Elemental Profiles of Whisk(e)y Allow Differentiation by Type and Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Instrumentation
2.3. Data Processing and Analysis
3. Results and Discussion
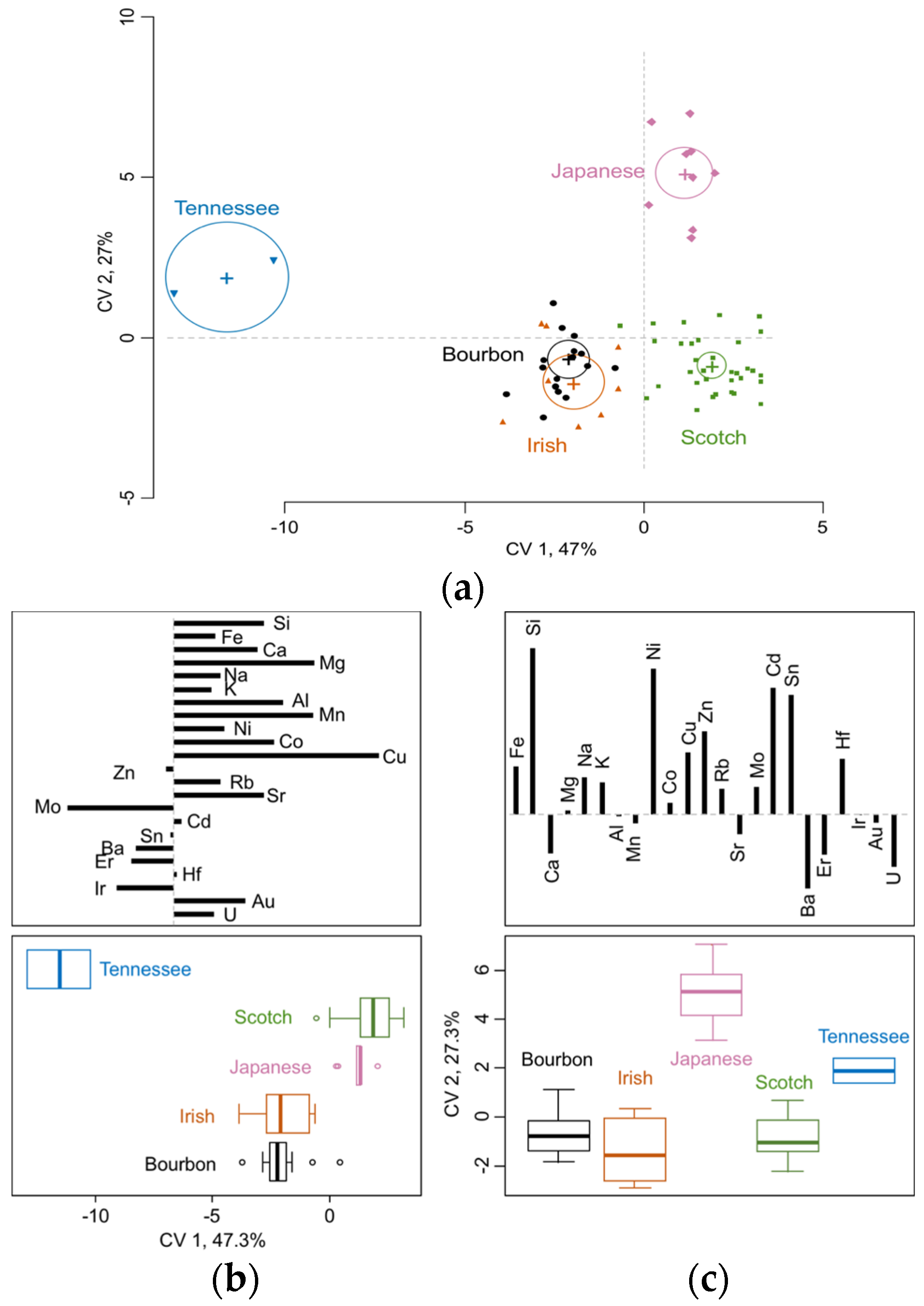
3.1. Whisk(e)y Types Differ Significantly in Elemental Composition
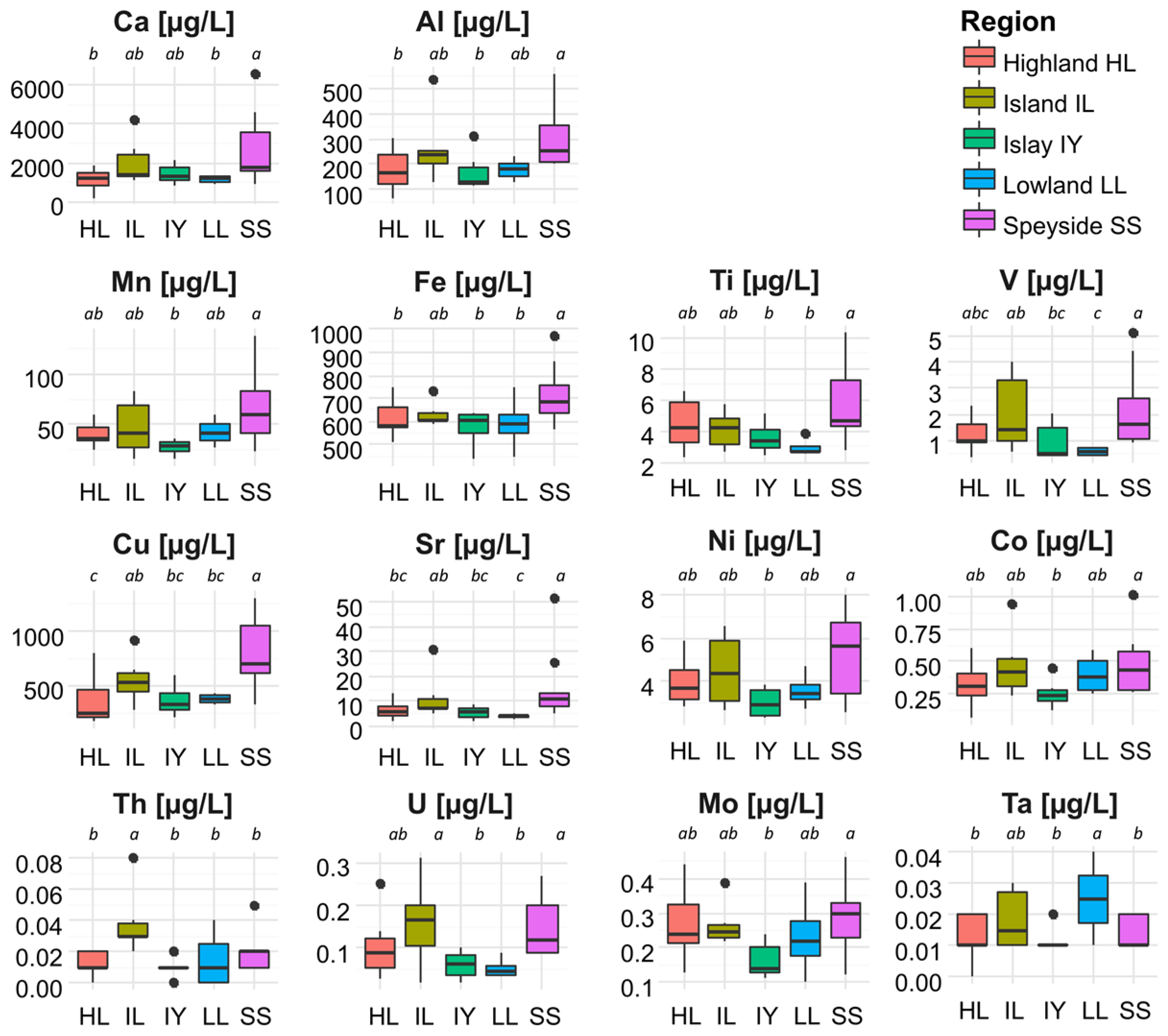
3.2. Single Malt Scotch Whiskies Differ in Their Elemental Composition across Production Areas
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| CVA | Canonical Variate Analysis |
| ICP-MS | Inductively Coupled Plasma-Mass Spectrometry |
| IS | Internal Standard |
| LSD | Fisher’s Least Significant Differences |
| MANOVA | Multivariate Analysis of Variance |
| MP-AES | Microwave Plasma-Atomic Emission Spectroscopy |
| QC | Quality Control |
References
- Stewart, G.; Russell, I.; Anstruther, A. An Introduction to Whisk(e)y and the Development of Scotch Whisky, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2014. [Google Scholar]
- Lyons, T.P. North American Whiskies: A Story of Evolution, Experience, and an Ongoing Entrepreneurial Spirit, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2014. [Google Scholar]
- Quinn, D. Irish Whiskey: Technology, Production & Marketing, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2014. [Google Scholar]
- Fukuyo, S.; Myojo, Y. Japanese Whisky, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2014. [Google Scholar]
- Aylott, R.I.; Clyne, A.H.; Fox, A.P.; Walker, D.A. Analytical Strategies to Confirm Scotch Whisky Authenticity. Analyst 1994, 119, 1741–1746. [Google Scholar] [CrossRef]
- Collins, T.S.; Zweigenbaum, J.; Ebeler, S.E. Profiling of nonvolatiles in whiskeys using ultra high pressure liquid chromatography quadrupole time-of-flight mass spectrometry (UHPLC–QTOF MS). Food Chem. 2014, 163, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arjona, D.; Gonzallez-Gallero, V.; Pablos, F.; Gustavo Gonzallez, A. Authentication and differentiation of Irish whiskeys by higher-alcohol congener analysis. Anal. Chim. Acta 1999, 381, 257–264. [Google Scholar] [CrossRef]
- Saxberg, B.E.H.; Duewer, D.L.; Booker, J.L.; Kowalski, B.R. Pattern recognition and blind assay techniques applied to forensic separation of whiskies. Anal. Chim. Acta 1978, 103, 201–212. [Google Scholar] [CrossRef]
- Lee, K.Y.M.; Paterson, A.; Piggott, J.R.; Richardson, G.D. Sensory discrimination of blended Scotch whiskies of different product categories. Food Qual. Prefer. 2001, 12, 109–117. [Google Scholar] [CrossRef]
- Jack, F.R.; Steele, G.M. Modelling the sensory characteristics of Scotch whisky using neural networks—A novel tool for generic protection. Food Qual. Prefer. 2002, 13, 163–172. [Google Scholar] [CrossRef]
- Aylott, R. Whisky Analysis, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2014. [Google Scholar]
- Woods, G. Measurement of Trace Elements in Malt Spirit Beverages (Whisky) by 7500cx ICP-MS Agilent Application Note. Available online: https://www.agilent.com/cs/library/applications/5989-7214EN.pdf (accessed on 1 January 2015).
- Adam, T.; Duthie, E.; Feldmann, J.; Park, H. Investigations into the use of copper and other metals as indicators for the authenticity of Scotch whiskies. J. Inst. Brew. 2002, 108, 459–464. [Google Scholar] [CrossRef]
- Pryde, J.; Conner, J.; Jack, F.; Lancaster, M.; Meek, L.; Owen, C.; Paterson, R.; Steele, G.; Strang, F.; Woods, J. Sensory and Chemical Analysis of “Shackleton”s’ Mackinlay Scotch Whisky. J. Inst. Brew. 2011, 117, 156–165. [Google Scholar] [CrossRef]
- Distilled Spirits Council of the United States Scotch: Take the High Road. Available online: http://www.discus.org/assets/1/7/Scotch_2015.pdf (accessed on 1 July 2016).
- Distilled Spirits Council of the United States on America’s Whiskey Trail. Available online: http://www.discus.org/assets/1/7/Bourbon_and_Tennessee_Whiskey_2014.pdf (accessed on 1 July 2016).
- Heymann, H.; Robinson, A.L.; Buscema, F.; Stoumen, M.E.; King, E.S.; Hopfer, H.; Boulton, R.B.; Ebeler, S.E. Effect of Region on the Volatile Composition and Sensory Profiles of Malbec and Cabernet Sauvignon Wines. In Advances in Wine Research; Ebeler, S.E., Ed.; American Chemical Society: Washington, DC, USA, 2015; pp. 109–122. [Google Scholar]
- Nelson, J.; Hopfer, H.; Gilleland, G.; Cuthbertson, D.; Boulton, R.B.; Ebeler, S.E. Elemental Profiling of Malbec Wines under Controlled Conditions Using Microwave Plasma-Atomic Emission Spectroscopy. Am. J. Enol. Vitic. 2015, 66, 373–378. [Google Scholar] [CrossRef]
- Hopfer, H.; Nelson, J.; Collins, T.S.; Heymann, H.; Ebeler, S.E. The combined impact of vineyard origin and processing winery on the elemental profile of red wines. Food Chem. 2015, 172, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Woods, G. Trace Elemental Analysis of Distilled Alcoholic Beverages Using the Agilent 7700x ICP-MS with Octopole Collision/Reaction Cell Agilent Application Note. Available online: https://www.agilent.com/cs/library/applications/5990-9971EN_AppNote_7700_Alcohol.pdf (accessed on 3 March 2015).
- Goossens, J.; Smaele, T.; Moens, L.; Dams, R. Accurate determination of lead in wines by inductively coupled plasma mass spectrometry. Fresenius J. Anal. Chem. 1993, 347, 119–125. [Google Scholar] [CrossRef]
- Boorn, A.W.; Browner, R.F. Effects of organic solvents in inductively coupled plasma atomic emission spectrometry. Anal. Chem. 1982, 54, 1402–1410. [Google Scholar] [CrossRef]
- Dams, R.F.J.; Goossens, J.; Moens, L. Spectral and non-spectral interferences in inductively coupled plasma mass-spectrometry. Mikrochim. Acta 1995, 119, 277–286. [Google Scholar] [CrossRef]
- Rossano, E.C.; Szilágyi, Z.; Malorni, A.; Pocsfalvi, G. Influence of winemaking practices on the concentration of rare earth elements in white wines studied by inductively coupled plasma mass spectrometry. J. Agric. Food Chem. 2007, 55, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Drvodelic, N.; Cauduro, J. Determination of Metals in Wine Using the Agilent 4100 Microwave Plasma-Atomic Emission Spectrometer Agilent Application Note. Available online: https://www.agilent.com/cs/library/applications/5991-1586EN_AppNote_4100MP-AES_Food_wine.pdf (accessed on 2 July 2016).
- Thomsen, V.; Schatzlein, D.; Mercuro, D. Limits of Detection in Spectroscopy. Spectroscopy 2003, 18, 112–114. [Google Scholar]
- Hopfer, H.; Nelson, J.; Mitchell, A.E.; Heymann, H.; Ebeler, S.E. Profiling the trace metal composition of wine as a function of storage temperature and packaging type. J. Anal. At. Spectrom. 2013, 28, 1288–1291. [Google Scholar] [CrossRef]
- Croghan, C.W.; Egeghy, P.P. Methods of Dealing with Values below the Limit of Detection Using SAS; Southern SAS User Group: St. Petersburg, FL, USA, 2003. [Google Scholar]
- Chatfield, C.; Collins, A.J. Introduction to Multivariate Analysis; CRC Press: Boca Raton, FL, USA, 1980. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. Available online: http://cran.r-project.org/package=agricolae (accessed on 3 January 2014).
- Friendly, M.; Fox, J. Candisc: Generalized Canonical Discriminant Analysis. Available online: http://cran.r-project.org/package=candisc (accessed on 3 January 2014).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions. Available online: http://cran.r-project.org/package=cluster (accessed on 3 January 2014).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Wickham, H. Reshaping Data with the reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F.F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Husson, F.; Josse, J.; Lê, S.; Mazet, J. FactoMineR: Multivariate Exploratory Data Analysis and Data Mining with R. Available online: http://cran.r-project.org/package=FactoMineR (accessed on 3 January 2016).
- Bartlett, M.S. Further aspects of the theory of multiple regression. Math. Proc. Camb. Philos. Soc. 1938, 34, 33–40. [Google Scholar] [CrossRef]
- State of Tennessee Public Chapter No. 341 House Bill 1084. Available online: http://www.tn.gov/sos/acts/108/pub/pc0341.pdf (accessed on 10 August 2016).
- International Molybdenum Association Metallurgy of Mo in Stainless Steel. Available online: http://www.imoa.info/molybdenum-uses/molybdenum-grade-stainless-steels/metallurgy-of-molybdenum-in-stainless-steel.php (accessed on 10 August 2016).
- International Stainless Steel Forum (ISSF) Commercial Food Equipment: The Ferritic Solution. Available online: http://www.outokumpu.com/sitecollectiondocuments/commercialfoodequipmenttheferriticsolution1.pdf (accessed on 10 August 2016).
- Harrison, B.; Fagnen, O.; Jack, F.; Brosnan, J. The Impact of Copper in Different Parts of Malt Whisky Pot Stills on New Make Spirit Composition and Aroma. J. Ind. Microbiol. Biotechnol. 2011, 117, 106–112. [Google Scholar] [CrossRef]
- Clayhammer Supply Copper Stills vs. Stainless Steel Stills. Available online: http://www.clawhammersupply.com/blogs/moonshine-still-blog/54804996-copper-stills-vs-stainless-steel-stills (accessed on 15 August 2016).
- Ibanez, J.G.; Carreon-Alvarez, A.; Barcena-Soto, M.; Casillas, N. Metals in alcoholic beverages: A review of sources, effects, concentrations, removal, speciation, and analysis. J. Food Compos. Anal. 2008, 21, 672–683. [Google Scholar] [CrossRef]
- Copper Development Association Inc. Copper Facts. Available online: https://www.copper.org/education/c-facts/ (accessed on 10 August 2016).
- Fermentation Waters. Available online: http://whiskyscience.blogspot.com/2014/04/fermentation-waters.html (accessed on 8 January 2016).
- American Water Works Association Strontium. Available online: http://www.drinktap.org/water-info/whats-in-my-water/strontium.aspx (accessed on 8 January 2016).
- Daguillard, R. EPA Makes Preliminary Determination to Regulate Strontium in Drinking Water. Available online: https://www.gpo.gov/fdsys/pkg/FR-2016-01-04/html/2015-32760.htm (accessed on 10 August 2016).
- US Environmental Protection Agency (EPA). 40 CRF Part 141. Announcement of Final Regulatory Determinations for Contaminants on the Third Drinking Water Contaminant Candidate List; US Environmental Protection Agency (EPA): Washington, DC, USA, 2016.
- British Geological Survey Strontium in Stream Sediment. Available online: http://www.largeimages.bgs.ac.uk/iip/mapsportal.html?id=1007723 (accessed on 12 August 2016).
- Volpe, M.G.; la Cara, F.; Volpe, F.; de Mattia, A.; Serino, V.; Petitto, F.; Zavalloni, C.; Limone, F.; Pellecchia, R.; de Prisco, P.P.; et al. Heavy metal uptake in the enological food chain. Food Chem. 2009, 117, 553–560. [Google Scholar] [CrossRef]
- Teissedre, P.L.; Krosniak, M.; Portet, K.; Gasc, F.; Waterhouse, A.L.; Serrano, J.J.; Cabanis, J.C.; Cros, G. Vanadium levels in French and Californian wines: Influence on vanadium dietary intake. Food Addit. Contam. 1998, 15, 585–591. [Google Scholar] [CrossRef] [PubMed]
- 1While distilled grain spirits from Scotland and Ireland are spelled whisky and whiskies, respectively, whiskey and whiskeys are the forms used for New World products. Japanese products are spelled whisky, an indication of the strong influence of the Scottish process.
| Code 1 | Age 2 | Proof 3 | Distillery 4 | Code 1 | Age 2 | Proof 3 | Distillery 4 |
|---|---|---|---|---|---|---|---|
| B1 | 7 | 107 | D1 | S1 | 10 | 86 | D17 (IL) |
| B2 | 8 | 80 | D1 | S2 | 10 | 92 | D18 (IY) |
| B3 | N.A. | 90 | D2 | S3 | 12 | 80 | D19 (LL) |
| B4 | N.A. | 100 | D2 | S4 | 18 | 86 | D19 (LL) |
| B5 | 10 | 90 | D2 | S5 | 27 | 116 | D19 (LL) |
| B6 | N.A. | 86.6 | D2 | S6 | 12 | 92.6 | D20 (IY) |
| B7 | N.A. | 100 | D3 | S7 | 12 | 86 | D21 (IY) |
| B8 | 12 | 86 | D1 | S8 | N.A. | 88 | D22 (HL) |
| B9 | N.A. | 101 | D4 | S9 | 10 | 80 | D23 (HL) |
| B10 | N.A. | 90 | D5 | S10 | 10 | 80 | D23 (HL) |
| B11 | 9 | 100 | D1 | S11 | 12 | 80 | D24 (SS) |
| B12 | N.A. | 114 | D4 | S12 | 15 | 92 | D24 (SS) |
| B13 | N.A. | 90.2 | D6 | S13 | 12 | 80 | D25 (SS) |
| B14 | N.A. | 90.4 | D7 | S14 | 10 | 80 | D26 (SS) |
| B15 | 12 | 90 | D2 | S15 | 15 | 92 | D26 (SS) |
| B16 | N.A. | 113 | D8 | S16 | 21 | 86 | D26 (SS) |
| I1 | N.A. | 80 | D9 | S17 | 12 | 80 | D27 (SS) |
| I2 | N.A. | 80 | D10 | S18 | 15 | 80 | D27 (SS) |
| I3 | N.A. | 80 | D11 | S19 | 12 | 86 | D28 (LL) |
| I4 | 8 | 80 | D12 | S20 | 12 | 86 | D29 (HL) |
| I5 | N.A. | 80 | D12 | S21 | 10 | 80 | D30 (HL) |
| I6 | 12 | 115 | D13 | S22 | 12 | 80 | D31 (IL) |
| I7 | 15 | 92 | D13 | S23 | 18 | 86 | D31 (IL) |
| I8 | 12 | 92 | D13 | S24 | 10 | 80 | D32 (IL) |
| J1 | 12 | 86 | D14 | S25 | 16 | 80 | D32 (IL) |
| J2 | 12 | 86 | D14 | S26 | 16 | 86 | D33 (IY) |
| J3 | N.A. | 96 | D15 | S27 | 10 | 80 | D34 (IY) |
| J4 | N.A. | 110 | D15 | S28 | 15 | 86 | D34 (IY) |
| J5 | 10 | 90 | D16 | S29 | 12 | 80 | D35 (SS) |
| J6 | 12 | 90 | D16 | S30 | 12 | 80 | D36 (HL) |
| J7 | N.A. | 80 | D16 | S31 | 16 | 80 | D37 (IL) |
| J8 | 17 | 86 | D16 | S32 | 15 | 92 | D38 (CA) |
| J9 | 12 | 80 | D16 | S33 | 10 | 86 | D30 (HL) |
| T1 | N.A. | 90 | D39 | ||||
| T2 | N.A. | 80 | D40 |
| Element | LOD (µg/L) | Min-Max (µg/L) | Element | LOD (µg/L) | Min-Max (µg/L) |
|---|---|---|---|---|---|
| 27 Al | 0.442 | 47.2–1940 | 140 Ce | 0.0006 | <LOD–0.708 |
| 47 Ti | 0.146 | 1.88–12.9 | 141 Pr | 0.0003 | 0.001–0.100 |
| 51 V | 0.007 | 0.161–18.7 | 146 Nd | 0.0013 | <LOD–0.393 |
| 52 Cr | 10.1 | 175–1380 | 147 Sm | 0.0005 | <LOD–0.094 |
| 55 Mn | 0.019 | 1.05–233 | 153 Eu | 0.0003 | 0.0003–0.056 |
| 56 Fe | 7.01 | 393–1100 | 157 Gd | 0.0006 | <LOD–0.178 |
| 58 Ni | 0.027 | 1.52–37.5 | 163 Dy | 0.0004 | <LOD–0.142 |
| 59 Co | 0.002 | 0.024–3.13 | 165 Ho | 0.0001 | 0.0001–0.029 |
| 63 Cu | 0.022 | 4.99–2380 | 166 Er | 0.0002 | 0.0009–0.136 |
| 66 Zn | 0.125 | 0.013–586 | 169 Tm | 0.0002 | 0.0003–0.014 |
| 71 Ga | 0.002 | 0.034–0.490 | 172 Yb | 0.0004 | <LOD–0.085 |
| 75 As | 0.002 | 0.098–22.4 | 175 Lu | 0.024 | <LOD–1.32 |
| 82 Se | 0.016 | <LOD–0.720 | 178 Hf | 0.008 | 0.048–0.558 |
| 85 Rb | 0.013 | 3.35–56.0 | 181 Ta | 0.002 | 0.005–0.042 |
| 88 Sr | 0.009 | 1.29–52.2 | 182 W | 0.006 | 0.090–0.832 |
| 90 Zr | 0.010 | <LOD–1.55 | 185 Re | 0.0004 | <LOD–0.012 |
| 93 Nb | 0.002 | 0.020–0.159 | 193 Ir | 0.014 | <LOD–0.084 |
| 97 Mo | 0.007 | <LOD–3.72 | 195 Pt | 0.007 | <LOD–0.298 |
| 101 Ru | 0.001 | <LOD–0.014 | 197 Au | 0.015 | <LOD–0.105 |
| 103 Rh | 0.002 | 0.056–0.131 | 205 Tl | 0.001 | <LOD–0.467 |
| 105 Pd | 0.041 | 2.04–5.05 | 208 Pb | 0.004 | <LOD–9.68 |
| 107 Ag | 0.004 | <LOD–1.15 | 232 Th | 0.001 | <LOD–0.092 |
| 114 Cd | 0.003 | <LOD–9.82 | 238 U | 0.001 | <LOD–0.754 |
| 118 Sn | 0.006 | 0.411–337 | Ca 1 | 2.79 | 214–8560 |
| 123 Sb | 0.004 | 0.04–2.01 | K 1 | 9.74 | 3210–55400 |
| 125 Te | 0.002 | <LOD–0.018 | Mg 1 | 7.66 | 14.4–4780 |
| 133 Cs | 0.002 | 0.039-02–2.76 | Na 1 | 8.87 | 434–26800 |
| 137 Ba | 0.016 | 0.174–31.3 | Si 1 | 49.6 | 259–21600 |
| 139 La | 0.0005 | <LOD–0.355 |
| Bourbon | Irish | Japanese | Scotch | Tennessee | |
|---|---|---|---|---|---|
| Si | 1005 b | 852 b | 6803 a | 1424 b | 1206 b |
| Ca | 1182 b | 981 b | 1042 b | 2003 a | 921 b |
| Mg | 517 a | 2578 b | 861 a | 1088 a | 137 c |
| Na | 6238 b | 9370 a | 13368 a | 9685 a | 9251 ab |
| K | 18787 a | 8290 b | 21556 a | 20508 a | 16164 ab |
| Fe | 554 b | 545 b | 688 a | 647 a | 531 b |
| Cu | 203 b | 49.9 c | 749 a | 584 a | 15.2 c |
| Zn | 151 b | 41.56 b | 107 a | 19.9 b | 178 ab |
| Al | 151 b | 117 b | 175 ab | 285 a | 117 ab |
| Mn | 49.7 a | 20.7 b | 42.2 a | 52.6 a | 5.82 c |
| Ni | 5.18 b | 2.50 c | 14.7 a | 4.44 b | 3.03 bc |
| Rb | 21.4 a | 9.67 b | 22.7 a | 23.6 a | 17.0 ab |
| Sr | 8.23 a | 3.24 b | 5.71 ab | 10.8 a | 2.23 b |
| Sn | 6.88 b | 60.2 b | 76.5 a | 11.0 b | 12.5 b |
| Ba | 7.59 a | 5.12 bc | 1.02 c | 3.69 b | 2.57 bc |
| Co | 0.213 b | 0.233 ab | 0.412 ab | 0.473 a | 0.090 b |
| Mo | 0.526 b | 0.156 c | 0.326 bc | 0.264 bc | 1.98 a |
| Cd | 0.152 c | 1.71 b | 2.189 a | 0.286 bc | 0.355 bc |
| Er | 0.046 a | 0.008 b | 0.008 b | 0.015 b | 0.005 b |
| Hf | 0.104 c | 0.091 c | 0.180 ab | 0.162 b | 0.300 a |
| Ir | n.d. 1 b | n.d. 1 b | n.d. 1 b | n.d. 1 b | 0.040 a |
| Au | 0.018 b | 0.016 b | 0.026 ab | 0.036 a | 0.025 ab |
| U | 0.064 b | 0.054 b | 0.038 b | 0.132 a | 0.125 ab |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopfer, H.; Gilleland, G.; Ebeler, S.E.; Nelson, J. Elemental Profiles of Whisk(e)y Allow Differentiation by Type and Region. Beverages 2017, 3, 8. https://doi.org/10.3390/beverages3010008
Hopfer H, Gilleland G, Ebeler SE, Nelson J. Elemental Profiles of Whisk(e)y Allow Differentiation by Type and Region. Beverages. 2017; 3(1):8. https://doi.org/10.3390/beverages3010008
Chicago/Turabian StyleHopfer, Helene, Greg Gilleland, Susan E. Ebeler, and Jenny Nelson. 2017. "Elemental Profiles of Whisk(e)y Allow Differentiation by Type and Region" Beverages 3, no. 1: 8. https://doi.org/10.3390/beverages3010008






