Effects of Sterilization Cycles on PEEK for Medical Device Application
Abstract
:1. Introduction
2. Materials and Methods
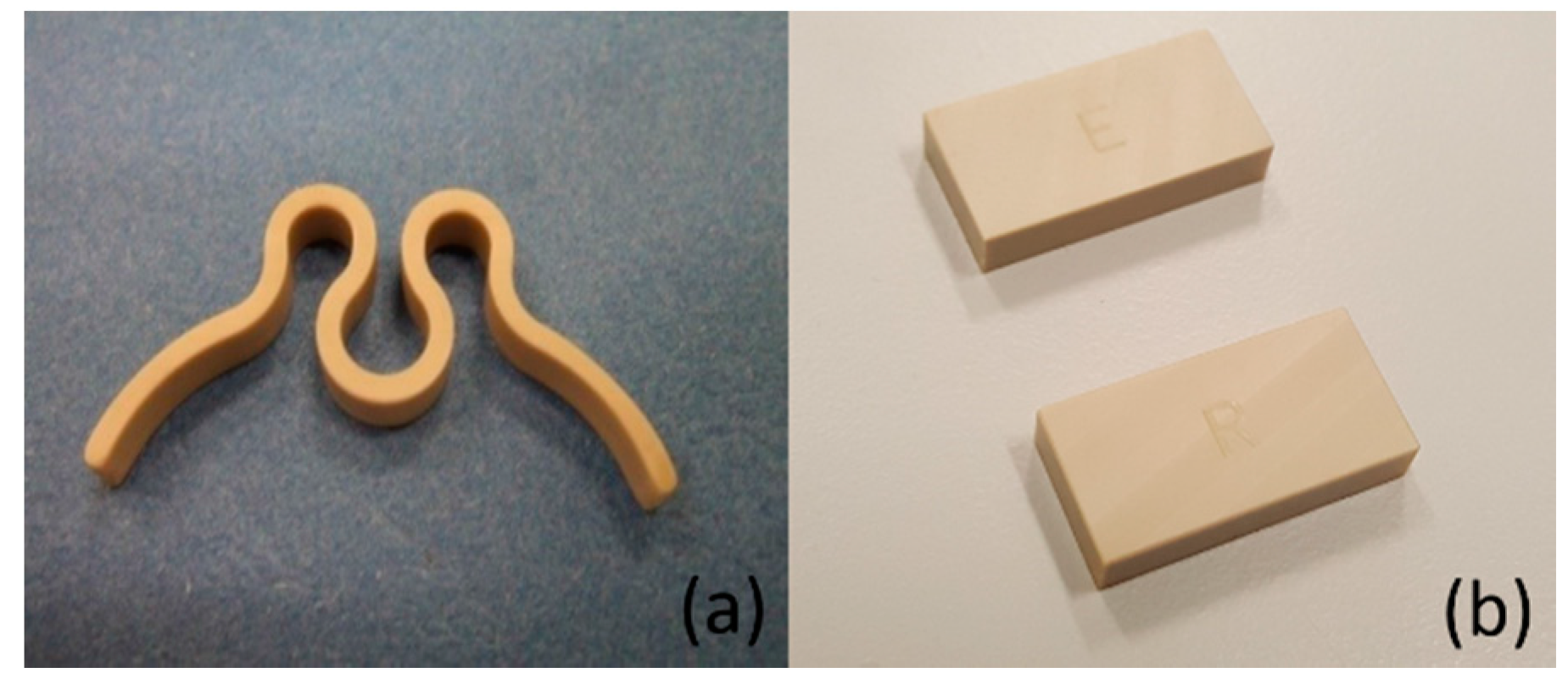
2.1. Specimen Preparation
2.2. Autoclave Sterilization
2.3. Characterization
2.3.1. Clip Characterization
2.3.2. Test Samples Characterization
3. Results
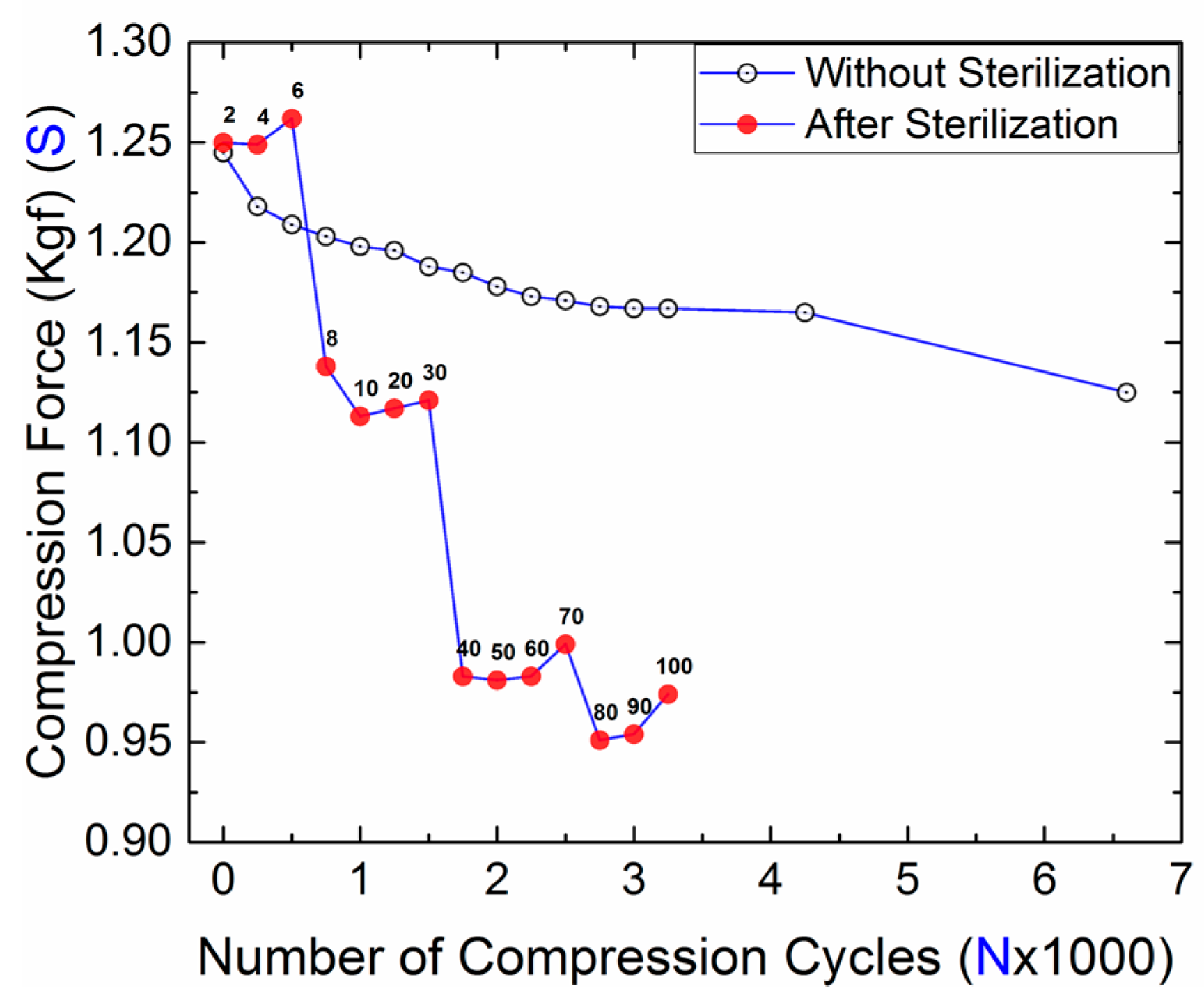
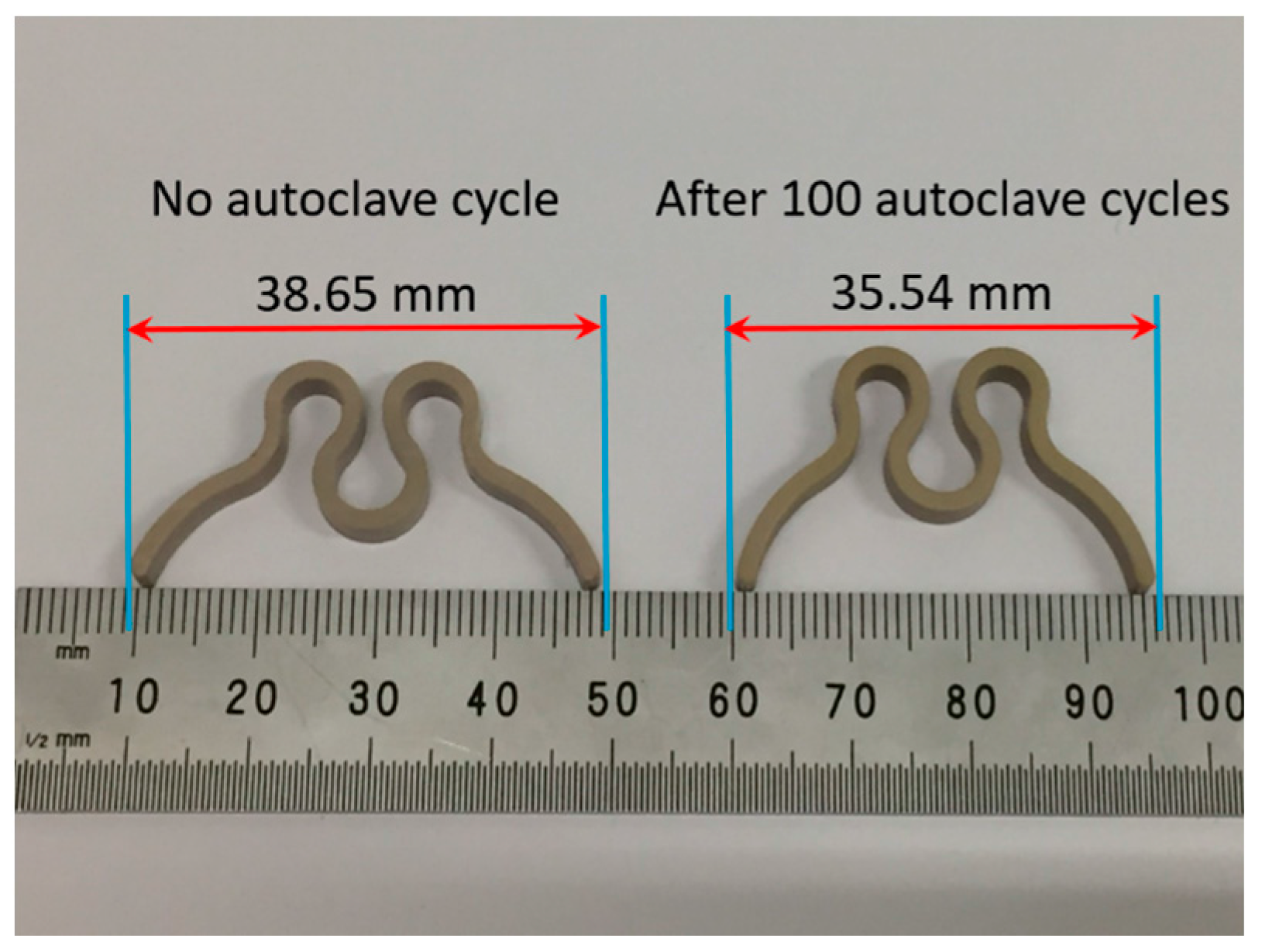
3.1. Compression Force and Dimension
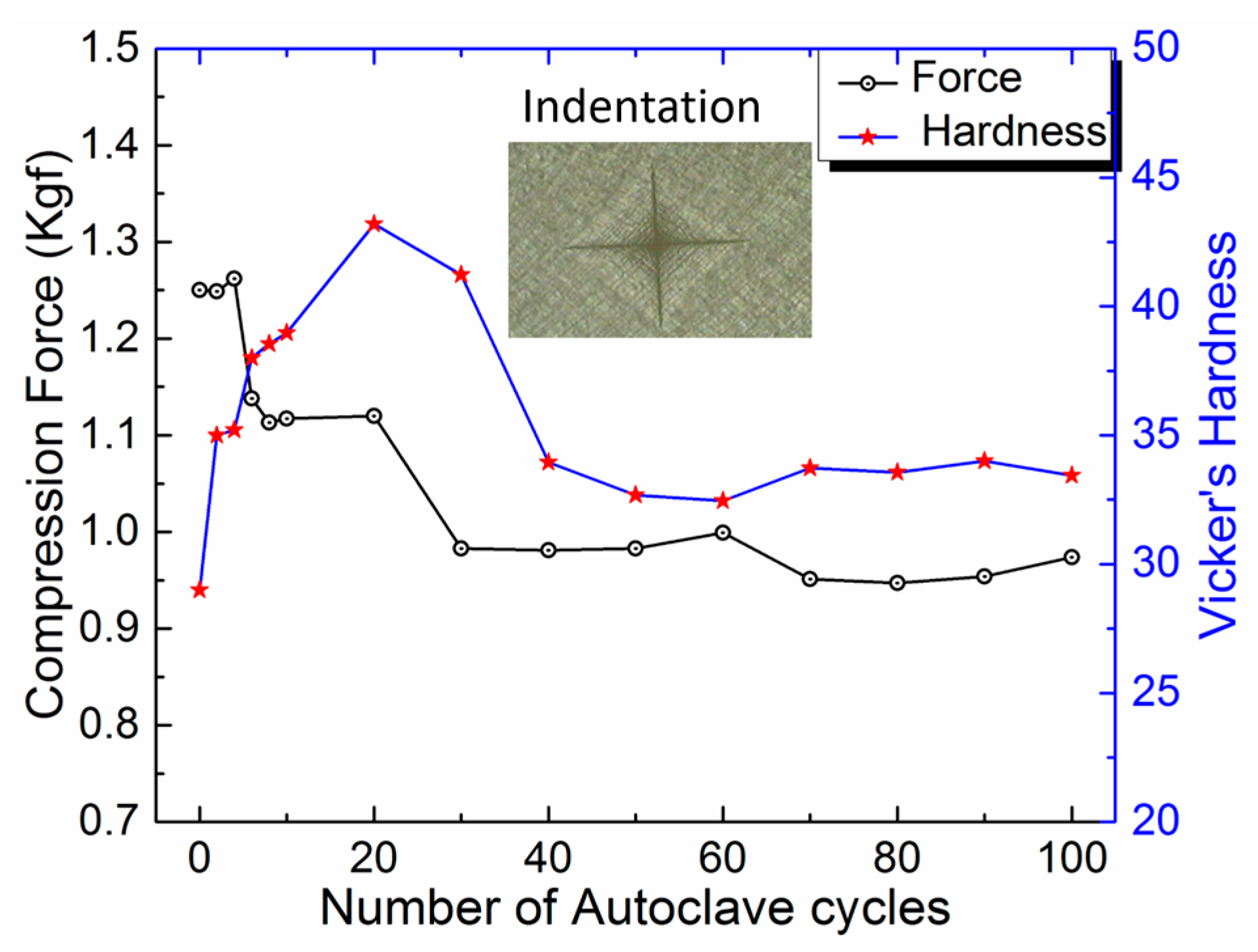
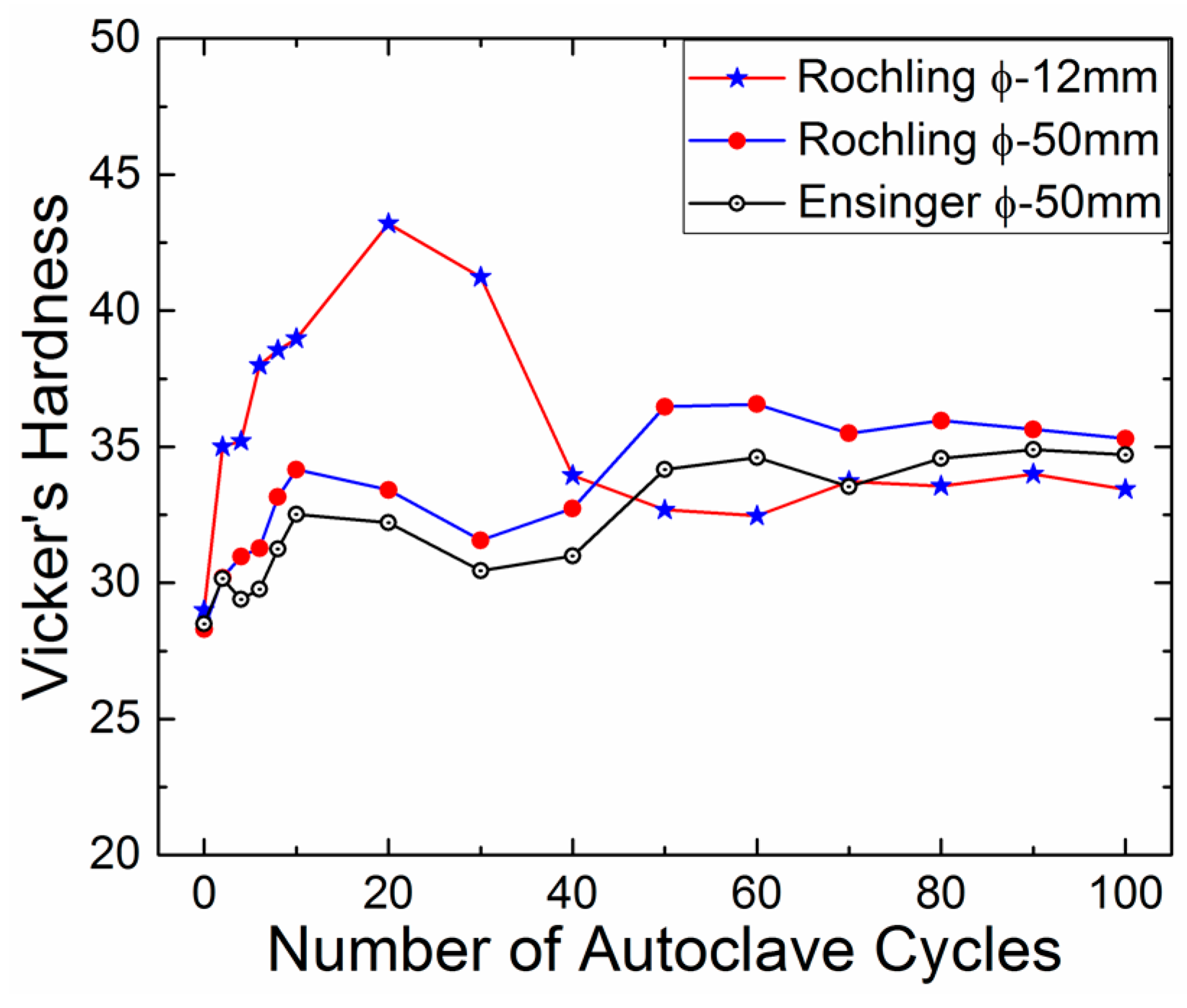
3.2. Hardness
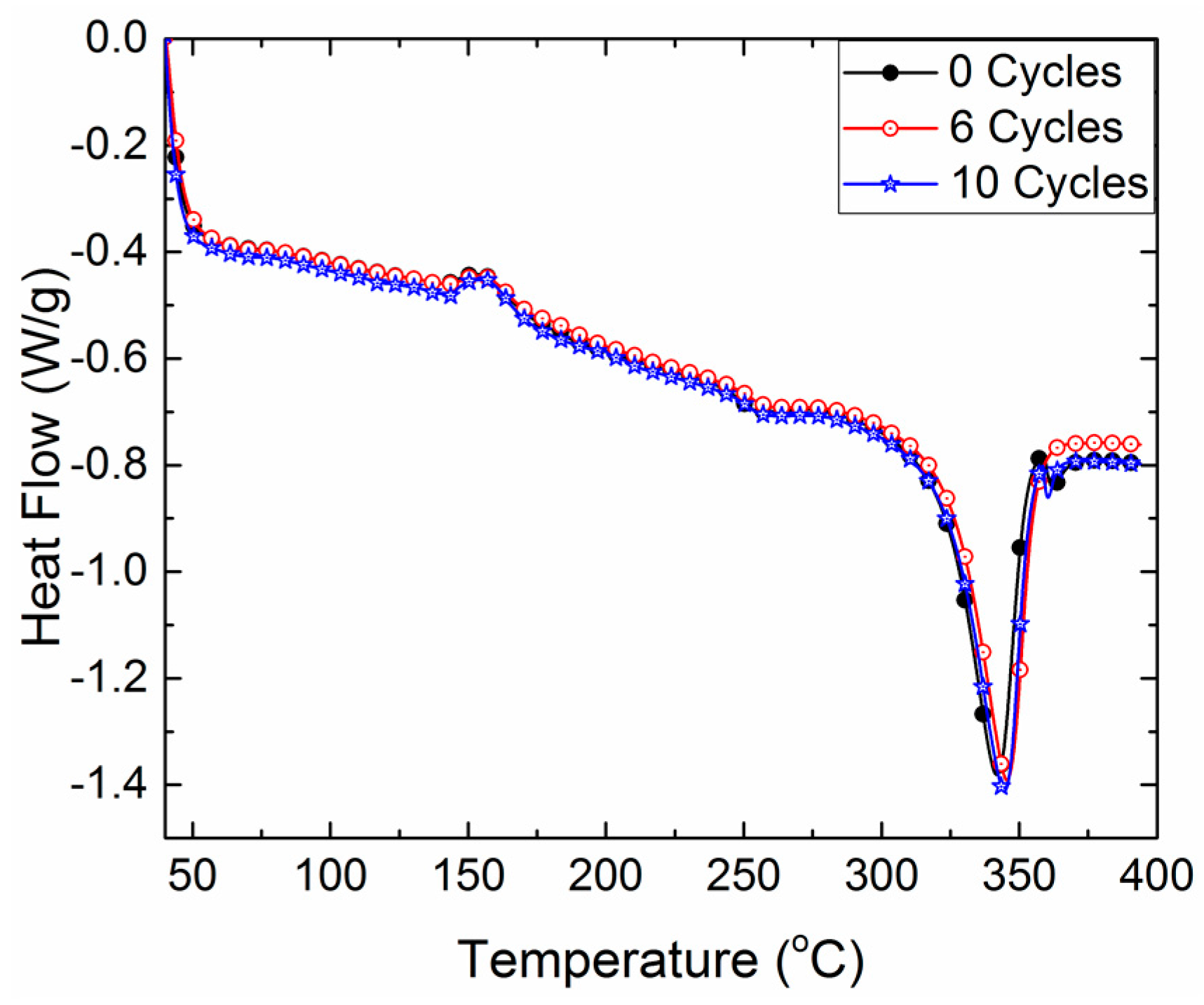
3.3. Differential Scanning Calorimetry
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liao, K. Performance characterization and modeling of a composite hip prosthesis. Exp. Tech. 1994, 18, 33–41. [Google Scholar] [CrossRef]
- Maharaj, G.R.; Jamison, R.D. Intraoperative impact: Characterization and laboratory simulation on composite hip prostheses. In Composite Materials for Implant Applications in the Human Body: Characterization and Testing; Jamison, R.D., Gilbertson, L.N., Eds.; ASTM: Philadelphia, PA, USA, 1993; pp. 98–108. [Google Scholar]
- Kelsey, D.J.; Springer, G.S.; Goodman, S.B. Composite implant for bone replacement. J. Compos. Mater. 1997, 31, 1593–1632. [Google Scholar] [CrossRef]
- Corvelli, A.A.; Biermann, P.J.; Roberts, C. Design, analysis, and fabrication of a composite segmental bone replacement implant. J. Adv. Mater. 1997, 28, 2–8. [Google Scholar]
- Williams, D. New horizons for thermoplastic polymers. Med. Device Technol. 2001, 12, 8–9. [Google Scholar] [PubMed]
- Wang, A.; Lin, R.; Stark, C.; Dumbleton, J.H. Suitability and limitations of carbon fiber reinforced PEEK composites as bearing surfaces for total joint replacements. Wear 1999, 225, 724–727. [Google Scholar] [CrossRef]
- Jones, E.; Wang, A.; Streicher, R. Validating the limits for a PEEK composite as an acetabular wear surface. In Proceedings of the 27th annual meeting of Society of Biomaterials, St Paul, MN, USA, 24–29 April 2001; p. 27. [Google Scholar]
- Joyce, T.J.; Rieker, C.; Unsworth, A. Comparative in vitro wear testing of PEEK and UHMWPE capped metacarpophalangeal prostheses. Biomed. Mater. Eng. 2006, 16, 1–10. [Google Scholar] [PubMed]
- Manley, M.; Ong, K.; Kurtz, S.M.; Rushton, N.; Field, R.E. Biomechanics of a PEEK horseshoe-shaped cup: Comparisons with a predicate deformable cup. In Proceedings of the Transactions of the 53rd Orthopedic Research Society, San Diego, CA, USA, 11–14 February 2007; Volume 32, p. 1717. [Google Scholar]
- Arias, A.; Rodríguez-Martínez, J.A.; Rusinek, A. Numerical simulations of impact behaviour of thin steel plates subjected to cylindrical, conical and hemispherical non-deformable projectiles. Eng. Fract. Mech. 2007, 75, 1635–1656. [Google Scholar] [CrossRef] [Green Version]
- Sobieraj, M.; Rimnac, C. Fracture, fatigue and noch behavior of PEEK. In PEEK Biomaterials Handbook; Kurtz, S., Ed.; William Andrew Books: Norwich, NY, USA, 2012; pp. 61–73. [Google Scholar]
- El-Qoubaa, Z.; Othman, R. Characterization and modeling of the strain rate sensitivity of polyetheretherketone’s compressive yield stress. Mater. Des. 2015, 66, 336–345. [Google Scholar] [CrossRef]
- Kurtz, S.; Devine, J. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Rae, P.; Brown, E.; Orler, E. The mechanical properties of poly(ether-ether-ketone) (PEEK) with emphasis on the large compressive strain response. Polymer 2007, 48, 598–615. [Google Scholar] [CrossRef]
- Godara, A.; Raabe, D.; Green, S. The influence of sterilization processes on the micromechanical properties of carbon fiber-reinforced PEEK composites for bone implant applications. Acta Biomater. 2007, 3, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Tai, N.H.; Yip, M.C.; Tseng, C.M. Influences of thermal cycling and low-energy impact on the fatigue behavior of carbon/PEEK laminates. Compos. Part B Eng. 1999, 30, 849–865. [Google Scholar] [CrossRef]
- Xin, H.; Shephered, D.E.T.; Dearn, K.D. Strength of poly-ether-ether-ketone: Effects of sterilisation and thermal ageing. Polym. Test. 2013, 32, 1001–1005. [Google Scholar] [CrossRef]
- Ensinger Engineering plastics: Manual. Available online: https://www.ensingerplastics.com/downloads (accessed on 14 December 2017).
- Ensinger TECAPEEK. Available online: https://www.ensingerplastics.com/en/shapes/biocompatible-medical-grade/peek (accessed on 14 December 2017).
- Roechling SUSTAPEEK. Available online: https://www.roechling.com/sg/industrial/materials/thermoplastics/detail/sustapeek-mg-natural-203/ (accessed on 14 December 2017).
- Leong, F.S.; Wei, N.K.; Quan, G.J.; Hua, T.P.; Kheng, L.T.; Hui, W.Q.; Edmund, C.; Kesavan, E. System and apparatus for guiding an instrument. U.S. Patent US20160206383 A1, 21 July 2016. [Google Scholar]
- Ray, B.C. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J. Colloid Interface Sci. 2006, 298, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kaelble, D.H.; Dynes, P.J.; Maus, L. Hydrothermal aging of compositematerials. 1. Aspects. J. Adhes. 1976, 8, 121–144. [Google Scholar] [CrossRef]
- Marom, G.; Broutman, L.J. Moisture in epoxy resin composites. J. Adhes. 1981, 2, 153–164. [Google Scholar] [CrossRef]
- Mijovic, M.; Lin, K.F. The effects of hygrothermal fatigue on physical mechanical properties and morphology on neat epoxy-resin and graphite composite. J. Appl. Poly. Sci. 1985, 30, 2527–2549. [Google Scholar] [CrossRef]
- Barraza, H.J.; Aktas, L.; Hamidi, Y.K.; Long, J., Jr.; O’Rear, E.A.; Altan, M.C. Moisture absorption and wet-adhesion properties of resin transfer molded (RTM) composites containing elastomer-coated glass fibers. J. Adhes. Sci. Technol. 2003, 17, 217–242. [Google Scholar] [CrossRef]
- Zheng, Q.; Morgan, R.J. Synergistic thermal—Moisture damage mechanisms of epoxy and their carbon-fiber composites. J. Compos. Mater. 1993, 27, 1465–1478. [Google Scholar] [CrossRef]

| Autoclave Cycles | Compression Force (Kgf) | Dimension Change (mm) | Hardness Roechling (ϕ-12 mm) |
|---|---|---|---|
| Value (% Change) | Value (% Change) | Value (% Change) | |
| 0 | 1.250 | 38.65 | 29 ± 0.8 |
| 2 | 1.249 (↓0.08) | 38.63 (↓0.05) | 35 ± 1.6 (↑20.69) |
| 4 | 1.262 (↓0.96) | 36.79 (↓4.81) | 35 ± 1.4 (↑20.69) |
| 6 | 1.138 (↓8.96) | 36.61 (↓5.28) | 38 ± 2.0 (↑31.03) |
| 8 | 1.113 (↓10.96) | 36.43 (↓5.74) | 39 ± 1.8 (↑34.48) |
| 10 | 1.117 (↓10.64) | 36.44 (↓5.72) | 39 ± 3.7 (↑34.48) |
| 20 | 1.120 (↓10.4) | 36.37 (↓5.90) | 43 ± 1.6 (↑48.28) |
| 30 | 0.983 (↓21.36) | 36.19 (↓6.36) | 41 ± 2.3 (↑41.38) |
| 40 | 0.981 (↓21.52) | 36.03 (↓6.78) | 34 ± 1.7 (↑17.24) |
| 50 | 0.983 (↓21.36) | 35.71 (↓7.61) | 33 ± 1.6 (↑13.79) |
| 60 | 0.999 (↓20.08) | 35.75 (↓7.50) | 32 ± 2.0 (↑10.34) |
| 70 | 0.951 (↓23.92) | 35.64 (↓7.79) | 34 ± 2.0 (↑17.24) |
| 80 | 0.947 (↓24.24) | 35.65 (↓7.76) | 34 ± 1.2 (↑17.24) |
| 90 | 0.954 (↓23.68) | 35.57 (↓7.97) | 34 ± 1.4 (↑17.24) |
| 100 | 0.974 (↓22.08) | 35.54 (↓8.05) | 33 ± 1.3 (↑13.79) |
| Autoclave Cycles | Hardness Roechling (ϕ-12 mm) | Hardness Roechling (ϕ-50 mm) | Hardness Ensinger (ϕ-50 mm) |
|---|---|---|---|
| Value (% Change) | Value (% Change) | Value (% Change) | |
| 0 | 29 ± 0.8 | 28 ± 1.2 | 28 ± 0.6 |
| 2 | 35 ± 1.6 (↑20.69) | 30 ± 1.2 (↑7.14) | 30 ± 1.0 (↑7.14) |
| 4 | 35 ± 1.4 (↑20.69) | 31 ± 1.0 (↑10.71) | 29 ± 1.1 (↑3.57) |
| 6 | 38 ± 2.0 (↑31.03) | 31 ± 1.0 (↑10.71) | 30 ± 1.7 (↑7.14) |
| 8 | 39 ± 1.8 (↑34.48) | 33 ± 0.7 (↑17.86) | 31 ± 1.7 (↑10.71) |
| 10 | 39 ± 3.7 (↑34.48) | 34 ± 0.9 (↑21.43) | 33 ± 2.2 (↑17.86) |
| 20 | 43 ± 1.6 (↑48.28) | 33 ± 2.2 (↑17.86) | 32 ± 1.6 (↑14.29) |
| 30 | 41 ± 2.3 (↑41.38) | 32 ± 1.4 (↑14.29) | 30 ± 1.5 (↑7.14) |
| 40 | 34 ± 1.7 (↑17.24) | 33 ± 1.2 (↑17.86) | 31 ± 1.8 (↑10.71) |
| 50 | 33 ± 1.6 (↑13.79) | 37 ± 1.4 (↑32.14) | 34 ± 1.9 (↑21.43) |
| 60 | 32 ± 2.0 (↑10.34) | 37 ± 2.5 (↑32.14) | 35 ± 2.0 (↑25) |
| 70 | 34 ± 2.0 (↑17.24) | 35 ± 1.0 (↑25) | 34 ± 1.2 (↑21.43) |
| 80 | 34 ± 1.2 (↑17.24) | 36 ± 1.5 (↑28.57) | 35 ± 1.8 (↑25) |
| 90 | 34 ± 1.4 (↑17.24) | 36 ± 0.9 (↑28.57) | 35 ± 1.3 (↑25) |
| 100 | 33 ± 1.3 (↑13.79) | 35 ± 1.4 (↑25) | 35 ± 1.7 (↑25) |
| Repetitive Heating Cycles without Moisture | |||
| No. of cycles | Hardness Roechling (50 mm) MPa | Hardness Sterilized sample MPa (%) | Hardness heated sample MPa (%) |
| 10 | 28 ± 1.2 | 34 ± 0.9 (21.43↑) | 30 ± 1.3 (7.14↑) |
| 20 | 33 ± 2.2 (17.86↑) | 30 ± 1.0 (7.14↑) | |
| Long Heating Period (20 h, 100 °C) of Sterilized Sample | |||
| No. of cycles | Hardness Roechling (12 mm) MPa | Hardness Sterilized sample MPa (%) | Hardness heated sample MPa (%) |
| 20 | 29 ± 0.8 | 43 ± 1.6 (48.28↑) | 31 ± 1.1 (6.89↑) |
| 30 | 41 ± 2.3 (42.38↑) | 30 ± 0.8 (3.45↑) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Yap, W.T.; Foo, S.L.; Lee, T.K. Effects of Sterilization Cycles on PEEK for Medical Device Application. Bioengineering 2018, 5, 18. https://doi.org/10.3390/bioengineering5010018
Kumar A, Yap WT, Foo SL, Lee TK. Effects of Sterilization Cycles on PEEK for Medical Device Application. Bioengineering. 2018; 5(1):18. https://doi.org/10.3390/bioengineering5010018
Chicago/Turabian StyleKumar, Amit, Wai Teng Yap, Soo Leong Foo, and Teck Kheng Lee. 2018. "Effects of Sterilization Cycles on PEEK for Medical Device Application" Bioengineering 5, no. 1: 18. https://doi.org/10.3390/bioengineering5010018





