Drink Red: Phenolic Composition of Red Fruit Juices and Their Sensorial Acceptance
Abstract
:1. General Introduction
2. Red Fruit Juices
3. Composition of Red Fruit Juices
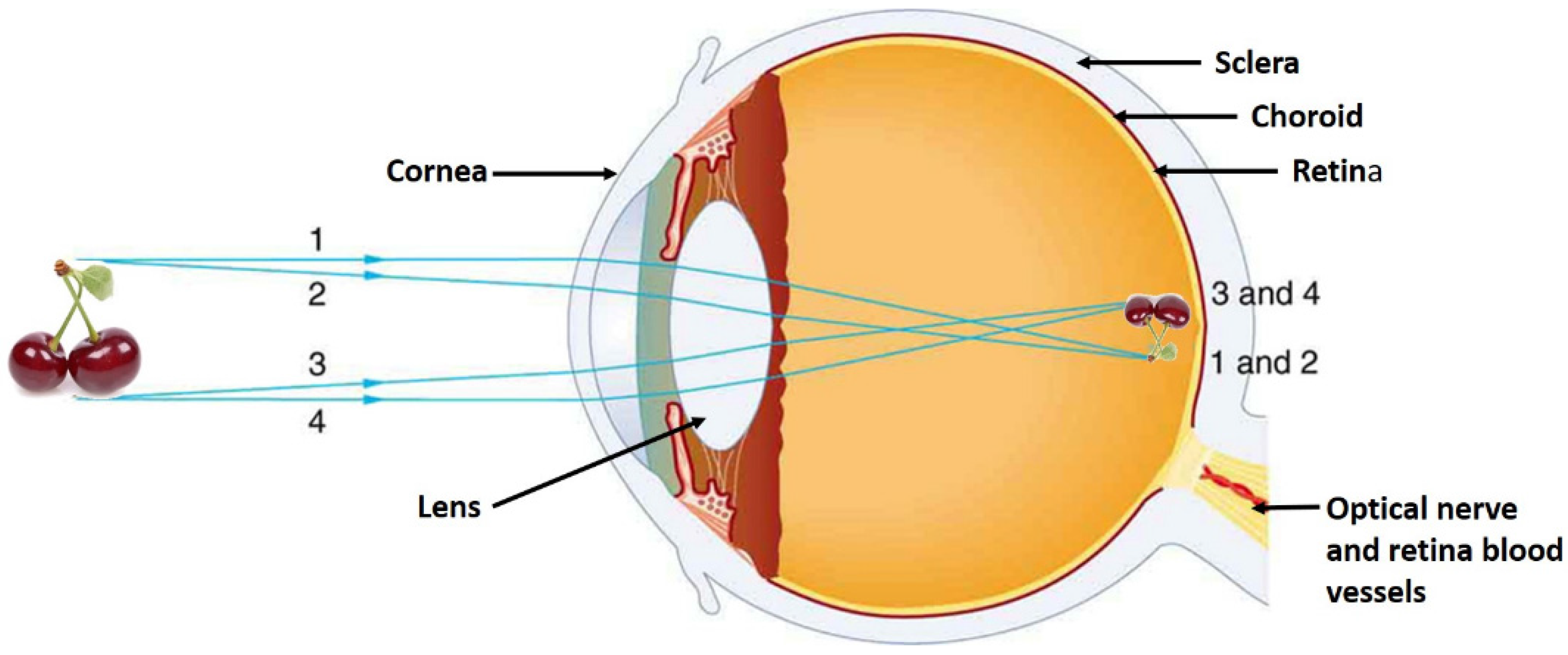
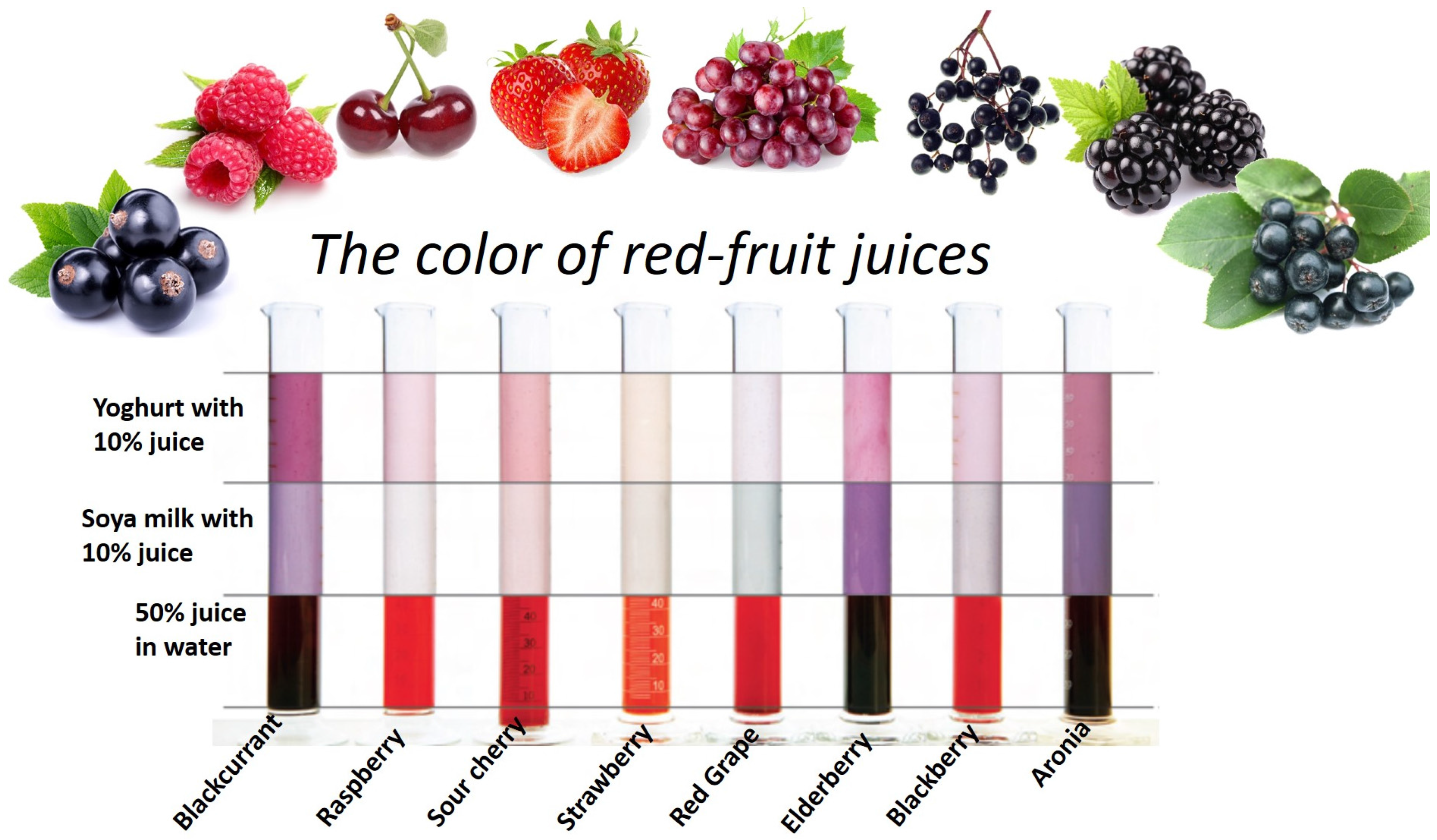
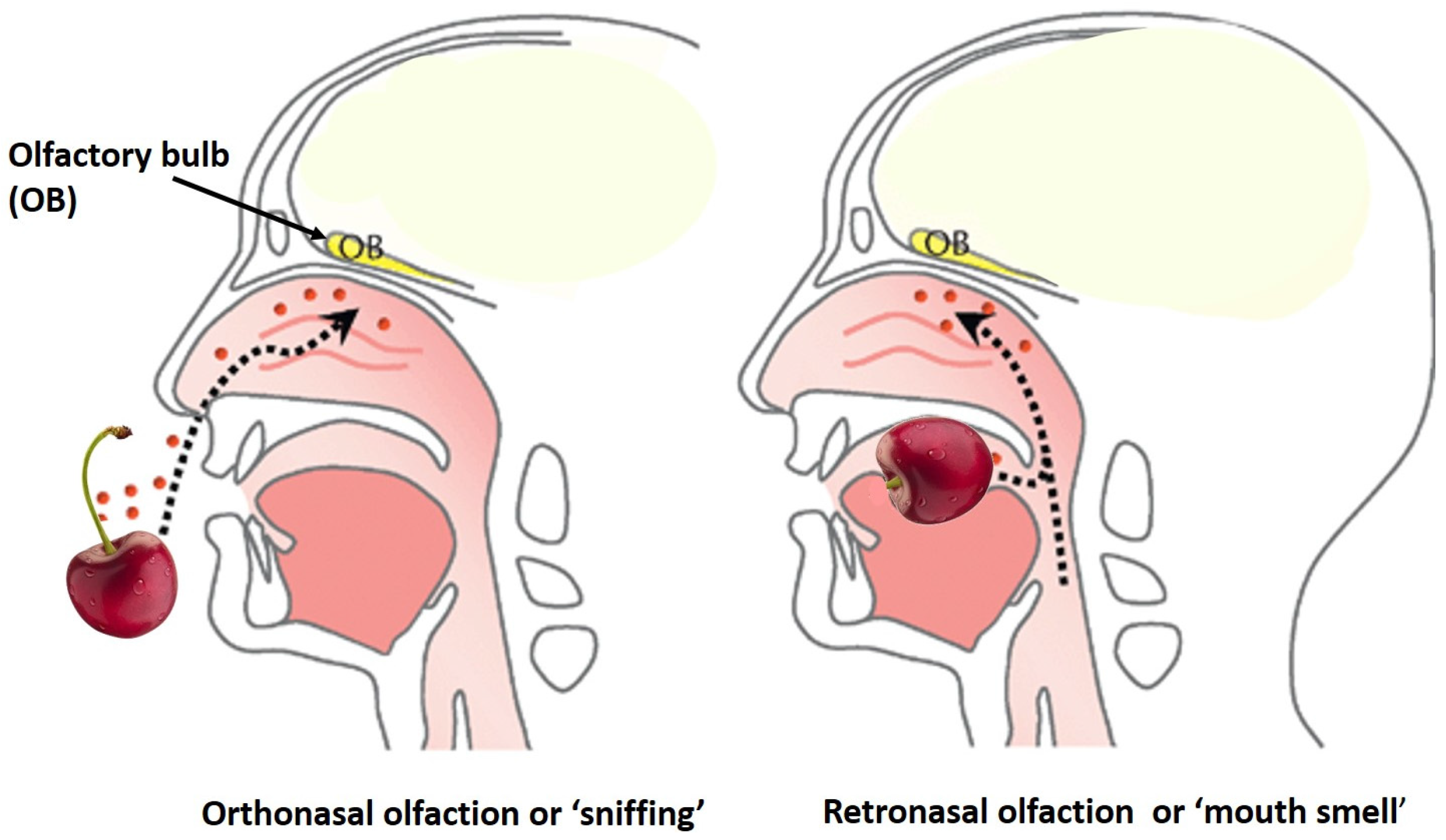
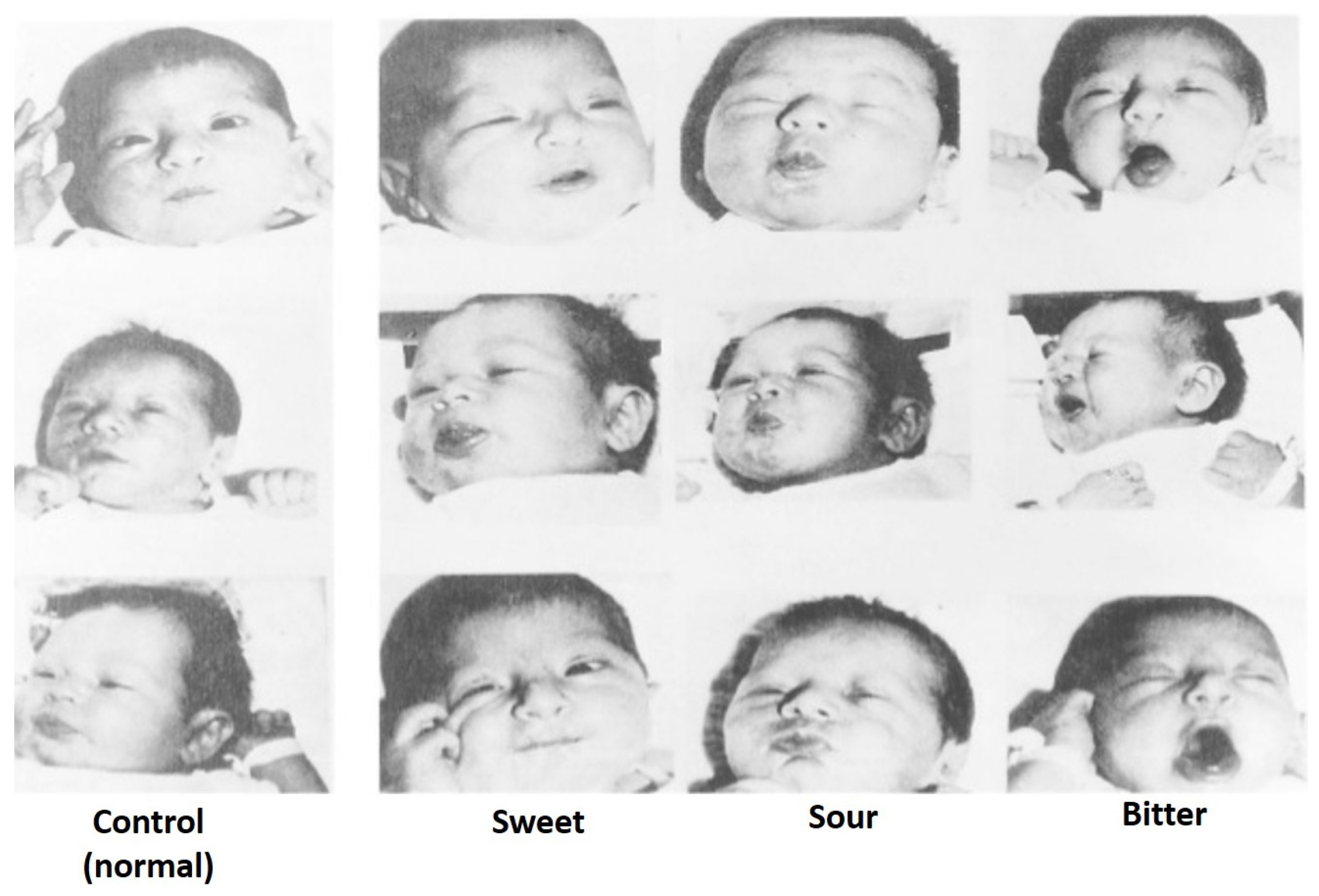
4. Color and Flavor Perception vs. Consumer Acceptance
5. Off Flavors Associated with Red Fruit Juices
5.1. Off Odors
5.2. Bitterness and Astringency
6. Taste and Flavor Modification Techniques
7. Final Remarks
Author Contributions
Conflicts of Interest
References
- IBISWorld. Global Fruit & Vegetables Processing; Industry Report; IBISWorld: Melbourne, Australia, 2016; p. 38. [Google Scholar]
- Hakkinen, S.; Heinonen, M.; Karenlampi, S.; Mykkanen, H.; Ruuskanen, J.; Torronen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food. Res. Int. 1999, 32, 345–354. [Google Scholar] [CrossRef]
- Asp, E.H. Factors affecting food decisions made by individual consumers. Food Policy 1999, 24, 287–294. [Google Scholar] [CrossRef]
- Bell, R.; Marshall, D.W. The construct of food involvement in behavioral research: Scale development and validation. Appetite 2003, 40, 235–244. [Google Scholar] [CrossRef]
- Delaney, M.; McCarthy, M. Food choice and health across the life course: A qualitative study examining food choice in older Irish adults. In Proceedings of the 113th EAAE Seminar “A Resilient European Food Industry and Food Chain in a Challenging World”, Chania, Crete, Greece, 3–6 September 2009.
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Ga, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Kyro, C.; Skeie, G.; Loft, S.; Landberg, R.; Christensen, J.; Lund, E.; Nilsson, L.M.; Palmqvist, R.; Tjonnelan, A.; Olsen, A. Intake of whole grains from different cereal and food sources and incidence of colorectal cancer in the Scandinavian HELGA cohort. Cancer Causes Control 2013, 24, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Virtanen, J.K.; Tuomainen, T.P.; Nurmi, T.; Voutilainen, S. Intake of fruit, berries, and vegetable and risk of type 2 diabetes in Finnish men: The kuopio ischaemic heart disease risk factor study. Am. J. Clin. Nutr. 2014, 9, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J. Association between vegetable, fruit and carbohydrate intake and breast cancer risk in relation to physical activity. Asian Pac. J. Cancer Prev. 2014, 15, 4429–4436. [Google Scholar] [CrossRef] [PubMed]
- Bates, R.P.; Morris, J.R.; Crandall, P.G. Principles and Practice of Small and Medium Scale Fruit Juice Processing; FAO Agricultural Services Bulletin; Food and Agricultural Organization: Rome, Italy, 2001; Volume 146. [Google Scholar]
- Prior, R.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R.L. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J. Agric. Food Chem. 1999, 47, 4638–4644. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Herrera, P.; Perez-Rodrıguez, M.-L.; Aguilera-Delgado, T.; Labari-Reyes, M.-J.; Olmedilla-Alonso, B.; Camara, M.; Pascual-Teresa, S. Anthocyanin profile of red fruits and black carrot juices, purees and concentrates by HPLC-DAD-ESI/MS-QTOF. Int. J. Food Sci. Technol. 2016, 51, 2290–2300. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Sánchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Mazza, G.J. Anthocyanins and heart health. Annali dell Istituto Superiore di Sanita 2007, 43, 369–374. [Google Scholar] [PubMed]
- Wang, L.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Serugi, M.; Medvidovic-Kosanovic, M.; Novak, I. Anthocyanin contente and antioxidante activity of various fruit juices. Deutsche Lebensmitlel-Rundschau 2007, 2, 58–64. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Burin, V.M.; Falcão, L.D.; Gonzaga, L.V.; Fett, R.; Rosier, J.P.; Bordignon-Luiz, M.T. Colour, phenolic content and antioxidant activity of grape juice. Ciência E Tecnologia De Alimentos 2010, 30, 1027–1032. [Google Scholar] [CrossRef]
- Rizzon, L.A.; Mielle, A. Analytical characteristics and discrimination of Brazilian commercial grape juice, nectar, and beverage. Ciência E Tecnologia De Alimentos 2012, 32, 93–97. [Google Scholar] [CrossRef]
- Voorpostel, C.R.; Dutra, M.B.L.; Bolini, H.M.A. Sensory profile and drivers of liking for grape néctar among smoker and nonsmoker consumers. Food Sci. Technol. 2014, 34, 164–173. [Google Scholar] [CrossRef]
- Yuste, J.; Fung, D.Y.C.; Thompson, L.K.; Crozier-Dodson, B.A. Combination of Carbon Dioxide and Cinnamon to Inactivate Escherichia coli O157:H7 in Apple Juice. J. Food Sci. 2002, 67, 3087–3090. [Google Scholar] [CrossRef]
- Manea, J. Evolution of bioactive compounds in fruit juices during preservation by refrigeration. Rev. Roum. Chim. 2013, 58, 619–622. [Google Scholar]
- Plaza, L.; Sánchez-Moreno, C.; Elez-Martínez, P.; de Ancos, B.; Martín-Belloso, O.; Cano, M.P. Effect of refrigerated storage on vitamin C and antioxidant activity of orange juice processes by high-pressure or pulsed electric fields with regard to low pasteurization. Eur. Food Res. Technol. 2006, 223, 487–493. [Google Scholar] [CrossRef]
- Kamal, R.A.; Romika, D.; Neeraj, K.A.; Ashish, A. Emerging preservation techniques for controlling spoilage and pathogenic microorganisms in fruit juices. Int. J. Food Microbiol. 2014, 2014, 758942. [Google Scholar]
- Obón, J.M.; Díaz-García, M.C.; Castellar, M.R. Red fruit juice quality and authenticity control by HPLC. J. Food Comp. Anal. 2011, 24, 760–771. [Google Scholar] [CrossRef]
- Da Silva, F.L.; Escribano-Bailón, M.T.; Alonso, J.J.P.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT-Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Stój, A.; Malik, A.; Targoński, Z. Comparative analysis of anthocyanin composition of juices obtained from selected species of berry fruits. Pol. J. Food Nutr. Sci. 2006, 15, 401–407. [Google Scholar]
- Goiffon, J.P.; Mouly, P.P.; Gaydou, E.M. Anthocyanic pigment determination in red fruit juices, concentrated juices and syrups using liquid chromatography. Anal. Chim. Acta 1999, 382, 39–50. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A. Evaluation of commercial red fruit juice concentrates as ingredients for antioxidant functional juices. Eur. Food Res. Technol. 2004, 219, 133–141. [Google Scholar] [CrossRef]
- Rubinskiene, M.; Jasutiene, I.; Venskutonis, P.R.; Viskelis, P. HPLC determination of the composition and stability of blackcurrant anthocyanins. J. Chromatogr. Sci. 2005, 43, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of procyanidins and anthocyanins in blueberry and cranberries (Vaccinium spp.) using high performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Versari, A.; Barbanti, D.; Biesenbruch, S.; Farnell, P.J. Analysis of anthocyanins in red fruits by use of HPLC/spectral array detection. Ital. J. Food Sci. 1997, 9, 141–148. [Google Scholar]
- Wu, X.; Prior, R. Systematic identification and characterization of anthocyanins by HPLC–ESI-MS/MS in common fruits in the United States: Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Lennie, P. The physiology of color vision. In The Science of Color; Elsevier: Amsterdam, The Netherlands, 2003; pp. 217–246. [Google Scholar]
- Kolb, H. Simple Anatomy of the Retina. The Organization of the Retina and Visual System. Webvision 2011. Available online: http://webvision.med.utah.edu/book/part-i-foundations/simple-anatomy-of-the-retina/ (accessed on 19 July 2016).
- Fairchild, M.D. Humam color vision. In Color Appearance Models, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 1–34. [Google Scholar]
- Lodish, H.; Berk, A.; Zipursky, S.L.; Matsudaria, P.; Baltimore, D.; Darnell, J. Sensory transduction. In Molecular Cell Biology, 4th ed.; W.H. Freeman: New York, NY, USA, 2000. [Google Scholar]
- Openstax. Physics of the Eye. 2016. Available online: http://cnx.org/contents/edFYqrQm@3/Physics-of-the-Eye (accessed on 19 July 2016).
- Piqueras-Fizman, B.; Spence, C. Sensory expectations based on product-extrinsic food cues: An interdisciplinary review of the empirical evidence and theoretical accounts. Food Qual. Prefer. 2015, 40, 165–179. [Google Scholar] [CrossRef]
- Roth, H.A.; Radle, L.J.; Gifford, S.R.; Clydesdale, F.M. Psychophysical relationships between perceived sweetness and color in lemon- and lime-flavored drinks. J. Food Sci. 1988, 53, 1116–1119. [Google Scholar] [CrossRef]
- Hutchings, J.B. Expectations and the Food Industry: The Impact of Color and Appearance; Kluwer Academic/Plenum Publisher: New York, NY, USA, 2003. [Google Scholar]
- Delwiche, J. The impact of perceptual interactions on perceived flavor. Food Qual. Prefer. 2004, 15, 137–146. [Google Scholar] [CrossRef]
- Morrot, G.; Brochet, F.; Dubourdieu, D. The color of odors. Brain Lang. 2001, 79, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Spence, C. On the psychological impact of food colour. Flavour 2015, 4. [Google Scholar] [CrossRef]
- Berryman, D. The use of fruit juices as natural colouring agents. New Food Beverage Process. Suppl. 2014, 17, 25–29. [Google Scholar]
- Keast, R.S.J.; Costanzo, A. Is fat the sixth taste primary? Evidence and implications. Flavour 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Spence, C. Multisensory flavour perception. Curr. Biol. 2013, 23, R365–R369. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.; Doolittle, N.; Nagata, H.; Breslin, P.A.S. The merging of the senses: Integration of subthreshold taste and smell. Nat. Neurosci. 2003, 3, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.E. Taste and flavour: Their importance in food choice and acceptance. Proc. Nutr. Soc. 1998, 57, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.E. Facial expressions of the neonate infant indicating the hedonics of food related chemical stimuli. In Taste and Development: The Genesis of Sweet Preference; DHEW Publication No. NIH 77-1068; United States Department of Health, Education, and Welfare: Bethesda, MD, USA, 1977; pp. 173–189. [Google Scholar]
- Spence, C.; Levitan, C.; Shankar, M.U.; Zampini, M. Does food color influence taste and flavor perception in humans? Chemosens. Percept. 2010, 3, 68–84. [Google Scholar] [CrossRef]
- Stevenson, R.J. The Psychology of Flavour; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Fallico, B.; Arena, E.; Chiappara, E.; Ballistreria, G. Colour and label evaluation of commercial pasteurised red juices and related drinks. Food Addit. Contam. B Surveill. 2010, 3, 201–211. [Google Scholar] [CrossRef] [PubMed]
- McGorrin, R.J. The significance of volatile sulfur compounds in food flavors: An overview. In Volatile Sulfur Compounds in Food; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011. [Google Scholar]
- Goeke, A. Sulfur-containing odorants in fragrance chemistry. Sulfur Rep. 2002, 23, 243–278. [Google Scholar] [CrossRef]
- Boelens, M.H.; van Gemert, L.J. Volatile character-impact sulfur compounds and their sensory properties. Perfum. Flavorist 1993, 18, 29–39. [Google Scholar]
- Darriet, P.; Lavigne, V.; Boidron, J.; Dubourdieu, D. Identification of a powerful aromatic component of Vitis. vinifera L. var. Sauvignon Wines: 4-Mercapto-4-methylpentan-2-one. Flavour Fragr. J. 1995, 10, 385–392. [Google Scholar] [CrossRef]
- Kolor, M.G. Identification of an important new flavor compound in Concord grape: ethyl-3-mercaptopropionate. J. Agric. Food Chem. 1983, 31, 1125–1127. [Google Scholar] [CrossRef]
- Schulbach, K.F.; Rouseff, R.L.; Sims, C.A.J. Changes in Volatile Sulfur Compounds in Strawberry Puree during Heating. Food Sci. 2004, 69, FCT268–FCT272. [Google Scholar] [CrossRef]
- Boccorh, R.K.; Paterson, A.; Piggot, J.R. Extraction of aroma components to quantify overall, sensory character in a processed blackcurrant (Ribes nigrum L.) concentrate. Flavour Fragr. J. 2002, 17, 385–391. [Google Scholar] [CrossRef]
- Varming, C.; Petersen, M.A.; Poll, L.J. Comparison of isolation methods for the determination of important aroma compounds in black currant (Ribes nigrum L.) juice, using nasal impact frequency profiling. Agric. Food Chem. 2004, 52, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Gerber, N.N. Three highly odorous metabolites from an Actinomycete: 2-isopropyl-3-methoxypyrazine, methylisoborneol and geosmin. J. Chem. Ecol. 1977, 3, 475–482. [Google Scholar] [CrossRef]
- Splittstoesser, D.F. Microorganisms involved in the spoilage of fermented fruit juice. J. Food Protect. 1982, 45, 874–877. [Google Scholar]
- Bevilacqua, A.; Corbo, M.R.; Campaniello, D.; D’Amato, D.; Gallo, M.; Speranza, B.; Sinigaglia, M. Shelf life prolongation of fruit juices through essential oils and homogenization: A review. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; Microbiology Series No 3; Volume 1, pp. 1157–1166. [Google Scholar]
- Siegmund, B.; Pöllinger-Zierler, B. Odor thresholds of microbially induced off-flavor compounds in apple juice. J. Agric. Food Chem. 2006, 54, 5984–5989. [Google Scholar] [CrossRef] [PubMed]
- Maga, J.A. Compound structure versus bitter taste. In Bitterness in Foods and Beverages. Developments in Food Science; Rousseff, R.L., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; Volume 25, pp. 35–48. [Google Scholar]
- Schobinger, U. Frucht-und Gemüsesäfte, Handbuch der Lebensmitteltechnologie; Verlag Eugen Ulmer: Stuttgart, Germany, 2001; pp. 506–635. [Google Scholar]
- Billing, J.; Sherman, P.W. Antimicrobial function of spices: Why some like it hot. Q. Rev. Biol. 1998, 73, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Profet, M.; Gold, L.S. Dietary pesticides (99.99% all natural). Proc. Natl. Acad. Sci. USA 1990, 87, 777–781. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Rouseff, R.L. Bitterness in food products: An overview. In Bitterness in Foods and Beverages. Developments in Food Science; Rousseff, R.L., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; Volume 25, pp. 1–14. [Google Scholar]
- Fenwick, G.R.; Curl, C.L.; Griffiths, N.M.; Heaney, R.K.; Price, K.R. Bitter principles in food plants. In Bitterness in Foods and Beverages. Developments in Food Science; Rousseff, R.L., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; Volume 25, pp. 205–250. [Google Scholar]
- Mikkelsen, B.B.; Poll, L. Decomposition and transformation of aroma compounds and anthocyanins during black currant (Ribes nigrum L.) juice processing. J. Food Sci. 2002, 67, 3447–3455. [Google Scholar] [CrossRef]
- Laaksonen, O.; Sandell, M.; Nordlund, E.; Heiniö, R.-L.; Malinen, H.-L.; Jaakkola, M.; Kallio, H. The effect of enzymatic treatment on blackcurrant (Ribes nigrum) juice flavour and its stability. Food Chem. 2012, 130, 31–41. [Google Scholar] [CrossRef]
- Laaksonen, O.; Ahola, J.; Sandell, M. Explaining and predicting individually experienced liking of berry fractions by the hTAS2R38 taste receptor genotype. Appetite 2013, 61, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Lawlor, J.B.; Chandra, S.; Chaya, C.; Hewson, L.; Hort, J. Using quantitative descriptive analysis and temporal dominance of sensations analysis as complementary methods for profiling commercial blackcurrant squashes. Food Qual. Prefer. 2012, 25, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Verbeke, W. Functional foods. Consumer willingness to compromise on taste for health? Food Qual. Prefer. 2006, 17, 126–131. [Google Scholar] [CrossRef]
- Muir, D.D.; Hunter, E.A.; Williams, S.A.R.; Brennan, R.M. Sensory profiles of commercial fruit juice drinks: Influence of sweetener type. J. Agric.Food. Chem. 1998, 77, 559–565. [Google Scholar] [CrossRef]
- Laaksonen, O.; Mäkilä, L.; Tahvonen, R.; Kallio, H.; Yang, B. Sensory quality and compositional characteristics of blackcurrant juices produced by different processes. Food Chem. 2013, 138, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, O.A.; Mäkilä, L.; Sandell, M.A.; Salminen, J.-P.; Liu, P.; Kallio, H.P.; Yang, B. Chemical-sensory characteristics and consumer responses of blackcurrant juices produced by different industrial processes. Food Bioprocess. Technol. 2014, 7, 2877–2888. [Google Scholar] [CrossRef]
| Red Fruit Juice | Anthocyanin Profile |
|---|---|
| Strawberry | Cyanidin 3-glucoside, pelargonidin 3-glucoside, and pelargonidin 3-rutinoside |
| Red raspberry | Cyanidin 3-sophoroside, cyanidin 3-glucosyl-rutinoside, cyanidin 3-glucoside, pelargonidin 3-sophoroside, and cyanidin 3-rutinoside |
| Blackcurrant | Delphinidin 3-glucoside, delphinidin 3-rutinoside, cyanidin 3-glucoside, cyanidin 3-rutinoside, and delphinidin 3-rutinoside |
| Blueberry | Dephinidin 3-galactoside, delphinidin 3-glucoside, cyanidin 3-galactoside, delphinidin 3-arabinoside, cyanidin 3-glucoside, petunidin 3-galactoside, cyanidin 3-arabinoside, petunidin 3-glucoside, peonidin 3-galactoside, petunidin 3-arabinoside, peonidin 3-glucoside, malvidin 3-galactoside, malvidin 3-glucoside, and malvidin 3-arabinoside |
| Grapes | Delphinidin 3-glucoside, cyanidin 3-glucoside, petunidin 3-glucoside, peonidin 3-glucoside and malvidin 3-glucoside |
| Character Impact Compound | Odor Description | Occurrence | Reference |
|---|---|---|---|
| 4-Methoxy-2-methyl-2-butanethiol | Blackcurrant sulfur | Blackcurrant | [59] |
| 8-Mercapto-p-menthan-3-one | Blackcurrant catty | Blackcurrant (synthetic) | [60] |
| 4-Mercapto-4-methyl-2-pentanone | Cat urine | Sauvignon grape | [61] |
| Ethyl-3-mercaptopropionate | Fresh grape foxy | Concord grape | [62] |
| Methyl thioacetate | Cheesy garlic | Strawberry | [63] |
| Methyl thiobutanoate | Cheesy cabbage | Strawberry | [63] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, A.; Cosme, F. Drink Red: Phenolic Composition of Red Fruit Juices and Their Sensorial Acceptance. Beverages 2016, 2, 29. https://doi.org/10.3390/beverages2040029
Vilela A, Cosme F. Drink Red: Phenolic Composition of Red Fruit Juices and Their Sensorial Acceptance. Beverages. 2016; 2(4):29. https://doi.org/10.3390/beverages2040029
Chicago/Turabian StyleVilela, Alice, and Fernanda Cosme. 2016. "Drink Red: Phenolic Composition of Red Fruit Juices and Their Sensorial Acceptance" Beverages 2, no. 4: 29. https://doi.org/10.3390/beverages2040029








