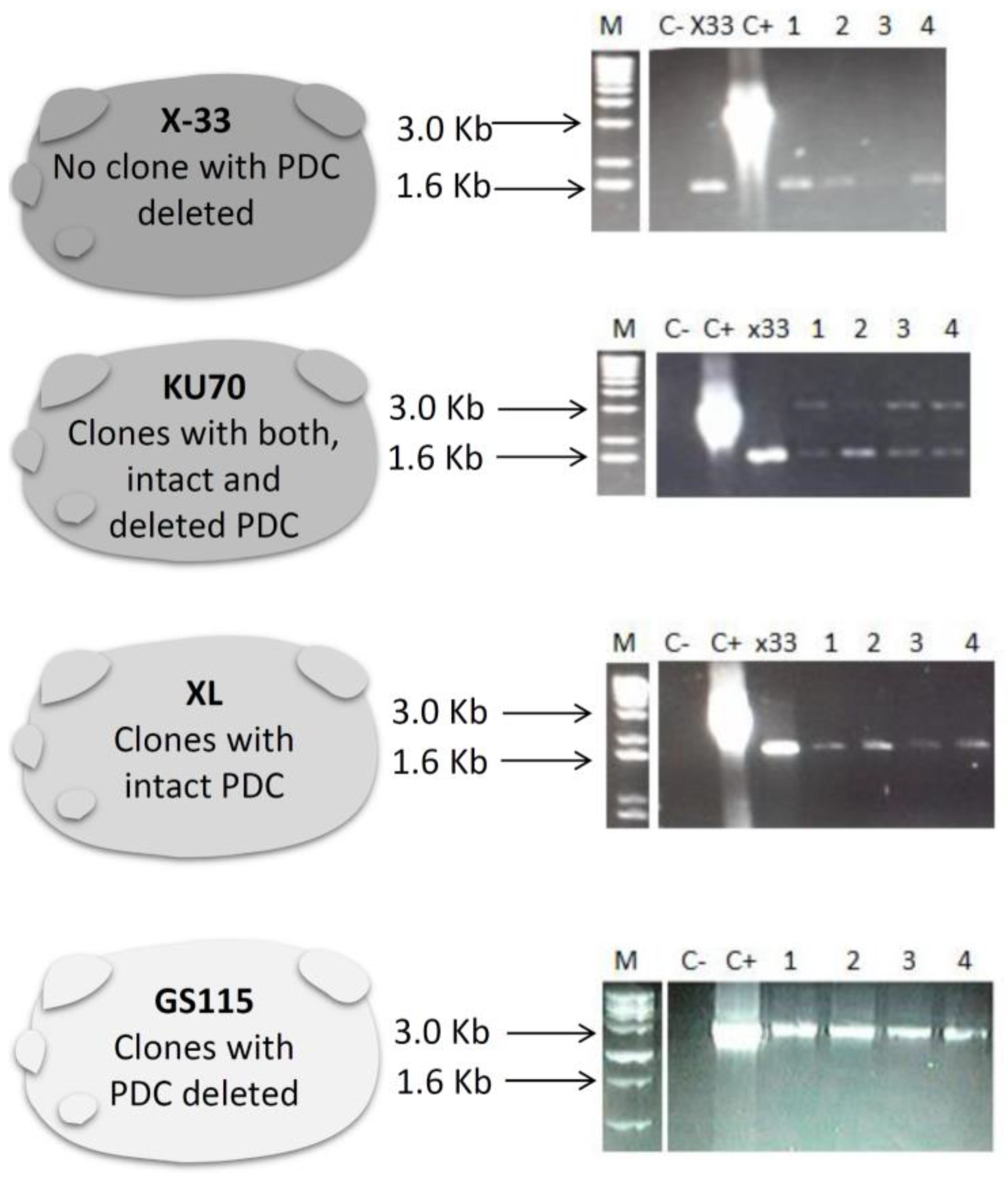
3.1. Construction of pUG6-PDCK and PDC Knockout P. pastoris Strains
3.2. Determination of Ploidy in Different P. pastoris Strains
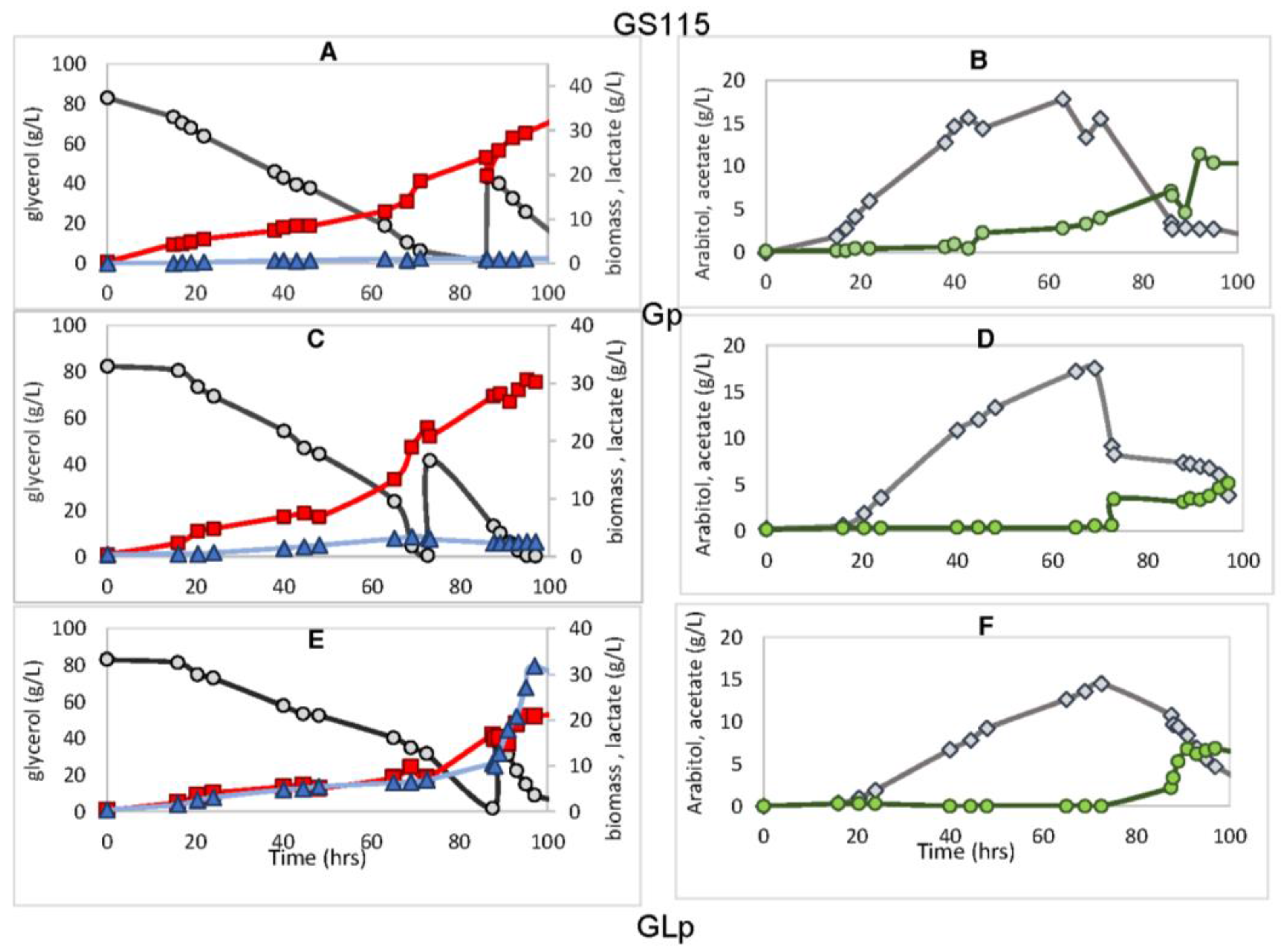
3.3. Product Distribution in P. pastoris Strains during Bioreactor Cultivation
3.4. Effect of PDC Interruption on l-Lactic Acid Production
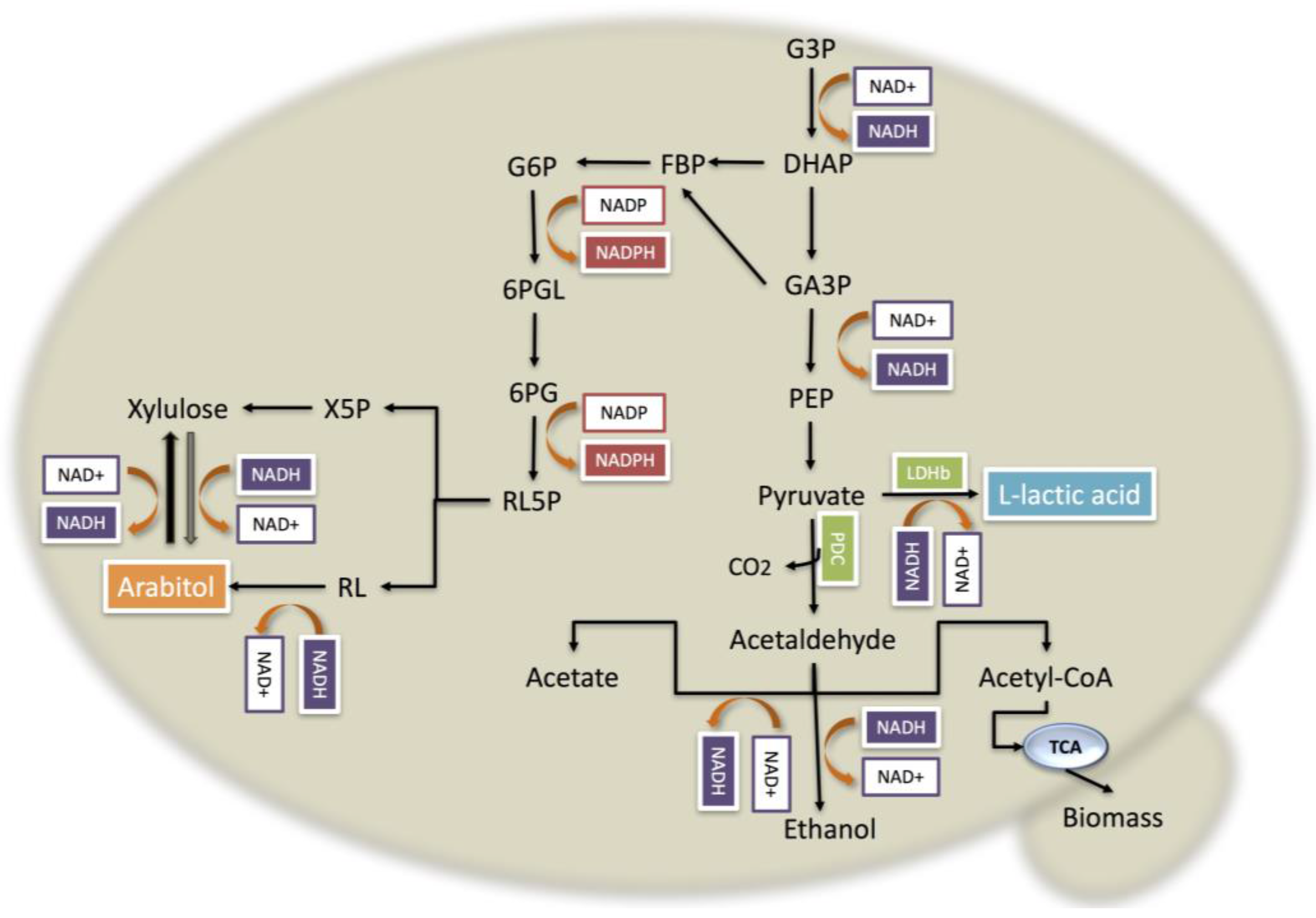
3.5. The Redox Mechanism When PDC Is Interrupted
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tsuji, H. Poly(lactic acid) stereocomplexes: A decade of progress. Adv Drug Deliv Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef] [PubMed]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Okino, S.; Suda, M.; Fujikura, K.; Inui, M.; Yukawa, H. Production of D-lactic acid by Corynebacterium glutamicum under oxygen deprivation. Appl. Microbiol. Biotechnol. 2008, 78, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: the latest development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Tokuhiro, K.; Ishida, N.; Nagamori, E.; Saitoh, S.; Onishi, T.; Kondo, A.; Takahashi, H. Double mutation of the PDC1 and ADH1 genes improves lactate production in the yeast Saccharomyces cerevisiae expressing the bovine lactate dehydrogenase gene. Appl. Microbiol. Biotechnol. 2009, 82, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Saitoh, S.; Onishi, T.; Tokuhiro, K.; Nagamori, E.; Kitamoto, K.; Takahashi, H. The Effect of Pyruvate Decarboxylase Gene Knockout in Saccharomyces cerevisiae on l-Lactic Acid Production. Biosci. Biotechnol. Biochem. 2006, 70, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Saitoh, S.; Tokuhiro, K.; Nagamori, E.; Matsuyama, T.; Kitamoto, K.; Takahashi, H. Efficient production of l-lactic acid by metabolically engineered Saccharomyces cerevisiae with a genome-integrated l-lactate dehydrogenase gene. Appl. Environ. Microbiol. 2005, 71, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.M.; Brambilla, L.; Protani, F.; Liu, C.L.; Lievense, J.; Porro, D. Efficient Homolactic Fermentation by Kluyveromyces lactis Strains Defective in Pyruvate Utilization and Transformed with the Heterologous LDH Gene. Appl. Environ. Microbiol. 2001, 67, 5621–5625. [Google Scholar] [CrossRef] [PubMed]
- Porro, D.; Bianchi, M.M.; Brambilla, L.; Menghini, R.; Bolzani, D.; Carrera, V.; Lievense, J.; Liu, C.L.; Ranzi, B.M.; Frontali, L.; Alberghina, L. Replacement of a metabolic pathway for large-scale production of lactic acid from engineered yeasts. Appl. Environ. Microbiol. 1999, 65, 4211–4215. [Google Scholar] [PubMed]
- Branduardi, P.; Valli, M.; Brambilla, L.; Sauer, M.; Alberghina, L.; Porro, D. The yeast Zygosaccharomyces bailii: A new host for heterologous protein production, secretion and for metabolic engineering applications. FEMS Yeast Res. 2004, 4, 493–504. [Google Scholar] [CrossRef]
- Ilmén, M.; Koivuranta, K.; Ruohonen, L.; Rajgarhia, V.; Suominen, P.; Penttilä, M. Production of l-lactic acid by the yeast Candida sonorensis expressing heterologous bacterial and fungal lactate dehydrogenases. Microb. Cell Factor. 2013, 12, 1–15. [Google Scholar] [CrossRef]
- Osawa, F.; Fujii, T.; Nishida, T.; Tada, N.; Ohnishi, T.; Kobayashi, O.; Komeda, T.; Yoshida, S. Efficient production of l-lactic acid by Crabtree-negative yeast Candida boidinii. Yeast 2009, 26, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Ilmén, M.; Koivuranta, K.; Ruohonen, L.; Suominen, P.; Penttilä, M. Efficient production of l-lactic acid from xylose by Pichia stipitis. Appl. Environ. Microbiol. 2007, 73, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P. Biotechnological strains of Komagataella (Pichia) pastoris are Komagataella pha Y i as determined from multigene. J. Ind. Microbiol. Biotechnol. 2009, 36, 1435–1438. [Google Scholar] [CrossRef]
- Kurtzman, C.P. Description of Komagataella phaffii sp . nov . and the transfer of Pichia pseudopastoris to the methylotrophic yeast genus Komagataella. Int. J. Syst. Evolut. Microbiol. 2005, 55, 973–976. [Google Scholar] [CrossRef]
- Looser, V.; Brühlmann, B.; Bumbak, F.; Stenger, C.; Costa, M.; Camattari, A.; Fotiadis, D.; Kovar, K. Cultivation strategies to enhance productivity of Pichia pastoris: A review. Biotechnol. Adv. 2015, 33, 1177–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastácio, G.S.; Santos, K.O.; Suarez, P.A.Z.; Torres, F.A.G.; De Marco, J.L.; Parachin, N.S. Utilization of glycerin byproduct derived from soybean oil biodiesel as a carbon source for heterologous protein production in Pichia pastoris. Bioresour. Technol. 2014, 152, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Cregg, J.M.; Barringer, K.J.; Hessler, A.Y.; Madden, K.R. Pichia pastoris as a host system for transformations. Mol. Cell. Biol. 1985, 5, 3376–3385. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, K.; Lin, Y.; Tiels, P.; Van Hecke, A.; Glinka, S.; Weber-Lehmann, J.; Rouzé, P.; Van de Peer, Y.; Callewaert, N. Genome sequence of the recombinant protein production host Pichia pastoris. Nat. Biotechnol. 2009, 27, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Näätsaari, L.; Mistlberger, B.; Ruth, C.; Hajek, T.; Hartner, F.S.; Glieder, A. Deletion of the Pichia pastoris ku70 homologue facilitates platform strain generation for gene expression and synthetic biology. PLoS ONE 2012, 7, e.39720. [Google Scholar] [CrossRef] [PubMed]
- De Lima, P.B.A.; Mulder, K.C.L.; Melo, N.T.M.; Carvalho, L.S.; Menino, G.S.; Mulinari, E.; de Castro, V.H.; dos Reis, T.F.; Goldman, G.H.; Magalhães, B.S.; Parachin, N.S. Novel homologous lactate transporter improves l-lactic acid production from glycerol in recombinant strains of Pichia pastoris. Microb. Cell Fact. 2016, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Uppada, V.; Noronha, S.B. Comparison of pyruvate decarboxylases from Saccharomyces cerevisiae and Komagataella pastoris (Pichia pastoris). Appl. Microbiol. Biotechnol. 2013, 97, 9439–9449. [Google Scholar] [CrossRef] [PubMed]
- Tarmy, E.M.; Kaplan, N.O. Chemical characterization of d-lactate dehydrogenase from Escherichia coli B. J. Biol. Chem. 1968, 243, 2579–2586. [Google Scholar] [PubMed]
- Maurer, M.; Kühleitner, M.; Gasser, B.; Mattanovich, D. Versatile modeling and optimization of fed batch processes for the production of secreted heterologous proteins with Pichia pastoris. Microb. Cell Fact. 2006, 5, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyon, G.; Gaudreau, G.; St-Gelais, D.; Beaulieu, Y.; Randall, C.J. Simultaneous HPLC Determination of Organic Acids, Sugars and Alcohols. Can. Inst. Food Sci. Technol. J. 1991, 24, 87–94. [Google Scholar] [CrossRef]
- Bianchi, M.M.; Tizzani, L.; Destruelle, M.; Frontali, L.; Wésolowski-Louvel, M. The “petite-negative” yeast Kluyveromyces lactis has a single gene expressing pyruvate decarboxylase activity. Mol. Microbiol. 1996, 19, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, S. Characterization of PDC6, a third structural gene for pyruvate decarboxylase in Saccharomyces cerevisiae. J. Bacteriol. 1991, 173, 7963–7969. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Anumanthan, A.; Gao, X.G.; Ilangovan, K.; Suzara, V.V.; Duzgunes, N.; Renugopalakrishnan, V. Expression of recombinant proteins in Pichia pastoris. Appl. Biochem. Biotechnol. 2007, 142, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Hyka, P.; Züllig, T.; Ruth, C.; Looser, V.; Meier, C.; Klein, J.; Melzoch, K.; Glieder, A.; Kovar, K.; Zu, T.; Meyer, H. Combined Use of Fluorescent Dyes and Flow Cytometry To Quantify the Physiological State of Pichia pastoris during the Production of Heterologous Proteins in High-Cell-Density Fed-Batch Cultures. Appl. Environ. Microbiol. 2010, 76, 4486–4496. [Google Scholar] [CrossRef] [PubMed]
- Küberl, A.; Schneider, J.; Thallinger, G.G.; Anderl, I.; Wibberg, D.; Hajek, T.; Jaenicke, S.; Brinkrolf, K.; Goesmann, A.; Szczepanowski, R.; et al. High-quality genome sequence of Pichia pastoris CBS7435. J. Biotechnol. 2011, 154, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Love, K.R.; Shah, K.A.; Whittaker, C.A.; Wu, J.; Bartlett, M.C.; Ma, D.; Leeson, R.L.; Priest, M.; Borowsky, J.; Young, S.K.; Love, J.C. Comparative genomics and transcriptomics of Pichia pastoris. BMC Genomics 2016, 17, 550. [Google Scholar] [CrossRef] [PubMed]
- Porro, D.; Brambilla, L.; Ranzi, B.M.; Martegani, E.; Alberghina, L. Development of metabolically engineered Saccharomyces cerevisiae cells for the production of lactic acid. Biotechnol. Prog. 1995, 11, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.; Porro, D.; Mattanovich, D. 16 years research on lactic acid production with yeast—Ready for the market? Biotechnol. Genet. Eng. Rev. 2010, 27, 229–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kang, C.D.; Lee, S.H.; Park, Y.K.; Cho, K.M. Engineering cellular redox balance in Saccharomyces cerevisiae for improved production of l-lactic acid. Biotechnol. Bioeng. 2015, 112, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Porro, D.; Brambilla, L.; Ranzi, B.M.; Martegani, E.; Generali, B.; Comparata, S.B.; Milano, U. Development of Metabolically Engineered. Society 1995, 294–298. [Google Scholar]
- Adachi, E.; Torigoe, M.; Sugiyama, M.; Nikawa, J.I.; Shimizu, K. Modification of metabolic pathways of Saccharomyces cerevisiae by the expression of lactate dehydrogenase and deletion of pyruvate decarboxylase genes for the lactic acid fermentation at low pH value. J. Ferment. Bioeng. 1998, 86, 284–289. [Google Scholar] [CrossRef]
- Turner, T.L.; Zhang, G.C.; Kim, S.R.; Subramaniam, V.; Steffen, D.; Skory, C.D.; Jang, J.Y.; Yu, B.J.; Jin, Y.S. Lactic acid production from xylose by engineered Saccharomyces cerevisiae without PDC or ADH deletion. Appl. Microbiol. Biotechnol. 2015, 99, 8023–8033. [Google Scholar] [CrossRef] [PubMed]
- Kordowska-Wiater, M. Production of arabitol by yeasts: current status and future prospects. J. Appl. Microbiol. 2015, 119, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Koganti, S.; Kuo, T.M.; Kurtzman, C.P.; Smith, N.; Ju, L.K. Production of arabitol from glycerol: Strain screening and study of factors affecting production yield. Appl. Microbiol. Biotechnol. 2011, 90, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lv, J.; Wang, H.; Wang, B.; Li, Z.; Deng, Z. Genetically engineered Pichia pastoris yeast for conversion of glucose to xylitol by a single-fermentation process. Appl. Microbiol. Biotechnol. 2014, 98, 3539–3552. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.; Carnicer, M.; Dragosits, M.; Graf, A.B.; Stadlmann, J.; Jouhten, P.; Maaheimo, H.; Gasser, B.; Albiol, J.; Mattanovich, D.; Ferrer, P. A multi-level study of recombinant Pichia pastoris in different oxygen conditions. BMC Syst. Biol. 2010, 4, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
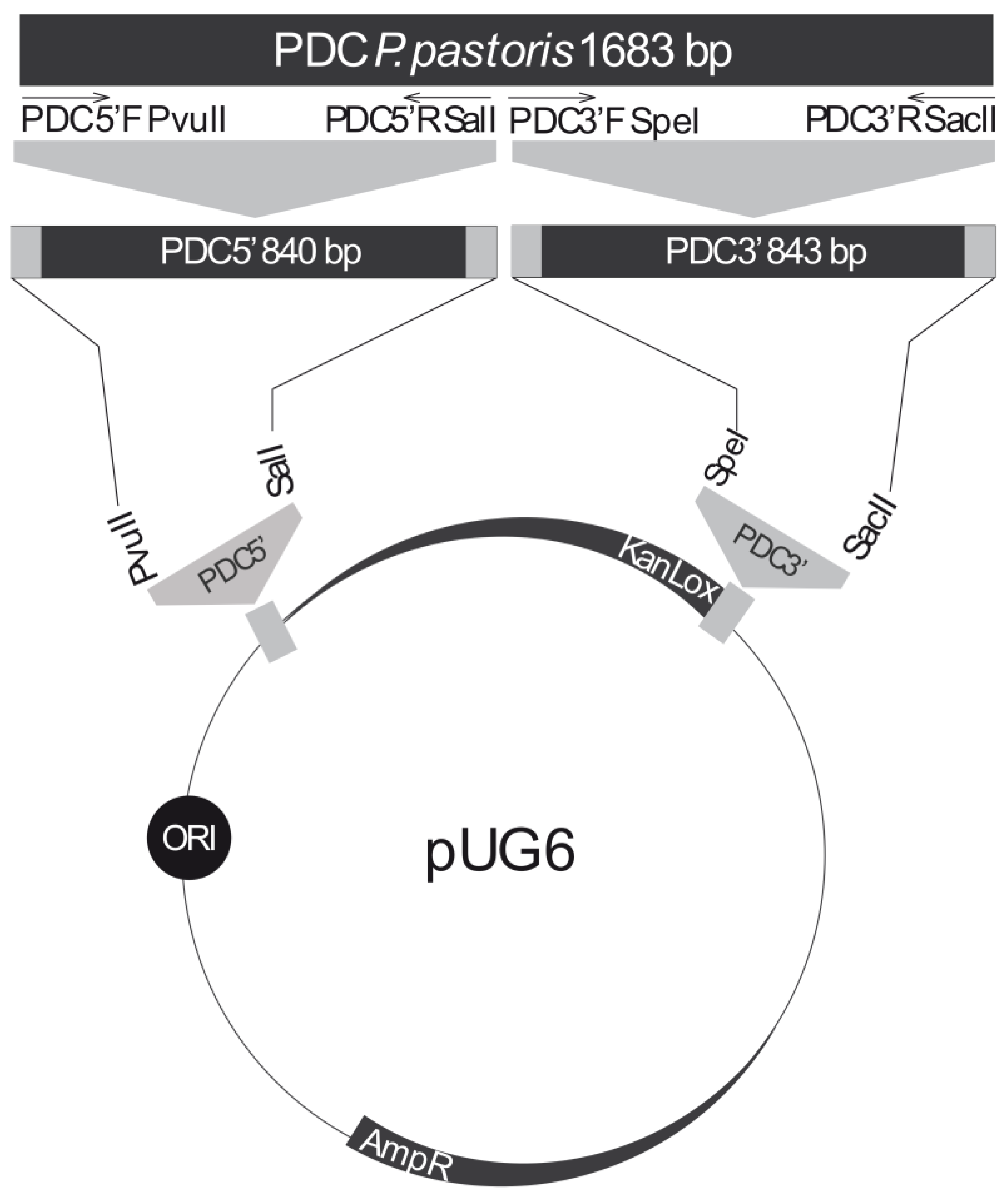
| Plasmids | Relevant Genotype | Ref. |
|---|---|---|
| pUG6 | loxP-PTEF-KanMX-TTEF-loxP | Life Technologies |
| pGAP-LDH | LDH+. Bos taurus gene encoding the LDH enzyme | [21] |
| pUG6-PDC | loxP-PTEF-KanMX-TTEF-loxP+PDC- | This work |
| Strains | Relevant Genotype | Ref. |
| DH5α™ | F-Φ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rK–, mK+) phoA supE44 λ-thi1 gyrA96 relA1 | Life Technologies |
| X-33 | Wildtype | Invitrogen |
| CBS7435 ku70 | Δku70 | [20] |
| GS115 | Δhis4 | [18] |
| XL | X-33 + pGAP-LDHBos taurus | [21] |
| GLp | GS115: Δpdc + pGAP-LDHBos taurus | This work |
| Gp | GS115:Δpdc | This work |
| Primer | Sequence 5’-3’ | Endonuclease |
|---|---|---|
| PDC5’F | CAGCTGATGGCTGAAATAACACTAGGAACT | PvuII |
| PDC5’R | GTCGACATCAGCCTTCTCCACGAACT | SalI |
| PDC3’F | ACTAGTCTTGTCATCTCTGTTGGTGC | SpeI |
| PDC3’R | CCGCGGTTAAGCTGCGTTGGTCTTGG | SacII |
| KanF | AGCTTGCCTCGTCCCC | |
| KanR | TCGACACTGGATGGCG |
| Strain | Yx/s | Ylac/s | Ylac/x | Yac/s | Yara/s | µ | qlac | qac | qara | |
|---|---|---|---|---|---|---|---|---|---|---|
| GS115 | 0.197 ± 0.025 | 0.015 ± 0.002 | 0.075 ± 0.002 | 0.062 ± 0.001 | 0.170 ± 0.064 | 0.023 ± 0.001 | 0.002 ± 0.001 | 0.007 ± 0.001 | 0.021 ± 0.011 | Aerobic |
| Gp | 0.204 ± 0.014 | 0.045 ± 0.001 | 0.219 ± 0.012 | 0.003 ± 0.001 | 0.247 ± 0.028 | 0.028 ± 0.005 | 0.006 ± 0.001 | 0.000 ± 0.000 | 0.033 ± 0.009 | |
| GLp | 0.138 ± 0.018 | 0.110 ± 0.003 | 0.807 ± 0.126 | 0.008 ± 0.001 | 0.308 ± 0.003 | 0.021 ± 0.003 | 0.017 ± 0.000 | 0.001 ± 0.000 | 0.047 ± 0.000 | |
| GS115 | 0.319 ± 0.021 | 0.007 ± 0.001 | 0.022 ± 0.002 | 0.102 ± 0.000 | 0.025 ± 0.016 | 0.015 ± 0.001 | 0.000 ± 0.000 | 0.005 ± 0.001 | 0.002 ± 0.001 | Oxygen Limited |
| Gp | 0.217 ± 0.014 | 0.013 ± 0.002 | 0.058 ± 0.014 | 0.026 ± 0.011 | 0.067 ± 0.023 | 0.017 ± 0.001 | 0.001 ± 0.000 | 0.002 ± 0.001 | 0.006 ± 0.002 | |
| GLp | 0.194 ± 0.012 | 0.646 ± 0.054 | 3.34 ± 0.072 | 0.081 ± 0.015 | 0.174 ± 0.038 | 0.015 ± 0.003 | 0.050 ± 0.009 | 0.006 ± 0.002 | 0.014 ± 0.007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, N.T.M.; Mulder, K.C.L.; Nicola, A.M.; Carvalho, L.S.; Menino, G.S.; Mulinari, E.; Parachin, N.S. Effect of Pyruvate Decarboxylase Knockout on Product Distribution Using Pichia pastoris (Komagataella phaffii) Engineered for Lactic Acid Production. Bioengineering 2018, 5, 17. https://doi.org/10.3390/bioengineering5010017
Melo NTM, Mulder KCL, Nicola AM, Carvalho LS, Menino GS, Mulinari E, Parachin NS. Effect of Pyruvate Decarboxylase Knockout on Product Distribution Using Pichia pastoris (Komagataella phaffii) Engineered for Lactic Acid Production. Bioengineering. 2018; 5(1):17. https://doi.org/10.3390/bioengineering5010017
Chicago/Turabian StyleMelo, Nadiele T. M., Kelly C. L. Mulder, André Moraes Nicola, Lucas S. Carvalho, Gisele S. Menino, Eduardo Mulinari, and Nádia S. Parachin. 2018. "Effect of Pyruvate Decarboxylase Knockout on Product Distribution Using Pichia pastoris (Komagataella phaffii) Engineered for Lactic Acid Production" Bioengineering 5, no. 1: 17. https://doi.org/10.3390/bioengineering5010017