Production of Nigella sativa Beverage Powder under Foam Mat Drying Using Egg Albumen as a Foaming Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nigella sativa Foam
2.2. Foaming Properties
2.2.1. Foam Density
2.2.2. Foam Expansion
2.2.3. Foam Stability
2.3. Drying Properties
2.3.1. Moisture Content
2.3.2. Water Activity
2.3.3. Water Solubility and Absorption Index
2.3.4. Colour
2.3.5. Bulk and Tapped Density
2.3.6. Experimental Design and Optimization
2.4. Chemical Analysis of the Nigella sativa Powder
2.4.1. Extraction of Sample
2.4.2. Determination of DPPH Free Radical-Scavenging Activity
2.4.3. Determination of Total Phenolic Content
2.5. Determination of Minerals Content
2.5.1. Digestion of Sample
Calcium
Sodium
Copper, Iron and Manganese
2.6. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Responses of Foaming Process
3.1.1. Foam Density
3.1.2. Foam Expansion
3.1.3. Foam Stability
3.1.4. Optimization of the Foaming Parameters
3.2. Analysis of Responses of Drying Process
3.2.1. Moisture Content and Water Activity
3.2.2. Water Solubility Index (WSI)
3.2.3. Water Absorption Index (WAI)
3.2.4. Colour (L* Value)
3.2.5. Bulk and Tapped Density
3.2.6. Optimization of the Drying Parameters
3.3. Quality of Nigella sativa Powder
3.3.1. Water Solubility Index
3.3.2. Antioxidant Activity
3.3.3. Total Phenolic Content
3.3.4. Composition of Minerals
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Fumagalli, F.; Silveira, A.M. Quality evaluation of microwave-dried Packham’s triumph pear. Dry. Technol. 2005, 23, 2215–2226. [Google Scholar] [CrossRef]
- Hertzendorf, M.S.; Moshy, R.J.; Seltzer, E. Foam mat drying in the food industry. Crit. Rev. Food Technol. 1970, 1, 25–70. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Chen, W.; Weng, Y.-M.; Tseng, C.-Y. Chemical composition, physical properties, and antioxidant activities of yams flours as affected by different drying methods. Food Chem. 2003, 83, 85–92. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Nindo, C.I.; Tang, J. Refractance window dehydration technology: A novel contact drying method. Dry. Technol. 2007, 25, 37–48. [Google Scholar] [CrossRef]
- Hasanah, H.; Chong, G.-L.; Suzana, S. Comparison of physicochemical analysis and antioxidant activities of Nigella sativa seeds and oils from Yemen, Iran and Malaysia. J. Sci. 2014, 43, 535–542. [Google Scholar]
- Chandrasekar, V.; Gabriela, J.; Kannan, K.; Sangamithra, A. Effect of foaming agent concentration and drying temperature on physicochemical and antimicrobial properties of foam mat dried powder. Asian J. Dairy Food Res. 2015, 34, 39–43. [Google Scholar] [CrossRef]
- Kadam, D.M.; Wilson, R.A.; Kaur, S. Determination of biochemical properties of foam-mat dried mango powder. Int. J. Food Sci. Technol. 2010, 45, 1626–1632. [Google Scholar] [CrossRef]
- Rajkumar, P.; Kailappan, R.; Viswanathan, R.; Raghavan, G.; Ratti, C. Foam mat drying of Alphonso mango pulp. Dry. Technol. 2007, 25, 357–365. [Google Scholar] [CrossRef]
- Kandasamy, P.; Varadharaju, N.; Kalemullah, S.; Moitra, R. Production of papaya powder under foam-mat drying using methyl cellulose as foaming agent. Asian J. Food Agro-Ind. 2012, 5, 374–387. [Google Scholar]
- Krasaekoopt, W.; Bhatia, S. Production of yogurt powder using foam-mat drying. AU J. Technol. 2012, 15, 166–171. [Google Scholar]
- Sankat, C.K.; Castaigne, F. Foaming and drying behaviour of ripe bananas. LWT-Food Sci. Technol. 2004, 37, 517–525. [Google Scholar] [CrossRef]
- Karim, A.A.; Wai, C.C. Foam-Mat drying of starfruit (Averrhoa carambola L.) puree. Stability and air drying characteristics. Food Chem. 1999, 64, 337–343. [Google Scholar] [CrossRef]
- Kato, A.; Takahashi, A.; Matsudomi, N.; Kobayashi, K. Determination of foaming properties of proteins by conductivity measurements. J. Food Sci. 1983, 48, 62–65. [Google Scholar] [CrossRef]
- Marinova, K.G.; Basheva, E.S.; Nenova, B.; Temelska, M.; Mirarefi, A.Y.; Campbell, B.; Ivanov, I.B. Physico-Chemical factors controlling the foamability and foam stability of milk proteins: Sodium caseinate and whey protein concentrates. Food Hydrocoll. 2009, 23, 1864–1876. [Google Scholar] [CrossRef]
- Jittanit, W.; Niti-Att, S.; Techanuntachaikul, O. Study of spray drying of pineapple juice using maltodextrin as an adjunct. Chiang Mai J. Sci. 2010, 37, 498–506. [Google Scholar]
- Grabowski, J.; Truong, V.D.; Daubert, C. Spray-Drying of amylase hydrolyzed sweetpotato puree and physicochemical properties of powder. J. Food Sci. 2006, 71, E209–E217. [Google Scholar] [CrossRef]
- Chegini, G.; Ghobadian, B. Effect of spray-drying conditions on physical properties of orange juice powder. Dry. Technol. 2005, 23, 657–668. [Google Scholar] [CrossRef]
- Patil, V.; Chauhan, A.K.; Singh, R.P. Optimization of the spray-drying process for developing guava powder using response surface methodology. Powder Technol. 2014, 253, 230–236. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chem. 2011, 127, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Ramesh, M.; Orsat, V. Spray drying for the production of nutraceutical ingredients—A review. Food Bioprocess Technol. 2012, 5, 3–14. [Google Scholar]
- Falade, K.O.; Adeyanju, K.I.; Uzo-Peters, P.I. Foam-Mat drying of cowpea (Vigna unguiculata) using glyceryl monostearate and egg albumin as foaming agents. Eur. Food Res. Technol. 2003, 217, 486–491. [Google Scholar] [CrossRef]
- Kudra, T.; Ratti, C. Foam-Mat drying: Energy and cost analyses. Can. Biosyst. Eng. 2006, 48, 3. [Google Scholar]
- Thuwapanichayanan, R.; Prachayawarakorn, S.; Soponronnarit, S. Effects of foaming agents and foam density on drying characteristics and textural property of banana foams. LWT-Food Sci. Technol. 2012, 47, 348–357. [Google Scholar] [CrossRef]
- Kampf, N.; Martinez, C.G.; Corradini, M.G.; Peleg, M. Effect of two gums on the development, rheological properties and stability of egg albumen foams. Rheol. Acta 2003, 42, 259–268. [Google Scholar]
- Karabulut, I.; Topcu, A.; Duran, A.; Turan, S.; Ozturk, B. Effect of hot air drying and sun drying on color values and β-carotene content of apricot (Prunus armenica L.). LWT-Food Sci. Technol. 2007, 40, 753–758. [Google Scholar] [CrossRef]
- Chun, K.P.; Nazimah, S.A.H.; Chin, P.T.; Mirhosseini, H.; Russly, A.R.; Gulam, R. Optimization of drum drying processing parameters for production of jackfruit (Artocarpus heterophyllus) powder using response surface methodology. LWT-Food Sci. Technol. 2010, 43, 343–349. [Google Scholar]
- Singh, A.K.; Selvam, R.P.; Sivakumar, T. Isolation, characterisation and formulation properties of a new plant gum obtained from mangifera indica. Int. J. Pharm. Biomed. Res. 2010, 1, 35–41. [Google Scholar]
- Hogekamp, S.; Schubert, H. Rehydration of food powders. Food Sci. Technol. Int. 2003, 9, 223–235. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.; Gai, G.; Yang, Y. Effect of superfine grinding on properties of ginger powder. J. Food Eng. 2009, 91, 217–222. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, N.; Ding, H.; Yao, R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Nurhanani, R.; Rasyidah, R.; Sarni, M.; Azlina, A. Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale). Food Chem. 2008, 111, 38–44. [Google Scholar]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Carughi, A. Polyphenol content and health benefits of raisins. Nutr. Res. 2010, 30, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.T.; Butt, M.S.; Anjum, F.M.; Jamil, A.; Akhtar, S.; Nasir, M. Nutritional profile of indigenous cultivar of black cumin seeds and antioxidant potential of its fixed and essential oil. Pak. J. Bot. 2009, 41, 1321–1330. [Google Scholar]

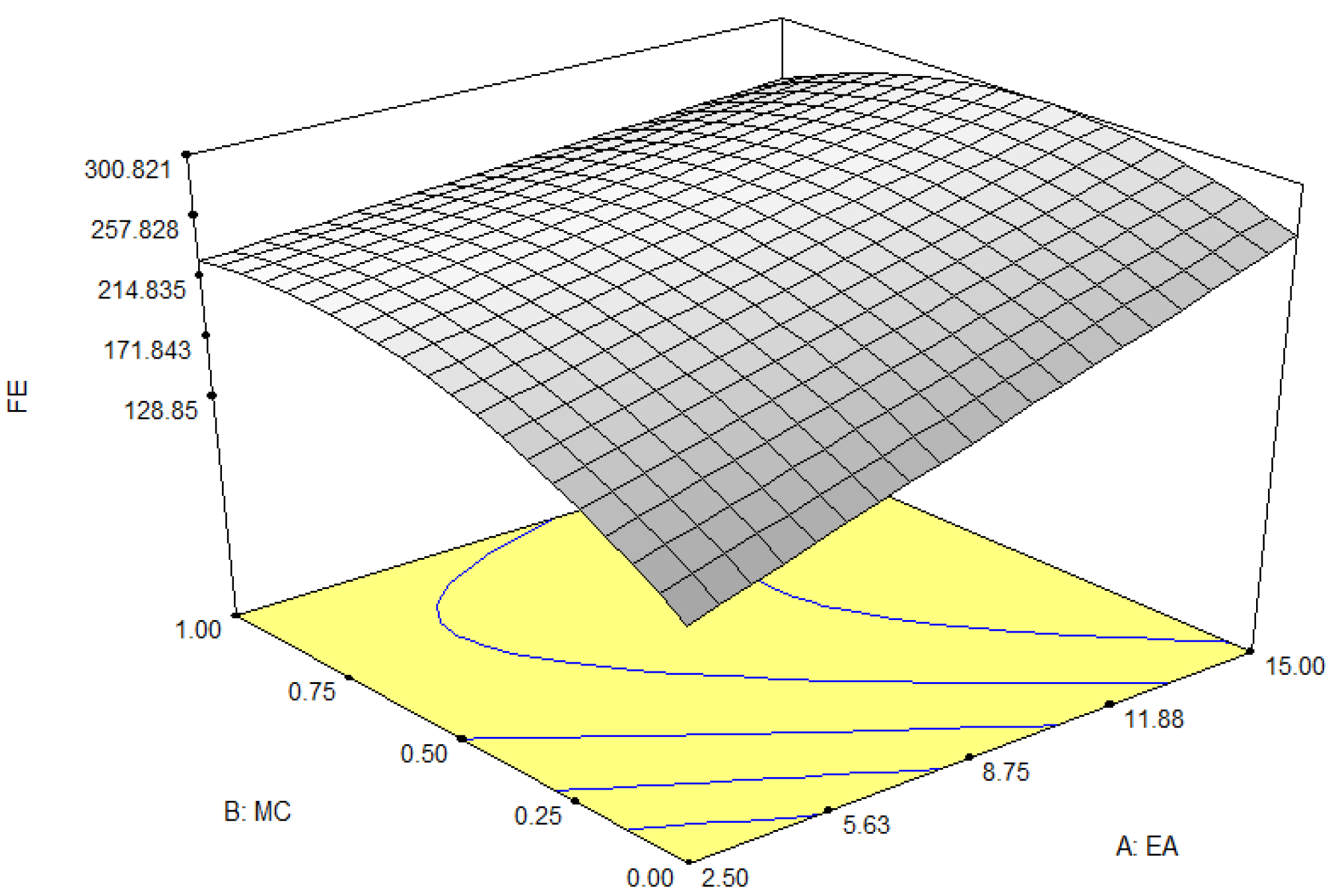

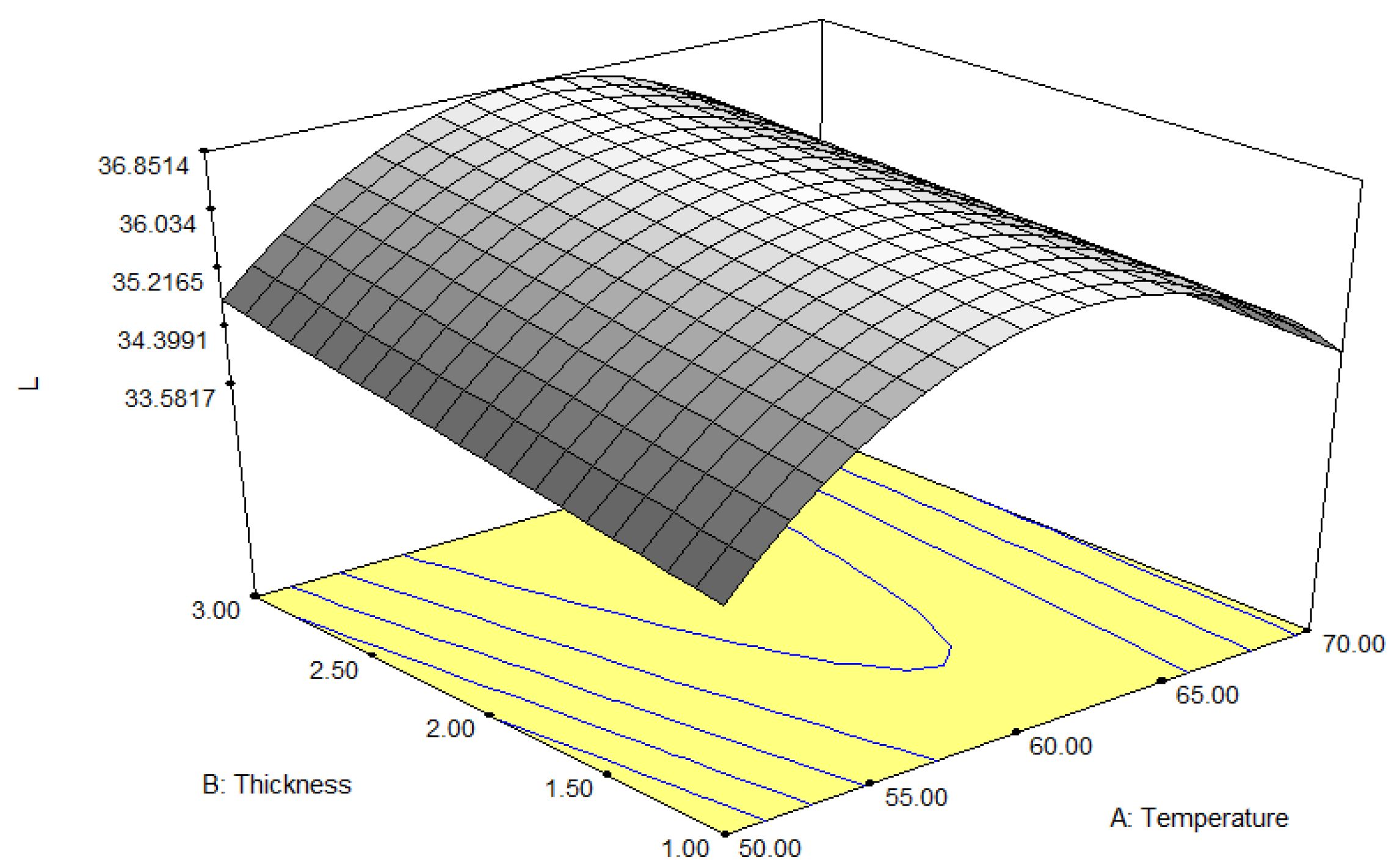
| Factors | Independent Variables | Units | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| A1 | Egg albumen | % | 2.5 | 8.75 | 15 |
| B1 | Methyl cellulose | % | 0 | 0.5 | 1 |
| C1 | Whipping time | min | 2 | 5 | 8 |
| Factors | Independent Variables | Units | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| A1 | Drying temperature | °C | 50 | 60 | 70 |
| B1 | Foam thickness | mm | 1 | 2 | 3 |
| Run No. | Process Variables | Responses | ||||
|---|---|---|---|---|---|---|
| % Egg Albumen | % Methyl Cellulose | Whipping Time (min) | Foam Density (g/cm3) | Foam Expansion (%) | Foam Stability (%) | |
| 1 | 2.50 | 0.00 | 8 | 0.44 | 110 | 82.22 |
| 2 | 8.75 | 0.50 | 5 | 0.35 | 244 | 85.95 |
| 3 | 8.75 | 0.50 | 2 | 0.38 | 135 | 87.50 |
| 4 | 2.50 | 0.00 | 2 | 0.49 | 45 | 71.00 |
| 5 | 8.75 | 0.50 | 5 | 0.35 | 220 | 83.00 |
| 6 | 15.00 | 0.00 | 2 | 0.35 | 105 | 85.71 |
| 7 | 8.75 | 0.50 | 5 | 0.31 | 272 | 88.25 |
| 8 | 2.50 | 1.00 | 2 | 0.40 | 144 | 72.49 |
| 9 | 8.75 | 0.50 | 5 | 0.34 | 256 | 87.76 |
| 10 | 15.00 | 1.00 | 2 | 0.38 | 169 | 98.70 |
| 11 | 8.75 | 0.00 | 5 | 0.36 | 210 | 78.19 |
| 12 | 2.50 | 0.50 | 5 | 0.37 | 233 | 78.75 |
| 13 | 2.50 | 1.00 | 8 | 0.36 | 215 | 95.24 |
| 14 | 8.75 | 0.50 | 5 | 0.29 | 266 | 88.00 |
| 15 | 8.75 | 0.50 | 5 | 0.32 | 264 | 87.91 |
| 16 | 15.00 | 1.00 | 8 | 0.36 | 252 | 100.00 |
| 17 | 8.75 | 1.00 | 5 | 0.40 | 240 | 94.68 |
| 18 | 15.00 | 0.00 | 8 | 0.23 | 328 | 83.11 |
| 19 | 8.75 | 0.50 | 8 | 0.26 | 300 | 93.27 |
| 20 | 15.00 | 0.50 | 5 | 0.27 | 308 | 91.12 |
| Response | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | R2 |
|---|---|---|---|---|---|---|
| Foam Density | 0.062 | 4 | 0.015 | 28.02 | <0.0001 | 0.8820 |
| Foam Expansion | 98,685.61 | 7 | 14,097.94 | 19.52 | <0.0001 | 0.9193 |
| Foam Stability | 1080.58 | 6 | 180.10 | 29.60 | <0.0001 | 0.9318 |
| Response | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | R2 |
|---|---|---|---|---|---|---|
| Moisture content | 10.39 | 4 | 2.6 | 64.68 | <0.0001 | 0.9700 |
| Water activity | 0.025 | 3 | 0.008334 | 24.27 | 0.0001 | 0.8900 |
| Water solubility index | 1077.66 | 3 | 359.22 | 108.54 | <0.0001 | 0.9731 |
| Water absorption index | 3.98 | 3 | 1.33 | 22.28 | 0.0002 | 0.8813 |
| L* | 18.76 | 2 | 9.38 | 36.08 | <0.0001 | 0.8783 |
| a* | 1.4 | 3 | 0.47 | 1.55 | 0.2689 | 0.3401 |
| b* | 2.15 | 4 | 0.54 | 1.31 | 0.3437 | 0.3961 |
| Bulk density | 0.006618 | 2 | 0.003309 | 16.21 | 0.0007 | 0.7643 |
| Tapped density | 0.011 | 2 | 0.005688 | 10.59 | 0.0034 | 0.6793 |
| Run No. | Process Variables | Responses | WAI | L* | a* | b* | Bulk Density (g/cm3) | Tapped Density (g/cm3) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Foam Thickness (mm) | MC (%) | Aw | WSI (%) | |||||||
| 1 | 70 | 1 | 5.61 | 0.32 | 55.45 | 3.218 | 34.38 | 12.84 | 21.72 | 0.263 | 0.334 |
| 2 | 50 | 2 | 8.16 | 0.489 | 25.45 | 4.559 | 34.02 | 12.81 | 22.56 | 0.334 | 0.386 |
| 3 | 60 | 2 | 7.65 | 0.451 | 45.69 | 3.825 | 37.06 | 12.79 | 23.47 | 0.335 | 0.359 |
| 4 | 60 | 2 | 7.69 | 0.45 | 42.16 | 3.986 | 36.83 | 12.14 | 22.02 | 0.315 | 0.388 |
| 5 | 60 | 1 | 6.88 | 0.414 | 45.95 | 4.126 | 35.35 | 13.03 | 22.71 | 0.315 | 0.36 |
| 6 | 70 | 2 | 6.1 | 0.409 | 49.51 | 2.743 | 35.3 | 12.74 | 22.34 | 0.28 | 0.36 |
| 7 | 60 | 3 | 7.5 | 0.466 | 47.18 | 3.328 | 36.85 | 11.82 | 21.66 | 0.359 | 0.457 |
| 8 | 50 | 3 | 8.68 | 0.51 | 27.38 | 4.664 | 34.68 | 12.38 | 22.04 | 0.335 | 0.457 |
| 9 | 60 | 2 | 7.78 | 0.473 | 46.36 | 4.147 | 37.07 | 11.89 | 22 | 0.313 | 0.358 |
| 10 | 50 | 1 | 8.04 | 0.46 | 26.12 | 4.326 | 33.86 | 13.38 | 22.87 | 0.335 | 0.386 |
| 11 | 60 | 2 | 7.53 | 0.461 | 45.69 | 3.825 | 37.22 | 11.3 | 21.17 | 0.314 | 0.386 |
| 12 | 60 | 2 | 7.68 | 0.483 | 44.51 | 4.045 | 36.83 | 11.9 | 22 | 0.313 | 0.358 |
| 13 | 70 | 3 | 5.78 | 0.482 | 48.02 | 2.839 | 34.22 | 12.29 | 21.06 | 0.28 | 0.36 |
| Samples | Water Solubility Index (%) | % Inhibition of DPPH | Total Phenolic Content (mg GAE/g) |
|---|---|---|---|
| Roasted Nigella sativa | 46.03 a ± 1.33 | 62.08 a ± 0.58 | 26.769 a ± 1.33 |
| Foam Mat Dried Nigella sativa Powder | 45.30 a ± 0.68 | 56.60 b ± 0.39 | 67.538 b ± 2.77 |
| Response | Ca | Na | Fe | Mn | Cu |
|---|---|---|---|---|---|
| Roasted Nigella sativa | 6.626 a ± 0.27 | 3.040 a ± 0.81 | 0.721 a ± 0.01 | 0.647 a ± 0.002 | 0.103 a ± 0.006 |
| Foam Mat Dried Nigella sativa Powder | 30.012 b ± 0.85 | 3.538 b ± 0.10 | 0.258 b ± 0.02 | 0.012 b ± 0.002 | 0.057 b ± 0.003 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Affandi, N.; Zzaman, W.; Yang, T.A.; Easa, A.M. Production of Nigella sativa Beverage Powder under Foam Mat Drying Using Egg Albumen as a Foaming Agent. Beverages 2017, 3, 9. https://doi.org/10.3390/beverages3010009
Affandi N, Zzaman W, Yang TA, Easa AM. Production of Nigella sativa Beverage Powder under Foam Mat Drying Using Egg Albumen as a Foaming Agent. Beverages. 2017; 3(1):9. https://doi.org/10.3390/beverages3010009
Chicago/Turabian StyleAffandi, Norhazirah, Wahidu Zzaman, Tajul Aris Yang, and Azhar Mat Easa. 2017. "Production of Nigella sativa Beverage Powder under Foam Mat Drying Using Egg Albumen as a Foaming Agent" Beverages 3, no. 1: 9. https://doi.org/10.3390/beverages3010009




