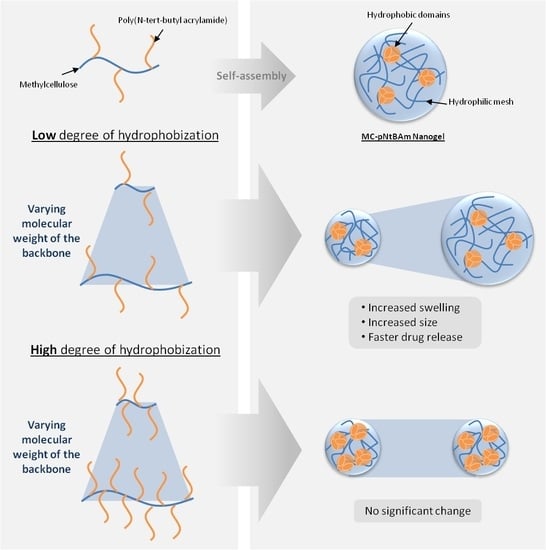
Effect of Methylcellulose Molecular Weight on the Properties of Self-Assembling MC-g-PNtBAm Nanogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MC-g-PNtBAm Nanogels
2.3. 1H NMR Analysis
2.4. Particle Size Measurements
2.5. Transmission Electron Microscopy Study
2.6. Loading of Dexamethasone
2.7. In Vitro Release of Dexamethasone
3. Results and Discussion
3.1. Synthesis
3.2. Impact of MC Molecular Weight on Particle Size and Morphology
3.3. Impact of MC Molecular Weight on Drug Release
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.H.; Robinsonx, J.R. Mechanistic and Quantitative Evaluation of Precorneal Pilocarpine Disposition in Albino Rabbits. J. Pharm. Sci. 1979, 68, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Rini, R.J.; Venkatraman, S.S. Drug delivery to the eye: What benefits do nanocarriers offer? Nanomedicine 2017, 12, 6. [Google Scholar]
- Diebold, Y.; Calonge, M. Applications of nanoparticles in ophthalmology. Prog. Retin. Eye Res. 2010, 29, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, S.; Weyenberg, W.; Adriaens, E.; Dhondt, M.M.; Vergote, V.; Vervaet, C.; De Prijck, K.; Nelis, H.J.; De Spiegeleer, B.; Ludwig, A.; et al. In vitro evaluation of gentamicin- and vancomycin-containing minitablets as a replacement for fortified eye drops. Drug Dev. Ind. Pharm. 2010, 36, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Salminen, L. Review: Systemic Absorption of Topically Applied Ocular Drugs in Humans. J. Ocul. Pharmacol. Ther. 1990, 6, 243–249. [Google Scholar] [CrossRef]
- Baudouin, C. Side effects of antiglaucomatous drugs on the ocular surface. Curr. Opin. Ophthalmol. 1996, 7, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Arici, M.K.; Arici, D.S.; Topalkara, A.; Guler, C. Adverse effects of topical antiglaucoma drugs on the ocular surface. Clin. Exp. Ophthalmol. 2000, 28, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jones, L.; Gu, F.X. Nanomaterials for ocular drug delivery. Macromol. Biosci. 2012, 12, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Paliwal, R.; Paliwal, S.R.; Vyas, S.P. Nanocarriers in Ocular Drug Delivery: An Update Review. Curr. Pharm. Des. 2009, 15, 2724–2750. [Google Scholar] [CrossRef] [PubMed]
- Nagarwal, R.C.; Kant, S.; Singh, P.N.; Maiti, P.; Pandit, J.K. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J. Control. Release 2009, 136, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.-C.; Chang, S.-F.; Liu, C.-Y.; Winston, W.-Y.; Kao, C.H.; Huang, J.L. Eye drop delivery of nano-polymericmicelle formulated genes with cornea-specific promoters. J. Gene Med. 2008, 10, 610–618. [Google Scholar]
- Zarbin, M.A.; Montemagno, C.; Leary, J.F.; Ritch, R. Nanomedicine in ophthalmology: The new frontier. Am. J. Ophthalmol. 2010, 150, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. Applications of Polymeric and Lipid Nanoparticles in Ophthalmic Pharmaceutical Formulations: Present and Future Considerations. J. Pharm. Pharm. Sci. 2014, 17, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Z.; Wang, M.; Sun, X.; Nie, S.; Pan, W. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int. J. Pharm. 2009, 379, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. Applications of poloxamers in ophthalmic pharmaceutical formulations: An overview. Expert Opin. Drug Deliv. 2013, 10, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Andreani, T.; Egea, M.A.; Garcia, M.L.; Souto, S.B.; Souto, E.B. Experimental factorial design applied to mucoadhesive lipid nanoparticles via multiple emulsion process. Colloids Surf. B 2012, 100, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Finke, J.H.; Schmolke, H.; Klages, C.P.; MűllerGoymann, C.C. Controlling solid lipid nanoparticle adhesion by polyelectrolyte multilayer surface modifications. Int. J. Pharm. 2013, 449, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Hreinsdóttir, D.; Stefánsson, E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: Aqueous dexamethasone eye drops. J. Pharm. Pharmacol. 2007, 59, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Jamard, M.; Hoare, T.; Sheardown, H. Nanogels of Methylcellulose Hydrophobized with N-tert-butylacrylamide for Ocular Drug Delivery. Drug. Deliv. Transl. Res. 2016, 6, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sun, L.; Fang, S.; Wang, S.; Chen, J.; Xiao, X.; Liu, C. Thermosensitive in situ nanogel as ophthalmic delivery system of curcumin: Development, characterization, in vitro permeation and in vivo pharmacokinetic studies. Pharm. Dev. Technol. 2016, 21, 576–582. [Google Scholar] [PubMed]
- Abd El-Rehim, H.A.; Swilem, A.E.; Klingner, A.; Hegazy, E.-S.; Hamed, A.A. Developing the potential ophthalmic applications of pilocarpine entrapped into polyvinylpyrrolidone-poly(acrylic acid) nanogel dispersions prepared by γ radiation. Biomacromolecules 2013, 14, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Moya-Ortega, M.D.; Alves, T.F.; Alvarez-Lorenzo, C.; Concheiro, A.; Stefánsson, E.; Thorsteinsdóttir, M.; Loftsson, T. Dexamethasone eye drops containing γ-cyclodextrin-based nanogels. Int. J. Pharm. 2013, 441, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Nagarwal, R.C.; Singh, P.N.; Kant, S.; Maiti, P.; Pandit, J.K. Chitosan nanoparticles of 5-fluorouracil for ophthalmic delivery: Characterization, in-vitro and in-vivo study. Chem. Pharm. Bull. 2011, 59, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, K.; Chaturvedi, K.; More, U.A.; Nadagouda, M.N.; Aminabhavi, T.M. Polysaccharide-based micro/nanohydrogels for delivering macromolecular therapeutics. J. Control. Release 2014, 193, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, L.; Li, H.; Han, Y.; Zhou, J.; Wang, X. Self-assembly and paclitaxel loading capacity of α-tocopherol succinate-conjugated hydroxyethyl cellulose nanomicelle. Colloid Polym. Sci. 2016, 294, 135–143. [Google Scholar] [CrossRef]
- Nichifor, M.; Mocanu, G.; Stanciu, M.C. Micelle-like association of polysaccharides with hydrophobic end groups. Carbohydr. Polym. 2014, 110, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Maysinger, D.; Eisenberg, A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf. B 1999, 16, 3–27. [Google Scholar] [CrossRef]
- Verma, M.S.; Liu, S.; Chen, Y.Y.; Meerasa, A.; Gu, F.X. Size-tunable nanoparticles composed of dextran-b-poly(d,l-lactide) for drug delivery applications. Nano Res. 2012, 5, 49–61. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, G.; Tang, G.; Wang, L.; Jiang, H. Synthesis, self-assembly, and cytotoxicity of well-defined trimethylated chitosan-O-poly(e-caprolactone): Effect of chitosan molecular weight. J. Biomed. Mater. Res. 2011, 98, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Calce, E.; Ringhieri, P.; Mercurio, F.A.; Leone, M.; Bugatti, V.; Saviano, M.; Vittoria, V.; De Luca, S. A biocompatible process to prepare hyaluronan-based material able to self-assemble into stable nano-particles. RSC Adv. 2015, 5, 29573–29576. [Google Scholar] [CrossRef]
- Nagahama, K.; Mori, Y.; Ohya, Y.; Ouchi, T. Biodegradable nanogel formation of polylactide-grafted dextran copolymer in dilute aqueous solution and enhancement of its stability by stereocomplexation. Biomacromolecules 2007, 8, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, X.; Shu, X.; Shen, Z.; Sun, R.C. Self-assembly and paclitaxel loading capacity of cellulose-graft- poly(lactide) nanomicelles. J. Agric. Food Chem. 2012, 60, 3900–3908. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chang, C.N.; Verma, M.S.; Hileeto, D.; Muntz, A.; Stahl, U.; Woods, J.; Jones, L.W.; Gu, F.X. Phenylboronic acid modified mucoadhesive nanoparticle drug carriers facilitate weekly treatment of experimentallyinduced dry eye syndrome. Nano Res. 2015, 8, 621–635. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Deguchi, S.; Tajima, H.; Nishikawa, T.; Sunamoto, J. Microscopic Structure and Thermoresponsiveness of a Hydrogel Nanoparticle by Self-Assembly of a Hydrophobized Polysaccharide. Macromolecules 1997, 9297, 857–861. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Shen, Z.; Shu, X.; Sun, R. Preparation of cellulose-graft-poly(ε-caprolactone) nanomicelles by homogeneous ROP in ionic liquid. Carbohydr. Polym. 2013, 92, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Q.; Wang, X.; Wang, C.; Jiang, X. Preparation, drug release and cellular uptake of doxorubicin-loaded dextran-b-poly(ε-caprolactone) nanoparticles. Carbohydr. Polym. 2013, 93, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Balan, V.; Redinciuc, V.; Tudorachi, N.; Verestiuc, L. Biotinylated N-palmitoyl chitosan for design of drug loaded self-assembled nanocarriers. Eur. Polym. J. 2016, 81, 284–294. [Google Scholar] [CrossRef]
- Vafaei, S.Y.; Esmaeili, M.; Amini, M.; Atyabi, F.; Ostad, S.N.; Dinarvand, R. Self assembled hyaluronic acid nanoparticles as a potential carrier for targeting the inflamed intestinal mucosa. Carbohydr. Polym. 2016, 144, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, C.; Montanari, E.; Manzi, L.; Villani, C.; Coviello, T.; Matricardi, P. Highly versatile nanohydrogel platform based on riboflavin-polysaccharide derivatives useful in the development of intrinsically fluorescent and cytocompatible drug carriers. Carbohydr. Polym. 2015, 115, 502–509. [Google Scholar] [CrossRef] [PubMed]




| MC Molecular Weight (kg/mol) | 30 | 85 | 165 | 230 | ||||
|---|---|---|---|---|---|---|---|---|
| CAN concentration (mol/L) | 1.82 × 10−3 | 9.12 × 10−3 | 1.82 × 10−3 | 9.12 × 10−3 | 1.82 × 10−3 | 9.12 × 10−3 | 1.82 × 10−3 | 9.12 × 10−3 |
| MC Molecular Weight (kg/mol) | 30 | 85 | 165 | 230 |
|---|---|---|---|---|
| Low initiation | 28.00% | 32.00% | 32.80% | 30.10% |
| High initiation | 50.80% | 50.20% | 43.20% | 51.60% |
| MC Molecular Weight (kg/mol) | 30 | 230 |
|---|---|---|
| DH = 30% | 71.61 ± 0.09% | 98.96 ± 0.02% |
| DH = 50% | 98.75 ± 0.09% | 93.54 ± 0.03% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamard, M.; Sheardown, H. Effect of Methylcellulose Molecular Weight on the Properties of Self-Assembling MC-g-PNtBAm Nanogels. Bioengineering 2018, 5, 39. https://doi.org/10.3390/bioengineering5020039
Jamard M, Sheardown H. Effect of Methylcellulose Molecular Weight on the Properties of Self-Assembling MC-g-PNtBAm Nanogels. Bioengineering. 2018; 5(2):39. https://doi.org/10.3390/bioengineering5020039
Chicago/Turabian StyleJamard, Marion, and Heather Sheardown. 2018. "Effect of Methylcellulose Molecular Weight on the Properties of Self-Assembling MC-g-PNtBAm Nanogels" Bioengineering 5, no. 2: 39. https://doi.org/10.3390/bioengineering5020039