Influence of Sample Storage on the Composition of Carbonated Beverages by MIR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carbonated Beverage Storage Trial
2.2. Basic Compositional Analyses
2.3. Attenuated Total Reflectance Mid-Infrared Spectroscopy (ATR-MIR)
2.4. Statistical Analysis
3. Results and Discussion
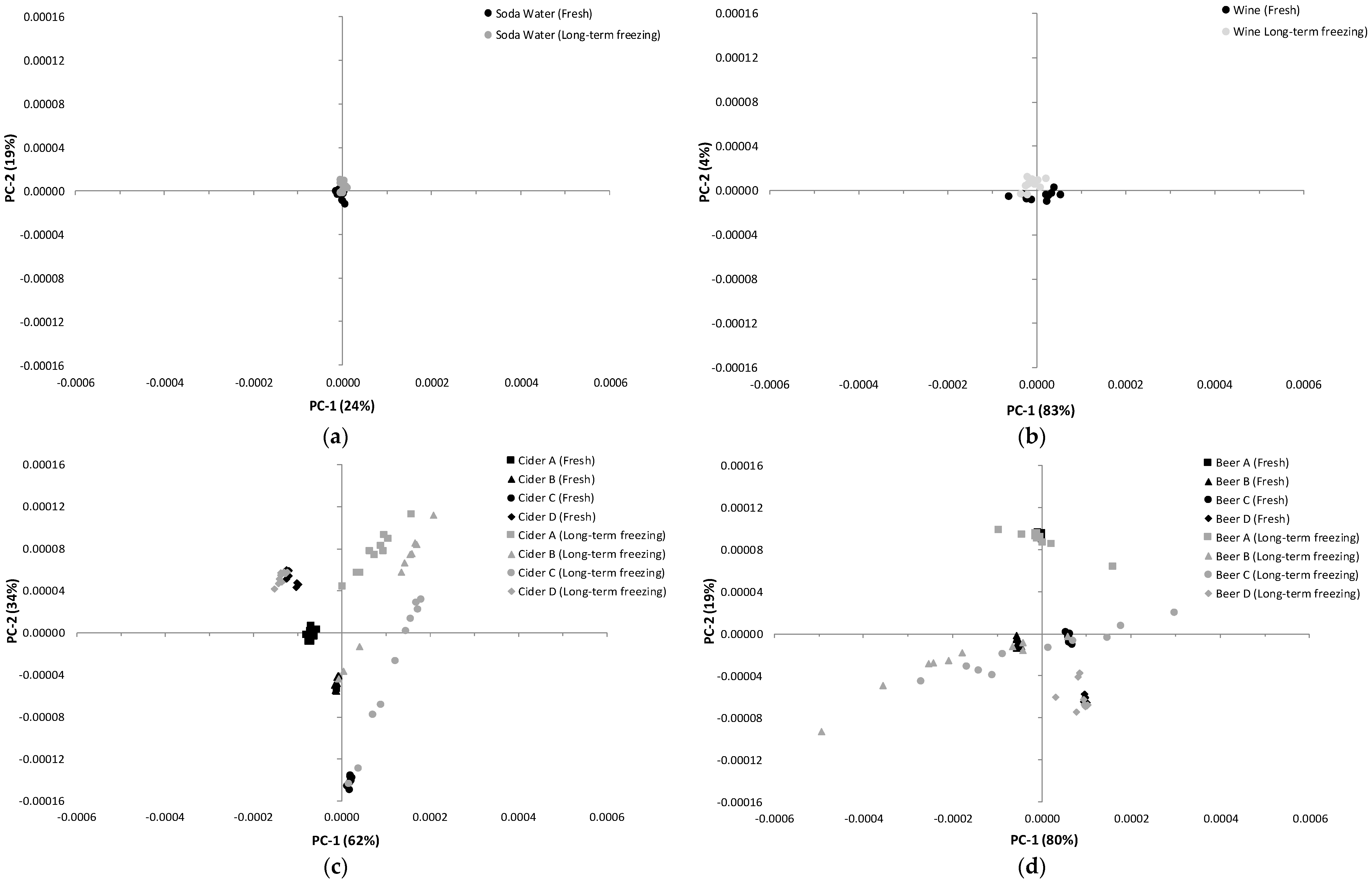
3.1. Influence of Storage Conditions on the Composition of Carbonated Beverages
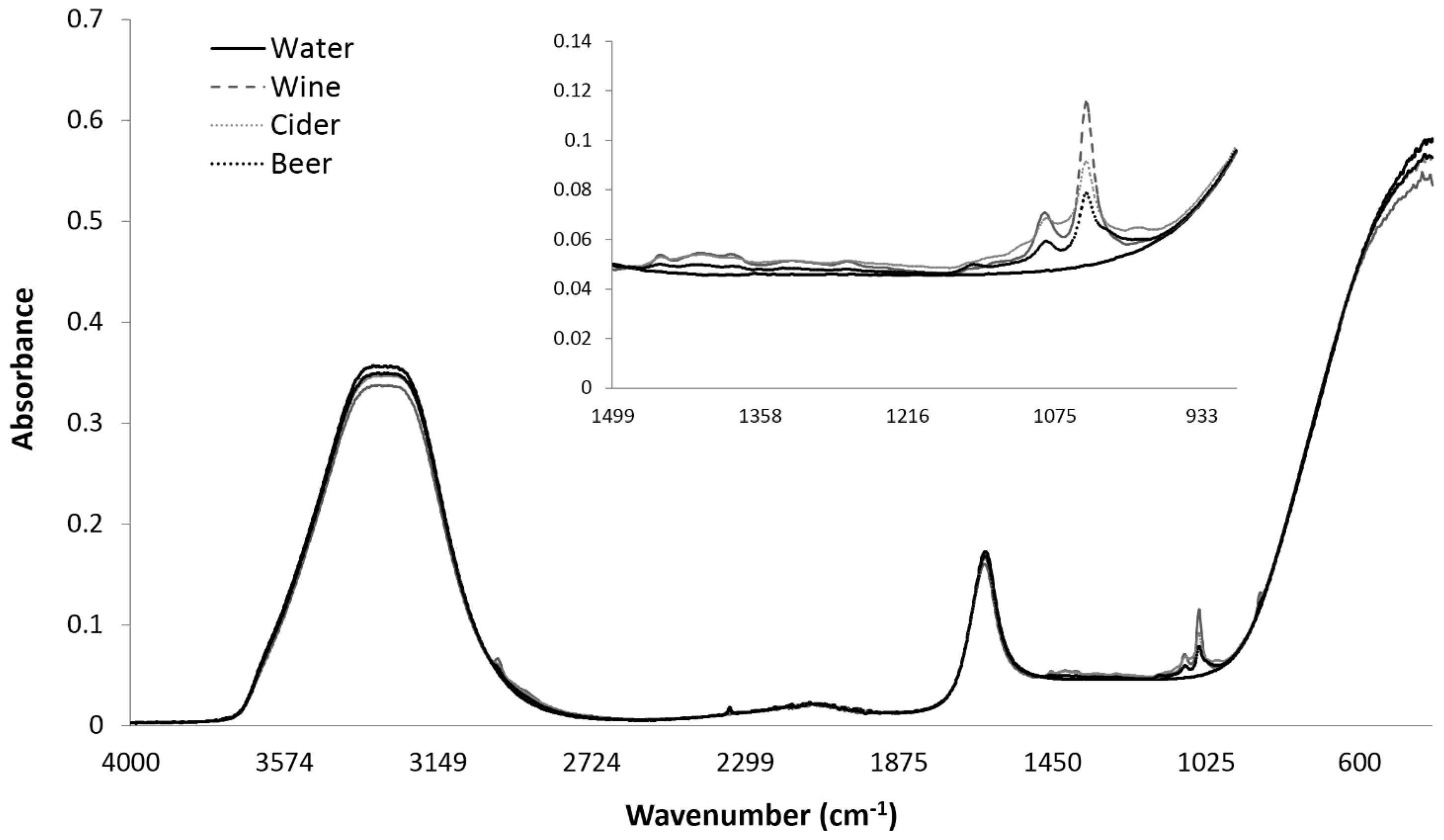
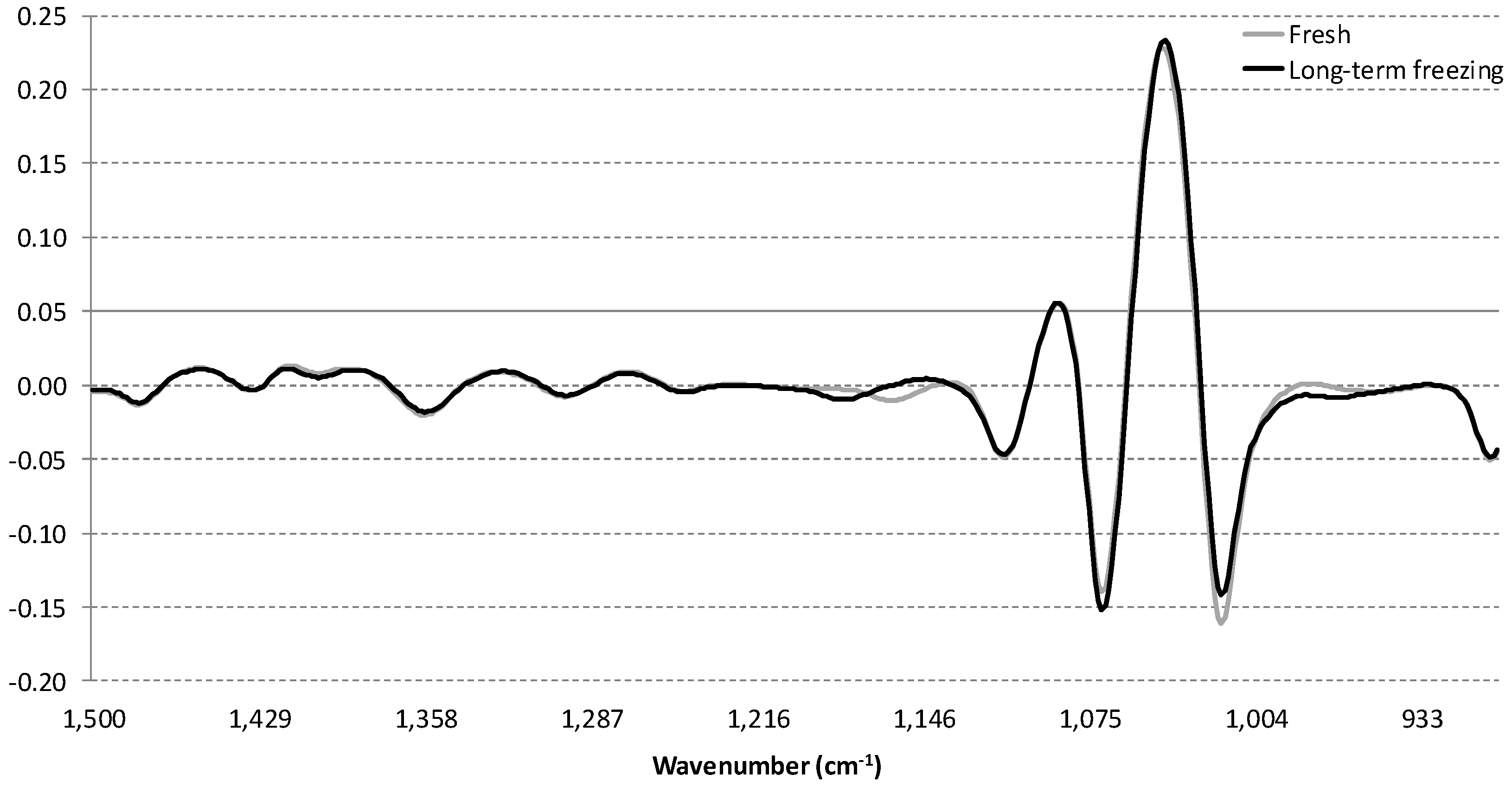
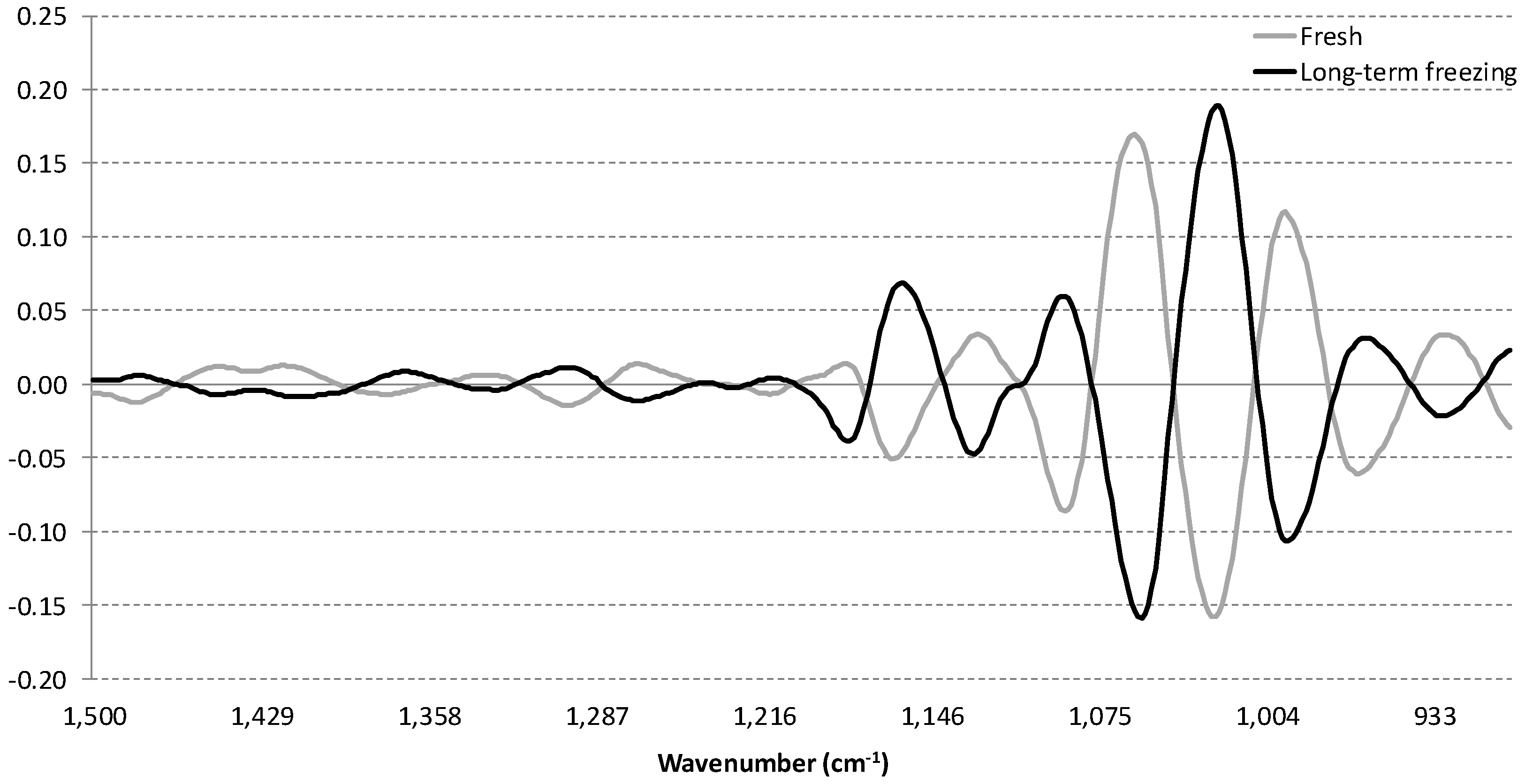
3.2. Influence of Storage Conditions on the MIR Spectra of Carbonated Beverages
4. Conclusions
Supplementary Material
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tandon, K.; Worobo, R.W.; Churey, J.J.; Padilla-Zakour, O.I. Storage quality of pasteurised and UV treated apple cider. J. Food Process. Preserv. 2003, 27, 21–35. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Delvaux, F.; Daenen, L.; Verachtert, H.; Delvaux, F.R. Aging characteristics of different beer types. Food Chem. 2007, 103, 404–412. [Google Scholar] [CrossRef]
- Pérez-Coello, M.; González-Viñas, M.; Garcıa-Romero, E.; Diaz-Maroto, M.; Cabezudo, M. Influence of storage temperature on the volatile compounds of young white wines. Food Control 2003, 14, 301–306. [Google Scholar] [CrossRef]
- Perez-Prieto, L.; Lopez-Roca, J.; Gómez-Plaza, E. Differences in major volatile compounds of red wines according to storage length and storage conditions. J. Food Compos. Anal. 2003, 16, 697–705. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Mensitieri, G.; Nicolais, L.; Masi, P. The influence of the thermal history on the shelf life of carbonated beverages bottled in plastic containers. J. Food Eng. 1997, 34, 1–13. [Google Scholar] [CrossRef]
- Heuberger, A.L.; Broeckling, C.D.; Lewis, M.R.; Salazar, L.; Bouckaert, P.; Prenni, J.E. Metabolomic profiling of beer reveals effect of temperature on non-volatile small molecules during short-term storage. Food Chem. 2012, 135, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Penner, M. Ultraviolet, Visible and Fluorescence Spectroscopy; Springer Science: New York, NY, USA, 2010. [Google Scholar]
- Bauer, R.; Nieuwoudt, H.; Bauer, F.F.; Kossman, J.; Koch, K.R.; Esbensen, K.H. FTIR spectroscopy for grape and wine analysis. Anal. Chem. 2008, 80, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.R. Spectroscopy in Food Research. J. Food Sci. 1938, 3, 121–125. [Google Scholar] [CrossRef]
- Cozzolino, D.; Holdstock, M.; Dambergs, R.G.; Cynkar, W.U.; Smith, P.A. Mid infrared spectroscopy and multivariate analysis: A tool to discriminate between organic and non-organic wines grown in Australia. Food Chem. 2009, 116, 761–765. [Google Scholar] [CrossRef]
- Bevin, C.J.; Dambergs, R.G.; Fergusson, A.J.; Cozzolino, D. Varietal discrimination of Australian wines by means of mid-infrared spectroscopy and multivariate analysis. Anal. Chim. Acta 2008, 621, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Cynkar, W.; Shah, N.; Smith, P. Technical solutions for analysis of grape juice, must, and wine: The role of infrared spectroscopy and chemometrics. Anal. Bioanal. Chem. 2011, 401, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W. Rapid quality control of spirit drinks and beer using multivariate data analysis of Fourier transform infrared spectra. Food Chem. 2007, 101, 825–832. [Google Scholar] [CrossRef]
- Gómez-Carracedo, M.; Andrade, J.; Fernández, E.; Prada, D.; Muniategui, S. Evaluation of the pure apple juice content in commercial apple beverages using FTMIR-ATR and potential curves. Spectrosc. Lett. 2004, 37, 73–93. [Google Scholar] [CrossRef]
- Sivakesava, S.; Irudayaraj, J.M.K.; Korach, R.L. Detection of adulteration in apple juice using mid infrared spectroscopy. Appl. Eng. Agric. 2001, 17, 815–820. [Google Scholar] [CrossRef]
- Reid, L.M.; Woodcock, T.; O’Donnell, C.P.; Kelly, J.D.; Downey, G. Differentiation of apple juice samples on the basis of heat treatment and variety using chemometric analysis of MIR and NIR data. Food Res. Int. 2005, 38, 1109–1115. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Kirsanov, D.; Legin, A.; Beullens, K.; Lammertyn, J.; Nicolaï, B.M.; Irudayaraj, J. Analysis of apples varieties—Comparison of electronic tongue with different analytical techniques. Sens. Actuators B 2006, 116, 23–28. [Google Scholar] [CrossRef]
- Fudge, A.L.; Wilkinson, K.L.; Ristic, R.; Cozzolino, D. Classification of Smoke Tainted Wines Using Mid-Infrared Spectroscopy and Chemometrics. J. Agric. Food Chem. 2011, 60, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Batten, G. Plant analysis using near infrared reflectance spectroscopy: The potential and the limitations. Anim. Prod. Sci. 1998, 38, 697–706. [Google Scholar] [CrossRef]
- Brereton, R.G. Introduction to multivariate calibration in analytical chemistry. Analyst 2000, 125, 2125–2154. [Google Scholar] [CrossRef]
- Garcia, S.; Santesteban, L.G.; Miranda, C.; Royo, J.B. Variety and storage time affect the compositional changes that occur in grape samples after frozen storage. Aust. J. Grape Wine Res. 2011, 17, 162–168. [Google Scholar] [CrossRef]
- Culbert, J.; Cozzolino, D.; Ristic, R.; Wilkinson, K. Classification of sparkling wine style and quality by MIR spectroscopy. Molecules 2015, 20, 8341–8356. [Google Scholar] [CrossRef] [PubMed]
- Iland, P.G.; Bruer, N.; Edwards, G.; Weeks, S.; Wilkes, E. Chemical Analysis of Grapes and Wine: Techniques and Concepts; Patrick Iland Wine Promotions: Adelaide, Australia, 2004. [Google Scholar]
- Moll, M.; European Brewery Convention. Method 8.3, E131. In Analytics-EBC, 4th ed.; Brauerei- und Getränke-Rundschau: Zurich, Switzerland, 1987. [Google Scholar]
- Hoff, S.; Lund, M.N.; Petersen, M.A.; Frank, W.; Andersen, M.L. Storage stability of pasteurized non-filtered beer. J. Inst. Brew. 2013, 119, 172–181. [Google Scholar] [CrossRef]
- Cozzolino, D.; Cynkar, W.; Shah, N.; Smith, P. Feasibility study on the use of attenuated total reflectance mid-infrared for analysis of compositional parameters in wine. Food Res. Int. 2011, 44, 181–186. [Google Scholar] [CrossRef]
- Gishen, M.; Cozzolino, D.; Dambergs, R.G. The analysis of grapes, wine, and other alcoholic beverages by infrared spectroscopy. In Handbook of Vibrational Spectroscopy; John Wiley and Sons: New York, NY, USA, 2006. [Google Scholar]
- Bevin, C.J.; Fergusson, A.J.; Perry, W.B.; Janik, L.J.; Cozzolino, D. Development of a rapid ”fingerprinting” system for wine authenticity by mid-infrared spectroscopy. J. Agric. Food Chem. 2006, 54, 9713–9718. [Google Scholar] [CrossRef] [PubMed]


| Carbonated Beverages | pH | TA (g/L) | Sugar (g/L) | Alcohol (% abv) | Total phenolics (au) | Color (au) | |
|---|---|---|---|---|---|---|---|
| Sparkling water | Mean a | 6.1 | 0.5 | nd | nd | nd | nd |
| Range | 5.9–6.1 | 0.1–0.9 | - | - | - | - | |
| Sparkling wine | Mean a | 3.1 | 6.6 | 12.7 | 11.7 | 3.6 | 0.1 |
| Range | 3.0–3.2 | 6.0–7.3 | 10.4–14.4 | 11.4–12.0 | 2.6–4.2 | 0.1–0.1 | |
| Beer A | Mean a | 4.1 | 2.0 | 0.2 | 4.5 | 11.1 | 8.8 |
| Range | 4.1–4.2 | 1.6–2.8 | 0.1–0.5 | 3.9–4.6 | 7.5–12.6 | 7.5–9.6 | |
| Beer B | Mean a | 4.1 | 2.0 | 0.1 | 4.5 | 14.1 | 10 |
| Range | 4.1–4.1 | 1.6–2.7 | 0.0–0.4 | 4.0–4.6 | 10.7–15.2 | 9.8–10.9 | |
| Beer C | Mean a | 4.1 | 1.8 | 0.2 | 3.4 | 11.0 | 9.0 |
| Range | 4.0–4.1 | 1.3–2.4 | 0.1–0.5 | 3.0–3.4 | 7.1–12.4 | 9.0–10.8 | |
| Beer D | Mean a | 4.1 | 1.2 | 0.2 | 2.4 | 9.0 | 10.9 |
| Range | 4.0–4.1 | 1.1–1.4 | 0.1–0.5 | 1.4–2.8 | 3.4–11.0 | 7.9–12.2 | |
| Cider A | Mean a | 3.3 | 6.8 | 20.5 | 5.0 | 17.0 | 1.5 |
| Range | 3.3–3.3 | 6.5–7.0 | 17.5-22.8 | 4.9–5.0 | 15.5–18.2 | 1.5–1.5 | |
| Cider B | Mean a | 3.6 | 6.4 | 21.7 | 4.7 | 29.7 | 3.0 |
| Range | 3.6–3.6 | 6.2–6.6 | 18.1–24.1 | 4.4–4.8 | 25.8–31.1 | 2.5–3.5 | |
| Cider C | Mean a | 3.5 | 7.1 | 21.7 | 4.9 | 18.1 | 2.0 |
| Range | 3.5–3.6 | 5.7–8.1 | 17.2–23.9 | 4.7–5.0 | 13.9–19.6 | 1.5–2.5 | |
| Cider D | Mean a | 3.1 | 5.4 | 11.0 | 4.9 | 14.7 | 1.5 |
| Range | 3.1–3.4 | 4.1–6.6 | 6.3–13.2 | 3.3–5.1 | 12.6–16.2 | 1.5–1.5 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pearce, K.; Culbert, J.; Cass, D.; Cozzolino, D.; Wilkinson, K. Influence of Sample Storage on the Composition of Carbonated Beverages by MIR Spectroscopy. Beverages 2016, 2, 26. https://doi.org/10.3390/beverages2040026
Pearce K, Culbert J, Cass D, Cozzolino D, Wilkinson K. Influence of Sample Storage on the Composition of Carbonated Beverages by MIR Spectroscopy. Beverages. 2016; 2(4):26. https://doi.org/10.3390/beverages2040026
Chicago/Turabian StylePearce, Karma, Julie Culbert, Diane Cass, Daniel Cozzolino, and Kerry Wilkinson. 2016. "Influence of Sample Storage on the Composition of Carbonated Beverages by MIR Spectroscopy" Beverages 2, no. 4: 26. https://doi.org/10.3390/beverages2040026






