Musculoskeletal Model Development of the Elbow Joint with an Experimental Evaluation
Abstract
:1. Introduction
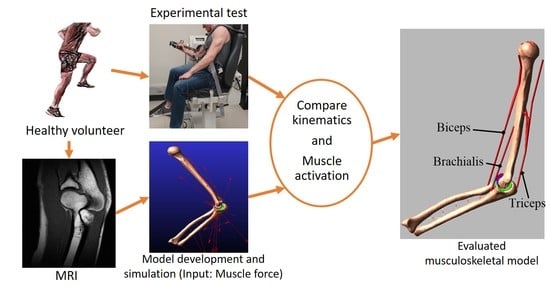
2. Materials and Methods
2.1. Experimental Measures
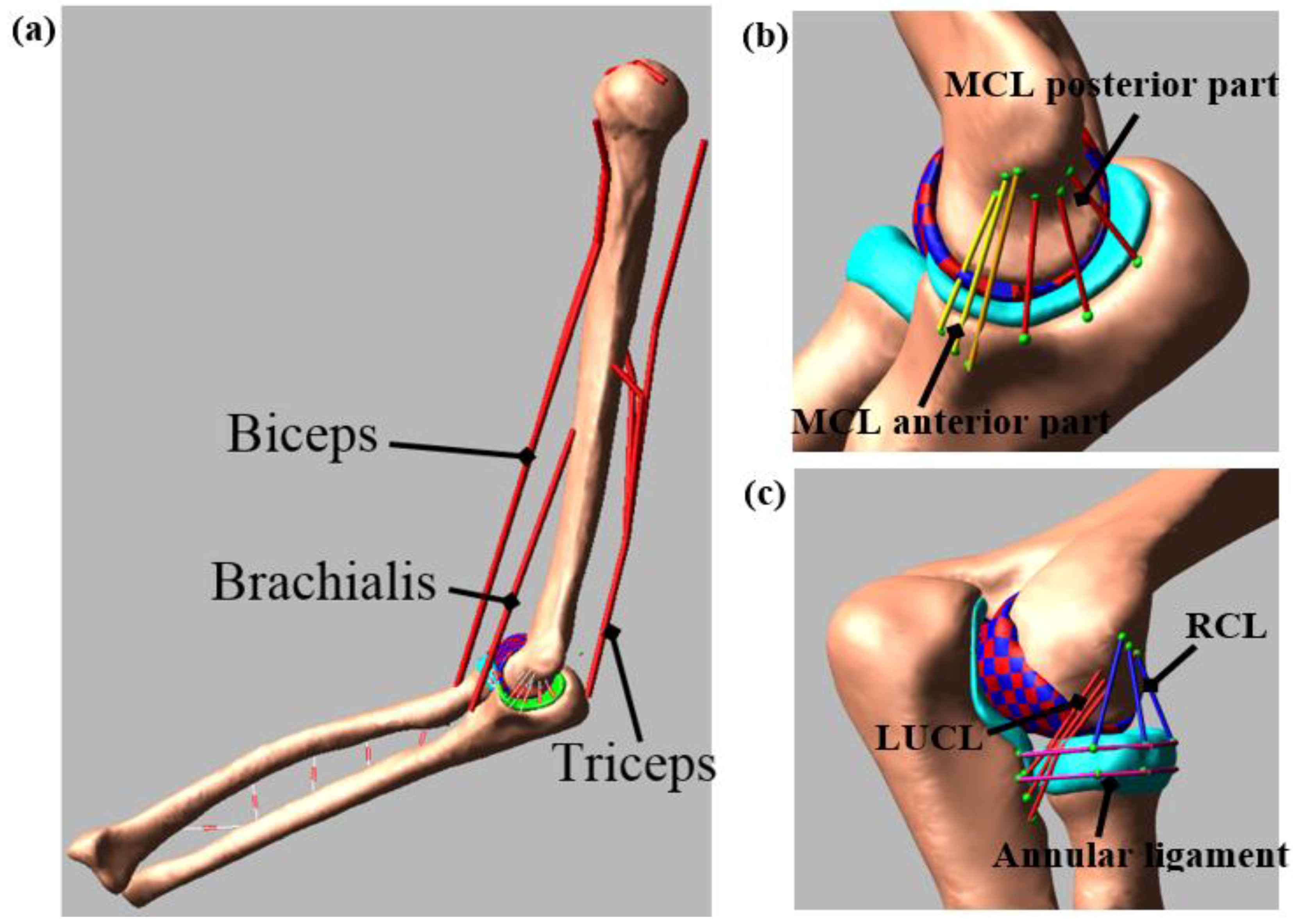
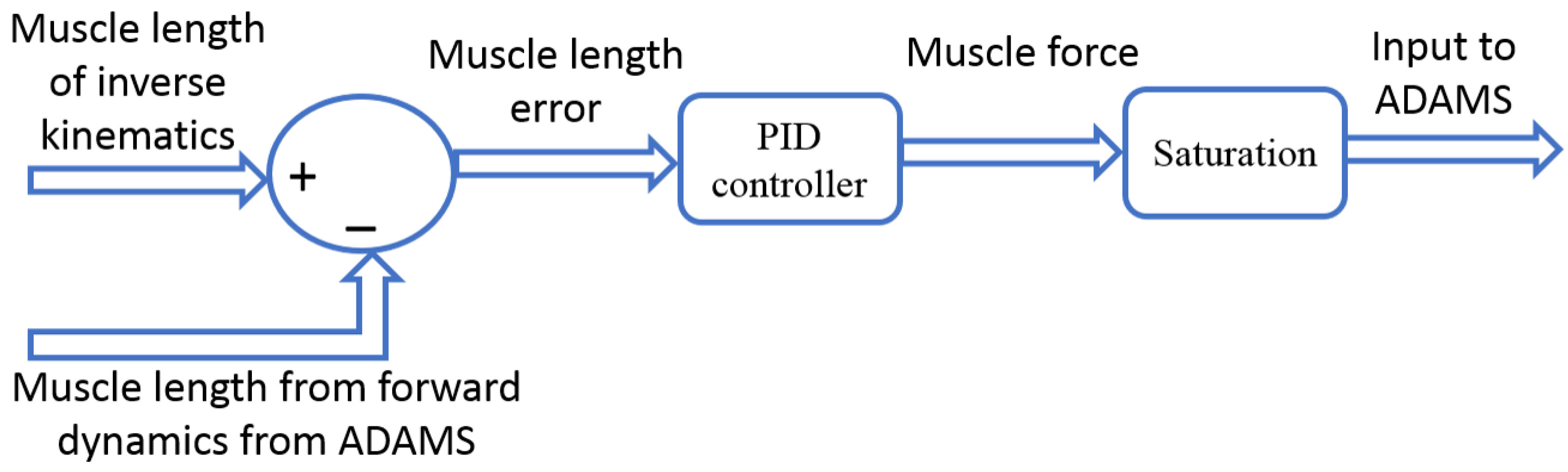
2.2. Computational Model
3. Results
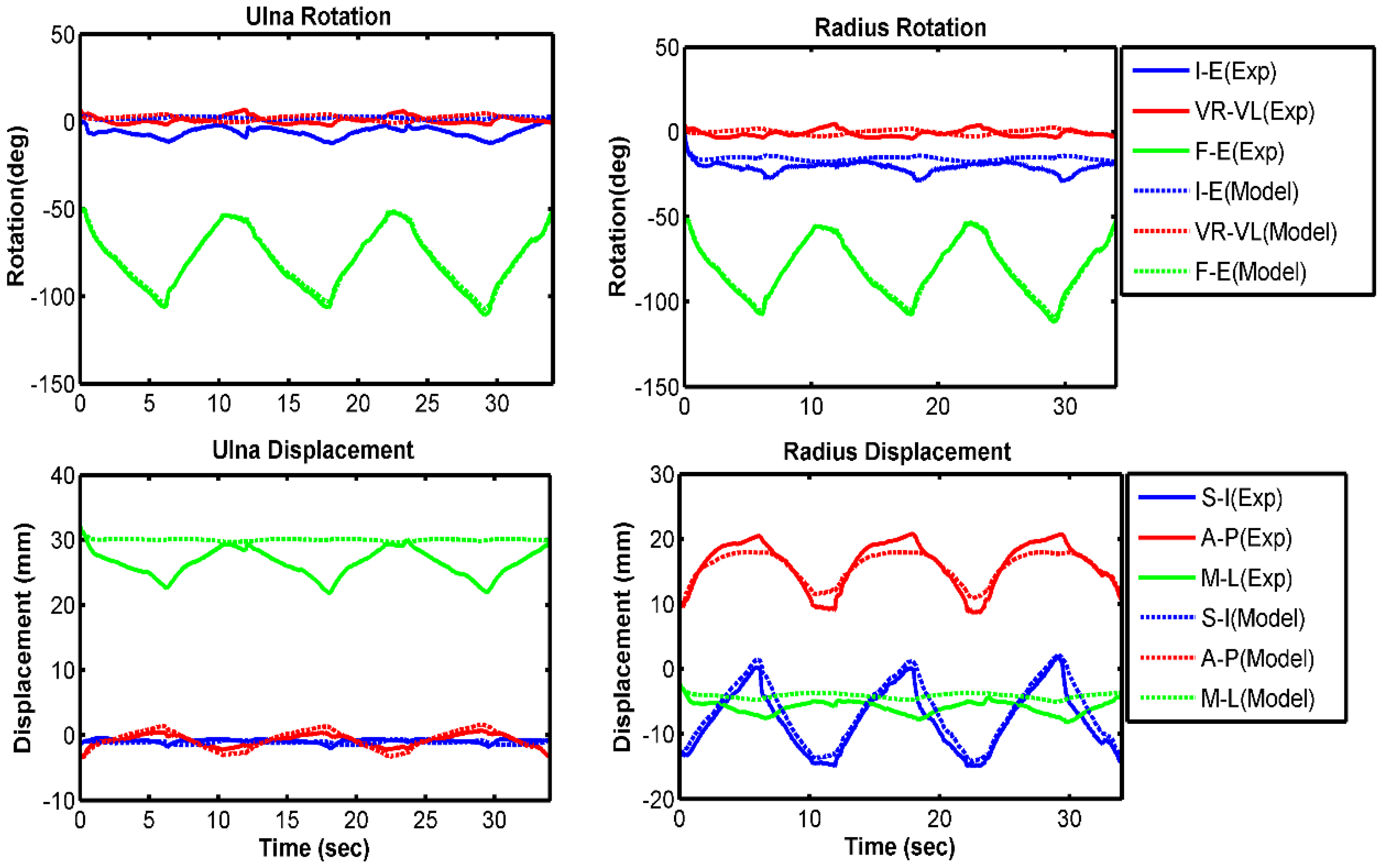
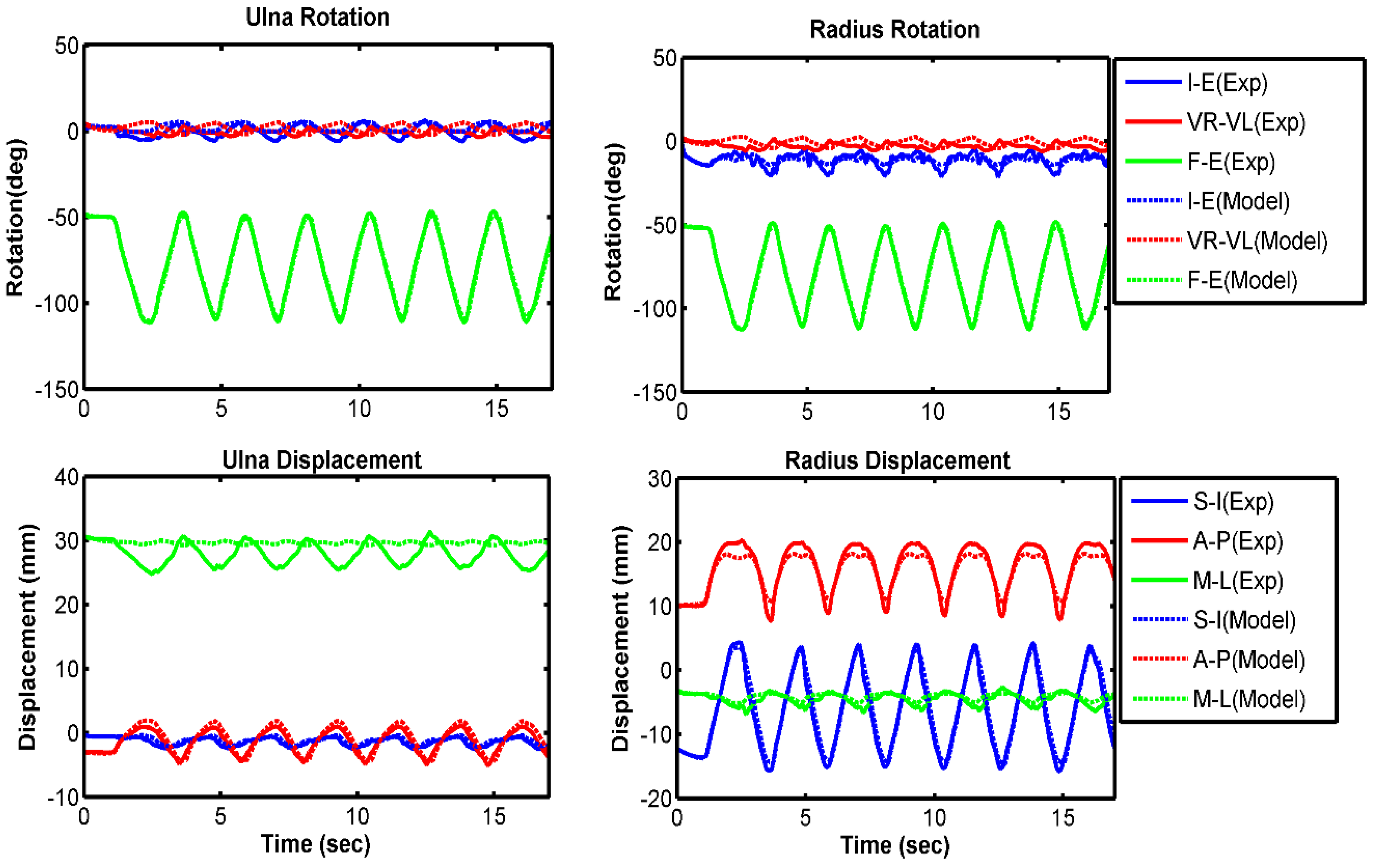
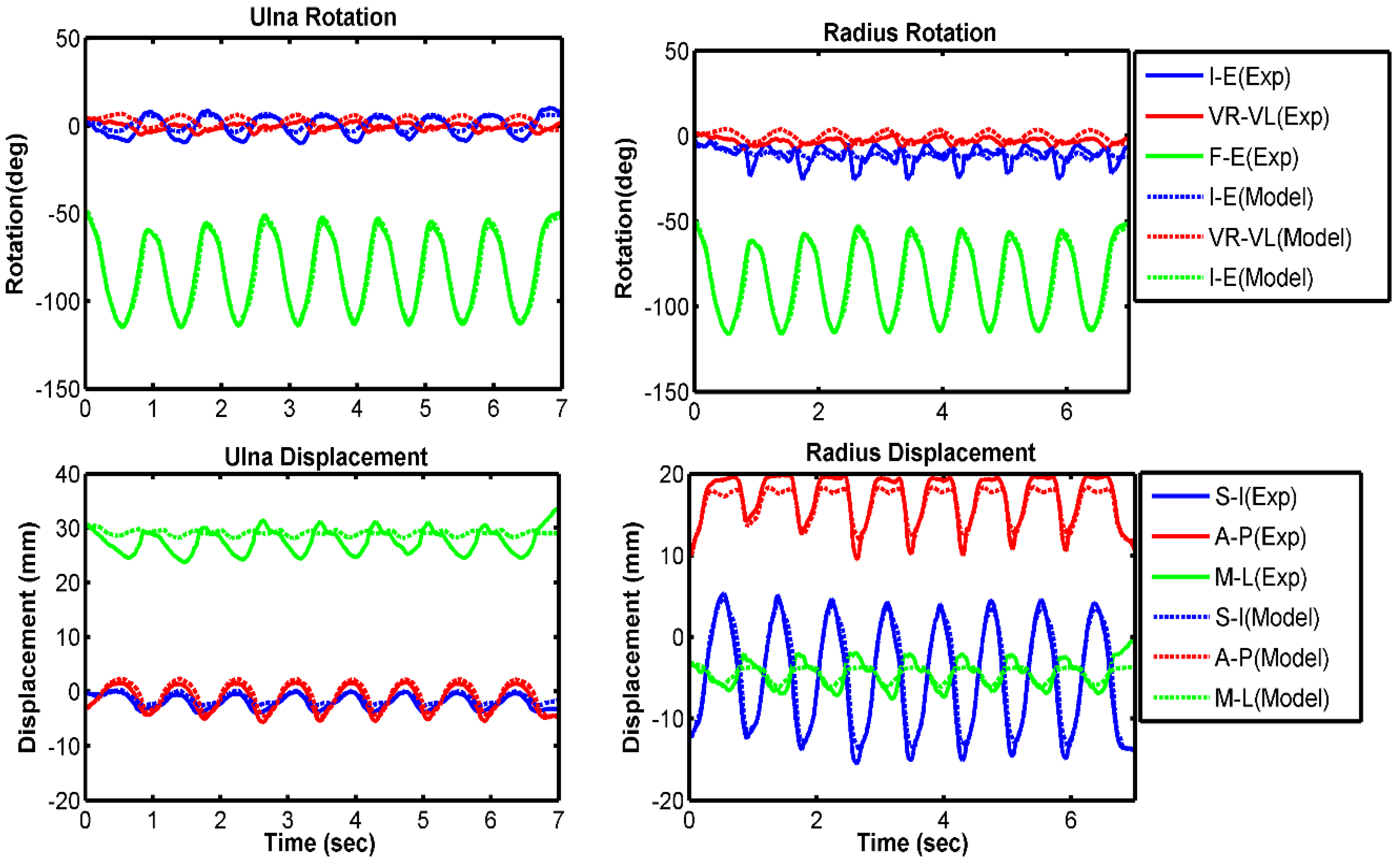
3.1. Model Evaluation
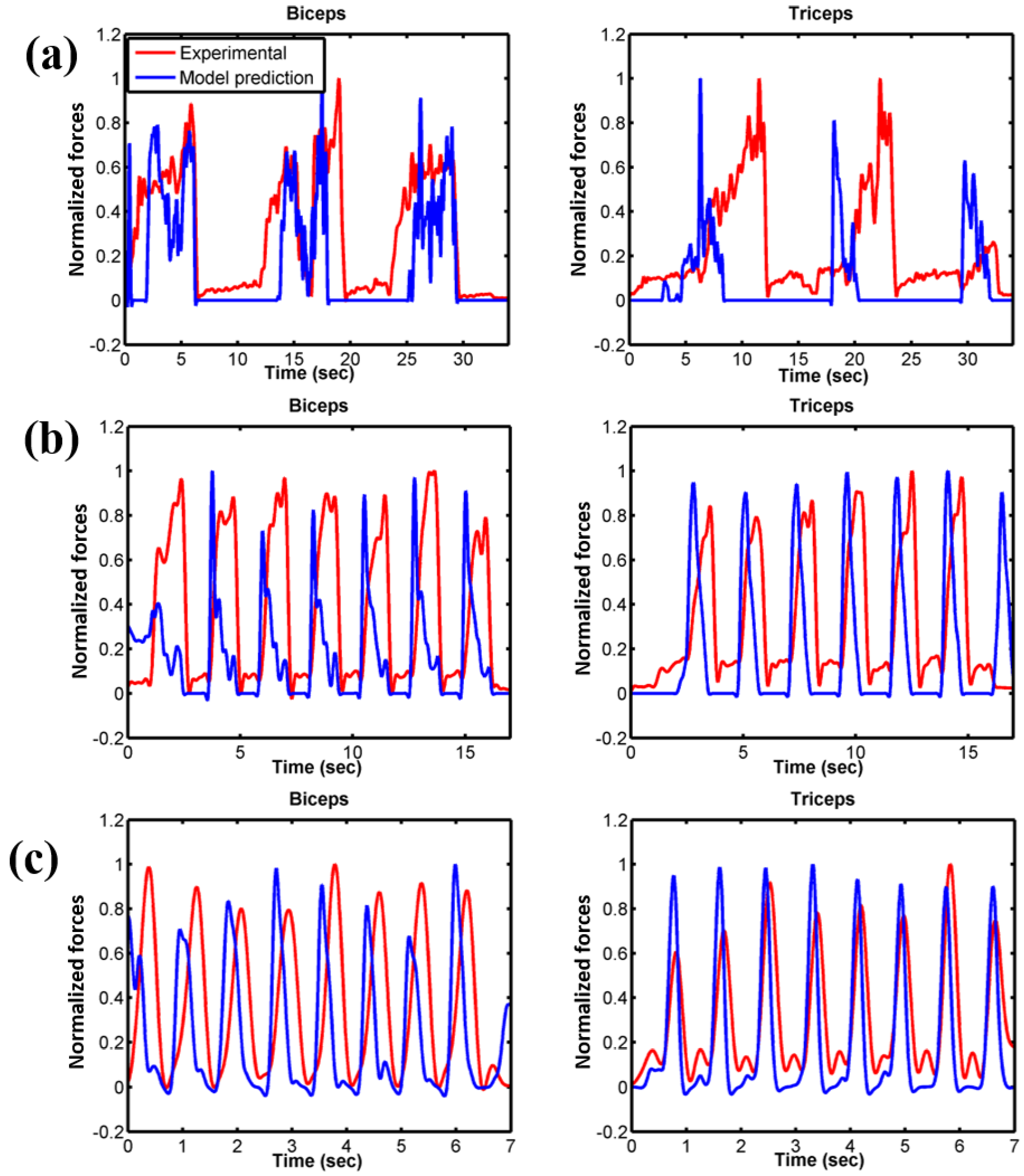
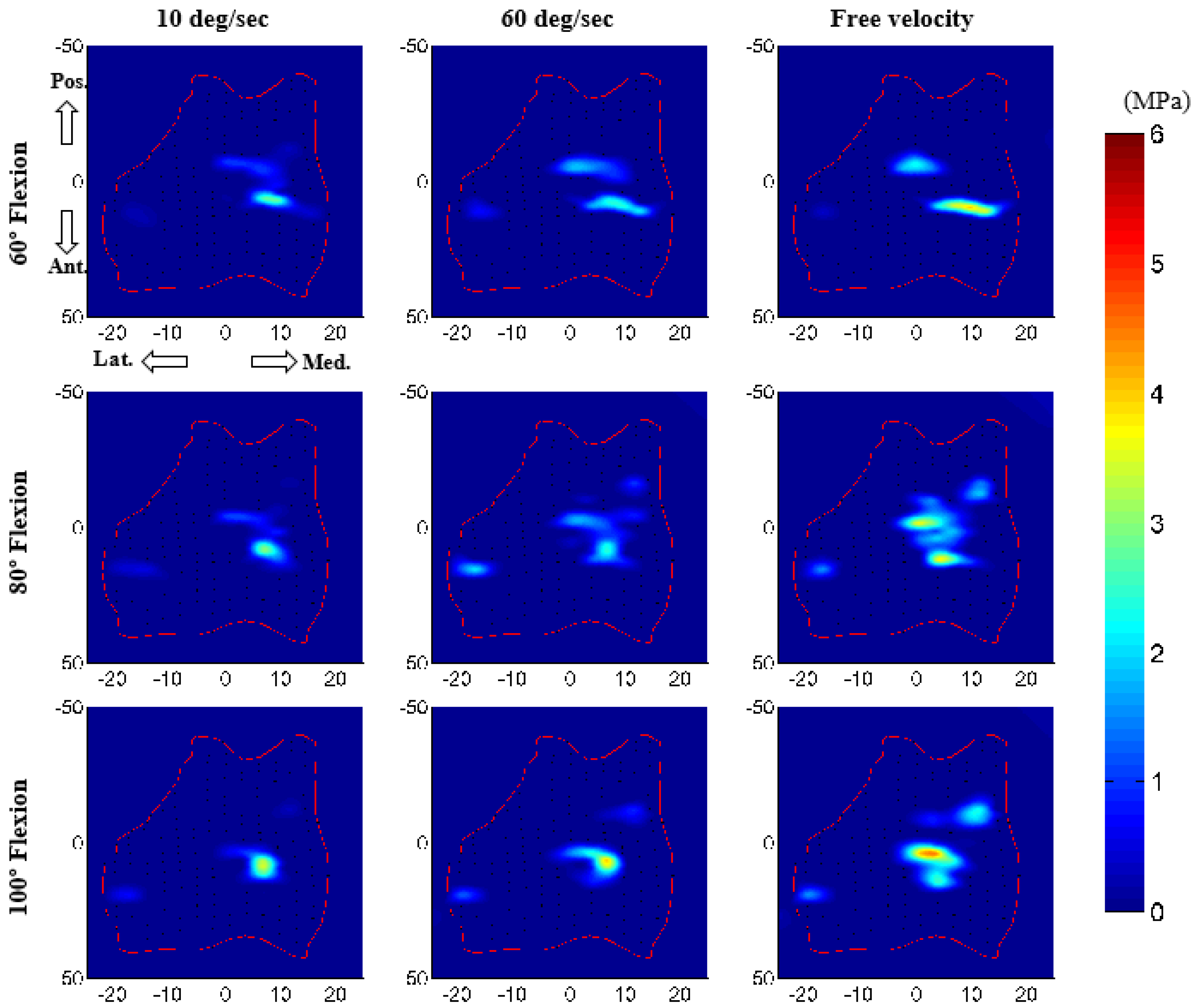
3.2. Force Prediction
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morrey, B.F. The Elbow and Its Disorders; W.B. Saunders: Philadelphia, PA, USA, 2000. [Google Scholar]
- Garner, B.A.; Pandy, M.G. Musculoskeletal model of the upper limb based on the visible human male dataset. Comput. Methods Biomech. Biomed. Eng. 2001, 4, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.V.; Hutchins, E.L.; Barr, R.E.; Abraham, L.D. Development and evaluation of a musculoskeletal model of the elbow joint complex. J. Biomech. Eng. 1996, 118, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Holzbaur, K.R.; Murray, W.M.; Delp, S.L. A model of the upper extremity for simulating musculoskeletal surgery and analyzing neuromuscular control. Ann. Biomed. Eng. 2005, 33, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Willing, R.T.; Nishiwaki, M.; Johnson, J.A.; King, G.J.; Athwal, G.S. Evaluation of a computational model to predict elbow range of motion. Comput. Aided Surg. 2014, 19, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Benham, M.P.; Wright, D.K.; Bibb, R. Modelling soft tissue for kinematic analysis of multi-segment human body models. Biomed. Sci. Instrum. 2001, 37, 111–116. [Google Scholar] [PubMed]
- Tanaka, S.; An, K.-N.; Morrey, B.F. Kinematics and laxity of ulnohumeral joint under valgus-varus stress. J. Musculoskelet. Res. 1998, 2, 45–54. [Google Scholar] [CrossRef]
- Fisk, J.P.; Wayne, J.S. Development and validation of a computational musculoskeletal model of the elbow and forearm. Ann. Biomed. Eng. 2009, 37, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Asraf Ali, M.; Sundaraj, K.; Badlishah Ahmad, R.; Ahamed, N.U.; Islam, A. Recent observations in surface electromyography recording of triceps brachii muscle in patients and athletes. Appl. Bionics Biomech. 2014, 11, 105–118. [Google Scholar] [CrossRef]
- Van Woensel, W.; Arwert, H. Effects of external load and abduction angle on emg level of shoulder muscles during isometric action. Electromyogr. Clin. Neurophysiol. 1993, 33, 185–191. [Google Scholar] [PubMed]
- Cignoni, P.; Callieri, M.; Corsini, M.; Dellepiane, M.; Ganovelli, F.; Ranzuglia, G. Meshlab: An open-source mesh processing tool. In Proceedings of the Sixth Eurographics Italian Chapter Conference, Salerno, Italy, 2–4 July 2008; pp. 129–136. [Google Scholar]
- Donahue, T.L.; Hull, M.L.; Rashid, M.M.; Jacobs, C.R. A finite element model of the human knee joint for the study of tibio-femoral contact. J. Biomech. Eng. 2002, 124, 273–280. [Google Scholar] [PubMed]
- Zielinska, B.; Donahue, T.L. 3D finite element model of meniscectomy: Changes in joint contact behavior. J. Biomech. Eng. 2006, 128, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Cil, A.; Johnson, M.; Lu, Y.; Guess, T.M. Development and validation of a computational multibody model of the elbow joint. Adv. Biomech. Appl. 2014, 1, 169–185. [Google Scholar] [CrossRef]
- Rahman, M.; Cil, A.; Bogener, J.W.; Stylianou, A.P. Lateral collateral ligament deficiency of the elbow joint: A modeling approach. J. Orthop. Res. 2016, 34, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Miyake, J.; Moritomo, H.; Masatomi, T.; Kataoka, T.; Murase, T.; Yoshikawa, H.; Sugamoto, K. In vivo and 3-dimensional functional anatomy of the anterior bundle of the medial collateral ligament of the elbow. J. Shoulder Elb. Surg. 2012, 21, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Morrey, B.F.; An, K.N. Functional anatomy of the ligaments of the elbow. Clin. Orthop. Relat. Res. 1985, 84–90. [Google Scholar] [CrossRef]
- Blankevoort, L.; Huiskes, R. Ligament-bone interaction in a three-dimensional model of the knee. J. Biomech. Eng. 1991, 113, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wismans, J.; Veldpaus, F.; Janssen, J.; Huson, A.; Struben, P. A three-dimensional mathematical model of the knee-joint. J. Biomech. 1980, 13, 677–685. [Google Scholar] [CrossRef]
- Li, G.; Gil, J.; Kanamori, A.; Woo, S.L. A validated three-dimensional computational model of a human knee joint. J. Biomech. Eng. 1999, 121, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Blankevoort, L.; Kuiper, J.H.; Huiskes, R.; Grootenboer, H.J. Articular contact in a three-dimensional model of the knee. J. Biomech. 1991, 24, 1019–1031. [Google Scholar] [CrossRef]
- Regan, W.D.; Korinek, S.L.; Morrey, B.F.; An, K.N. Biomechanical study of ligaments around the elbow joint. Clin. Orthop. Relat. Res. 1991, 170–179. [Google Scholar] [CrossRef]
- Bloemker, K.H.; Guess, T.M.; Maletsky, L.; Dodd, K. Computational knee ligament modeling using experimentally determined zero-load lengths. Open Biomed. Eng. J. 2012, 6, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Cil, A.; Stylianou, A.P. Prediction of elbow joint contact mechanics in the multibody framework. Med. Eng. Phys. 2016, 38, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Guess, T.M.; Stylianou, A.P.; Kia, M. Concurrent prediction of muscle and tibiofemoral contact forces during treadmill gait. J. Biomech. Eng. 2014, 136, 021032. [Google Scholar] [CrossRef] [PubMed]
- Kia, M.; Stylianou, A.P.; Guess, T.M. Evaluation of a musculoskeletal model with prosthetic knee through six experimental gait trials. Med. Eng. Phys. 2014, 36, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Renani, M.S.; Rahman, M.; Cil, A.; Stylianou, A.P. Calibrating multibody ulno-humeral joint cartilage using a validated finite element model. Multibody Syst. Dyn. 2018. [Google Scholar] [CrossRef]
- Stylianou, A.P.; Guess, T.M.; Cook, J.L. Development and validation of a multi-body model of the canine stifle joint. Comput. Methods Biomech. Biomed. Eng. 2014, 17, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.P.; Guess, T.M.; Kia, M. Multibody muscle driven model of an instrumented prosthetic knee during squat and toe rise motions. J. Biomech. Eng. 2013, 135, 041008. [Google Scholar] [CrossRef] [PubMed]
- Renani, M.S.; Rahman, M.; Cil, A.; Stylianou, A.P. Ulna-humerus contact mechanics: Finite element analysis and experimental measurements using a tactile pressure sensor. Med. Eng. Phys. 2017, 50, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, M.G.; Liu, D. Upper limb strength estimation of physically impaired persons using a musculoskeletal model: A sensitivity analysis. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 2015, 2438–2441. [Google Scholar] [PubMed]
- Gonzalez, R.V.; Abraham, L.D.; Barr, R.E.; Buchanan, T.S. Muscle activity in rapid multi-degree-of-freedom elbow movements: Solutions from a musculoskeletal model. Biol. Cybern. 1999, 80, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.V.; Andritsos, M.J.; Barr, R.E.; Abraham, L.D. Comparison of experimental and predicted muscle activation patterns in ballistic elbow joint movements. Biomed. Sci. Instrum. 1993, 29, 9–16. [Google Scholar] [PubMed]
- O’Driscoll, S.W.; An, K.N.; Korinek, S.; Morrey, B.F. Kinematics of semi-constrained total elbow arthroplasty. J. Bone Jt. Surg. Br. 1992, 74, 297–299. [Google Scholar] [CrossRef]
- Morrey, B.F.; An, K.N. Articular and ligamentous contributions to the stability of the elbow joint. Am. J. Sports Med. 1983, 11, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, P.; Yahia, L.; Feldman, A.G. Moment arms and lengths of human upper limb muscles as functions of joint angles. J. Biomech. 1996, 29, 1365–1370. [Google Scholar] [CrossRef]
- Eckstein, F.; Merz, B.; Muller-Gerbl, M.; Holzknecht, N.; Pleier, M.; Putz, R. Morphomechanics of the humero-ulnar joint: II. Concave incongruity determines the distribution of load and subchondral mineralization. Anat. Rec. 1995, 243, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.A. Joint contact stress: A reasonable surrogate for biological processes? Iowa Orthop. J. 2005, 25, 82–94. [Google Scholar] [PubMed]

| Parameters | Values |
|---|---|
| Contact type | Impact (deformable) |
| Contact element size | 3 mm × 3 mm |
| Friction | No |
| Stiffness (kc) | 40 N/mm |
| Damping coefficient (Bc(δ)) | 5 Ns/mm |
| Exponent (n) | 3.05 |
| Interpenetration of the geometries (δ) | 0.1 mm |
| Kinematics Description | 10 deg/s | 60 deg/s | Free Velocity | |||
|---|---|---|---|---|---|---|
| RMS Error | Correlation Coefficient | RMS Error | Correlation Coefficient | RMS Error | Correlation Coefficient | |
| Ulna I–E rotation | 7.4 | 0.48 | 2.9 | 0.86 | 4.0 | 0.92 |
| Ulna VR–VL rotation | 2.8 | −0.63 | 2.9 | −0.13 | 2.7 | 0.57 |
| Ulna F–E rotation | 1.3 | 0.99 | 1.2 | 0.99 | 2.7 | 0.99 |
| Radius I–E rotation | 5.5 | 0.44 | 2.3 | 0.55 | 3.6 | 0.08 |
| Radius VR–VL rotation | 2.6 | −0.60 | 2.8 | −0.15 | 2.6 | 0.61 |
| Radius F–E rotation | 1.1 | 0.99 | 1.1 | 0.99 | 2.7 | 0.99 |
| Ulna S–I displacement | 0.4 | 0.07 | 0.2 | 0.90 | 0.4 | 0.97 |
| Ulna A–P displacement | 0.4 | 0.98 | 0.4 | 0.99 | 0.6 | 0.98 |
| Ulna M–L displacement | 3.4 | −0.45 | 2.0 | −0.62 | 2.3 | −0.14 |
| Radius S–I displacement | 1.3 | 0.99 | 0.6 | 0.99 | 1.3 | 0.99 |
| Radius A–P displacement | 1.0 | 0.98 | 0.8 | 0.99 | 0.8 | 0.97 |
| Radius M–L displacement | 1.5 | 0.86 | 0.6 | 0.91 | 0.9 | 0.91 |
| Ligament | Bundles | Peak Ligament Load (N) | ||
|---|---|---|---|---|
| 10 deg/s | 60 deg/s | Free Velocity | ||
| MCL anterior part | Anterior | 23 | 37 | 101 |
| Central | 45 | 61 | 123 | |
| Posterior | 61 | 76 | 112 | |
| MCL posterior part | Anterior | 35 | 40 | 55 |
| Central | 30 | 44 | 87 | |
| Posterior | 24 | 47 | 125 | |
| LUCL | Anterior | 43 | 58 | 80 |
| Central | 23 | 33 | 44 | |
| Posterior | 26 | 25 | 50 | |
| RCL | Anterior | 21 | 44 | 131 |
| Central | 18 | 26 | 55 | |
| Posterior | 12 | 31 | 88 | |
| Annular ligament | Proximal | 46 | 51 | 52 |
| Distal | 36 | 41 | 41 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.; Sharifi Renani, M.; Cil, A.; Stylianou, A.P. Musculoskeletal Model Development of the Elbow Joint with an Experimental Evaluation. Bioengineering 2018, 5, 31. https://doi.org/10.3390/bioengineering5020031
Rahman M, Sharifi Renani M, Cil A, Stylianou AP. Musculoskeletal Model Development of the Elbow Joint with an Experimental Evaluation. Bioengineering. 2018; 5(2):31. https://doi.org/10.3390/bioengineering5020031
Chicago/Turabian StyleRahman, Munsur, Mohsen Sharifi Renani, Akin Cil, and Antonis P. Stylianou. 2018. "Musculoskeletal Model Development of the Elbow Joint with an Experimental Evaluation" Bioengineering 5, no. 2: 31. https://doi.org/10.3390/bioengineering5020031