Analysis of Volatile Components of Varietal English Wines Using Stir Bar Sorptive Extraction/Gas Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
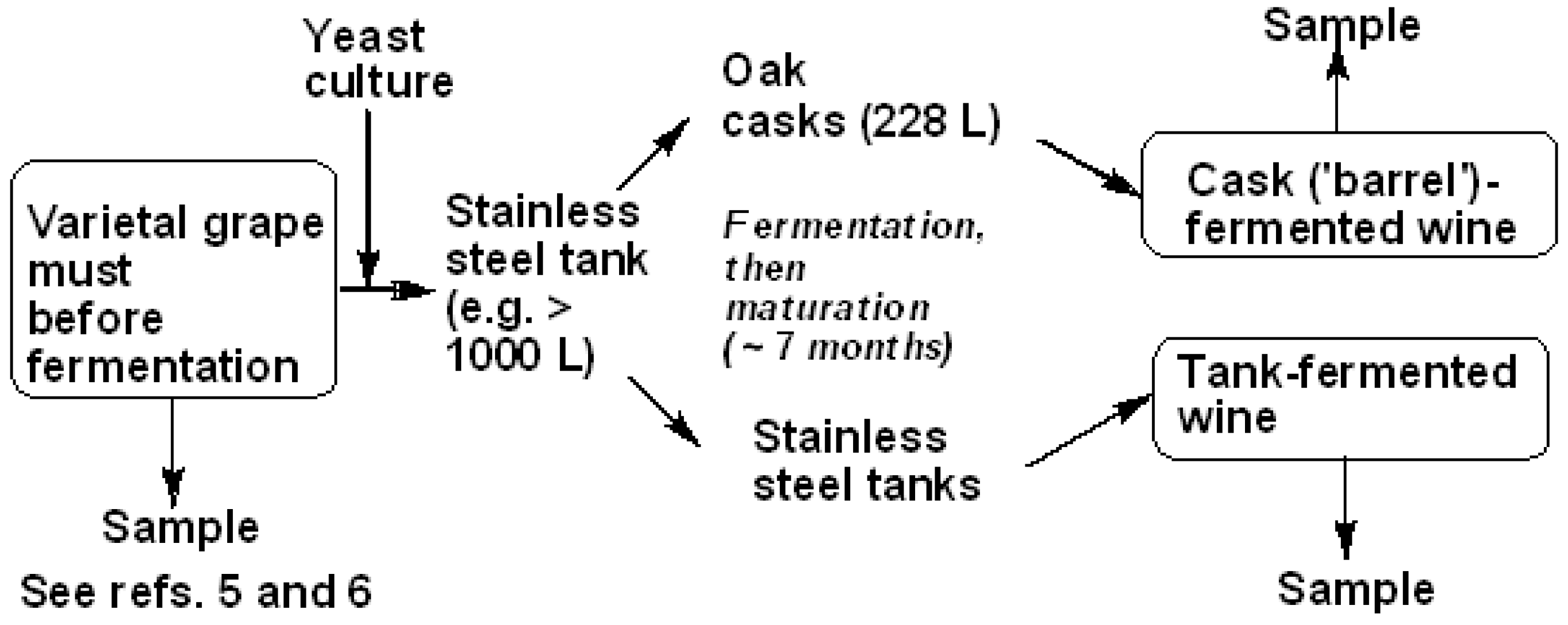
2.2. Wine Samples
2.3. Winemaking and Ageing
2.4. Determination of Routine Viticultural and Oenological Parameters
2.5. Sampling Conditions for SBSE
2.6. Instrumentation and Conditions
2.7. Identification of Volatile Components and Determination of Semi-Quantitative Concentrations
3. Results and Discussion
3.1. General
3.2. Wine Volatiles
3.2.1. Alcohols
3.2.2. Esters
3.2.3. Fatty Acids
3.2.4. Terpenoids and Norisoprenoids
3.2.5. Comparison of Volatile Compounds Identified in Tank- and Barrel-Fermented/Aged Wines
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rapp, A.; Hastrich, H.; Engel, L.; Knipser, W. Flavour of Foods and Beverages. Chemistry and Technology; Charalambous, G., Inglett, G.E., Eds.; Academic Press: New York, NY, USA, 1978; pp. 391–417. [Google Scholar]
- Rapp, A. Volatile flavour of wine: Correlation between instrumental analysis and sensory perception. Nahrung 1998, 42, 351–363. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Caven-Quantrill, D.J.; Buglass, A.J. Comparison of micro-scale simultaneous distillation-extraction and stir bar sorptive extraction for the determination of volatile organic constituents of grape juice. J. Chromatogr. A 2006, 1117, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Caven-Quantrill, D.J.; Buglass, A.J. Determination of volatile organic compounds in English vineyard grape juices by immersion stir bar sorptive extraction-gas chromatography/mass spectrometry. Flavour Fragr. J. 2007, 22, 206–213. [Google Scholar] [CrossRef]
- Caven-Quantrill, D.J.; Buglass, A.J. Seasonal variation of flavour content of English vineyard grapes, determined by stir-bar sorptive extraction-gas chromatography-mass spectrometry. Flavour Fragr. J. 2008, 23, 239–248. [Google Scholar] [CrossRef]
- Pedroza, M.A.; Zalacain, A.; Lara, J.F.; Salinas, M.R. Global grape aroma potential and its individual analysis by SBSE-GC-MS. Food Res. Int. 2010, 43, 1003–1008. [Google Scholar] [CrossRef]
- Perestrelo, R.; Nogueira, J.M.F.; Câmara, J.S. Potentialities of two solventless extraction approaches—Stir bar sorptive extraction and headspace solid-phase microextraction for determination of higher alcohol acetates, isoamyl esters and ethyl esters in wines. Talanta 2009, 80, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gil, A.M.; Garde-Cerdán, T.; Martínez, L.; Alonso, G.L.; Salinas, M.R. Effect of Oak Extract Application to Verdejo Grapevines on Grape and Wine Aroma. J. Agric. Food Chem. 2011, 59, 3253–3263. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gil, A.M.; Garde-Cerdán, T.; Zalacain, A.; Pardo-García, A.I.; Salinas, M.R. Applications of an oak extract on Petit Verdot grapevines. Influence on grape and wine volatile compounds. Food Chem. 2012, 132, 1836–1845. [Google Scholar] [CrossRef]
- Díez, J.; Domínguez, C.; Guillén, D.A.; Veas, R.; Barroso, C.G. Optimisation of stir bar sorptive extraction for the analysis of volatile phenols in wines. J. Chromatogr. A 2004, 1025, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.F.; Nascimento, A.M.D.; Nogueira, J.M.F. Characterization of the aroma profile of Madeira wine by sorptive extraction techniques. Anal. Chim. Acta 2005, 546, 11–21. [Google Scholar] [CrossRef]
- Marín, J.; Zalacain, A.; De Miguel, C.; Alonso, G.L.; Salinas, M.R. Stir bar sorptive extraction for the determination of volatile compounds in oak-aged wines. J. Chromatogr. A 2005, 1098, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.C.; Fang, Y.; Shellie, K. Volatile Composition of Merlot Wine from Different Vine Water Status. J. Agric. Food Chem. 2009, 57, 7459–7463. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Coimbra, M.A.; Nogueira, J.M.F.; Rocha, S.M. Quantification approach for assessment of sparkling wine volatiles from different soils, ripening stages, and varieties by stir bar sorptive extraction with liquid desorption. Anal. Chim. Acta 2009, 635, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Weldegergis, B.T.; Crouch, A.M. Analysis of volatiles in pinotage wines by stir bar sorptive extraction and chemometric profiling. J. Agric. Food Chem. 2008, 56, 10225–10236. [Google Scholar] [CrossRef] [PubMed]
- Caven-Quantrill, D.J.; Buglass, A.J. Comparison of volatile constituents extracted from model grape juice and model wine by stir bar sorptive extraction-gas chromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Buglass, A.J.; Lee, S.H. Applications of chiral ligand-exchange chromatography for the Analysis of D- and L-lactic acid content of wine and other foodstuffs. LCGC N. Am. 2003, 21, 554–562. [Google Scholar]
- Jackson, D.; Schuster, D. Grape-Growing and Winemaking; Alister Taylor Publishing Ltd.: Martinborough, New Zealand, 1981; pp. 177–180. [Google Scholar]
- Stackler, B.; Christensen, E.N. Quantitative determination of ethanol in wine by gas chromatography. Am. J. Enol. Vitic. 1974, 25, 202–207. [Google Scholar]
- Rood, D. A Practical Guide to the Care, Maintenance, and Troubleshooting of Capillary Gas Chromatographic Systems; Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Margolit, Y. Concepts in Wine Technology; Wine Appreciation Guild: San Francisco, CA, USA, 2004; Chapters III and V. [Google Scholar]
- Fowles, G.W.A. Acids in grapes and wine. A review. J. Wine Res. 1992, 3, 25–41. [Google Scholar] [CrossRef]
- Clarke, R.J.; Bakker, J. Wine Flavour Chemistry; Blackwell Publishing: Oxford, UK, 2004; pp. 120–188, 265–280. [Google Scholar]
- Jackson, R.S. Wine Science: Principles, Practice, Perception; Academic Press: London, UK, 2000; pp. 232–280. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology Volume 2, 2nd ed.; John Wiley and Sons Ltd.: Chichester, UK, 2006; pp. 51–64, 205–230. [Google Scholar]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Allured. Arctander’s Perfume and Flavor Chemicals and Perfume and Flavor Materials of Natural Origin [CD-ROM]; Allured: Carol Stream, IL, USA, 1999. [Google Scholar]
- Yue, T.; Chi, M.; Song, C.; Liu, M.; Meng, J.; Zhang, Z.; Li, M. Aroma characterization of Cabernet Sauvignon wine from the plateau of Yunnan (China) with different altitudes using SPME-GC/MS. Int. J. Food Prop. 2015, 18, 1584–1596. [Google Scholar] [CrossRef]
- Rocha, S.M.; Coutinho, P.; Coelho, E.; Barros, A.S.; Delgadillo, I.; Coimbra, M.A. Relationships between the varietal volatile composition of the musts and white wine aroma quality. A four year feasibility study. LWT-Food Sci. Technol. 2010, 43, 1508–1516. [Google Scholar] [CrossRef]
- Vilanova, M.; Cortés, S.; Santiago, J.L.; Martínez, C.; Fernández, E. Aromatic compounds in wines produced during fermentation: Effect of three red cultivars. Int. J. Food Prop. 2007, 10, 867–875. [Google Scholar] [CrossRef]
- Ong, P.K.C.; Acree, T.E. Similarities in the aroma chemistry of Gewurztraminer variety wines and lychee (Litchi chinesis Sonn.) fruit. J. Agric. Food Chem. 1999, 47, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Moreno, N.J.; Azpilicueta, C.A. Binding of oak volatile compounds by wine lees during simulation of wine ageing. LWT-Food Sci. Technol. 2007, 40, 619–624. [Google Scholar] [CrossRef]
- Ferreras, D.; Fernandez, E.; Falque, E. Effects of oak wood on the aromatic composition of Vitis vinifera L. var. Treixadura wines. Food Sci. Technol. Int. 2002, 8, 343–349. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Azpilicueta, C.A. Effect of oak barrel type on the volatile composition of wine: Storage time optimization. LWT-Food Sci. Technol. 2006, 39, 199–205. [Google Scholar] [CrossRef]
| Parameter | Huxelrebe 2004 must | Ortega 2004 must | Schönburger 2004 must | Siegerrebe 2004 must |
|---|---|---|---|---|
| °Brix (±0.2°) b | 19.4 | 21.6 | 19.6 | 21.6 |
| Tartaric acid concentration. (%w/w, %Relative standard deviation (RSD) = 2.7) (n = 3) | 0.57 | 0.41 | 0.50 | 0.30 |
| Malic acid concentration. (%w/w, %RSD = 1.4) (n = 3) | 0.38 | 0.16 | 0.33 | 0.13 |
| Retention Times (min) | RI b | Component c | Huxelrebe | Ortega | Schönburger | Siegerrebe | %RSD (CV) d | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 2004 | 2004 | 2004 | 2004 | |||||||
| Tank | Barrel | Tank | Barrel | Tank | Barrel | (Tank) | n = 3 | |||
| 2.24 | 92 | Acetaldehyde | 37 | 58 | 45 | 47 | 33 | 37 | 19 | 5.9 |
| 3.48 | 200 | Ethyl acetate | 820 | 380 | 1900 | 1300 | 12,000 | 1500 | 700 | 7.4 |
| 3.51 | 203 | 1,1-Diethoxyethane (Acetal) | - | 240 | - | 170 | - | 220 | 990 | 6.8 |
| 3.80 | 230 | 2-Methylbutanal | tr. | - | - | - | - | - | tr. | - |
| 3.86 | 235 | 3-Methylbutanal (Isovaleraldehyde) | tr. | tr. | tr. | tr. | - | tr. | 11 | 7.3 |
| 4.28 | 273 | 2,4,5-Trimethyl-1,3-dioxolane (Acetaldehyde-2,3-butanediol acetal) | - | 181 | - | 121 | - | 241 | 720 | 3.1 |
| 4.58 | 300 | Ethyl propionate | 41 | 33 | 43 | 30 | 119 | 83 | 101 | 4.9 |
| 4.75 | 308 | Ethyl isobutyrate | 92 | 88 | 150 | 118 | 330 | 350 | 123 | 6.2 |
| 5.06 | 323 | Diacetyl | tr. | tr. | tr. | tr. | tr. | tr. | - | - |
| 5.91 | 364/365 | α-Pinene/Isobutyl acetate | 410 | 129 | 300 | 230 | - | 190 | 870 | 7.0 |
| 5.91 | 365 | Isobutyl acetate | - | - | - | - | 520 | - | - | 7.6 |
| 6.59 | 400 | Ethyl butyrate | 1420 | 1270 | 1470 | 1470 | 2100 | 1060 | 3800 | 5.0 |
| 7.06 | 413 | Ethyl-2-methyl butyrate | 87 | 87 | 74 | 65 | 113 | 280 | 55 | 6.0 |
| 7.12 | 415 | Camphene | tr. | tr. | tr. | tr. | - | - | 25 | 5.3 |
| 7.60 | 430 | Ethyl isovalerate | 150 | 190 | 125 | 116 | 160 | 370 | 69 | 6.8 |
| 7.74 | 434 | n-Butyl acetate | 65 | 33 | 29 | 26 | 26 | 17 | 50 | 6.3 |
| 7.88 | 437 | 1,1-Diethoxy-3-methylbutane (Isovaleraldehyde diethyl acetal) | - | 50 | - | 26 | - | 41 | 67 | 4.2 |
| 8.00 | 440 | Hexanal | tr. | tr. | - | tr. | tr. | tr. | - | - |
| 8.30 | 449 | 2-Methyl-1-propanol (Isobutyl alcohol) | 68 | 26 | 110 | 71 | 710 | 170 | 190 | 7.4 |
| 8.43 | 452 | β-Pinene | 11 | tr. | tr. | tr. | - | tr. | tr. | 9.0 |
| 8.81 | 465 | 2,2,6-Trimethyl-6-vinyltetrahydropyran | 14 | 35 | tr. | 24 | 21 | 30 | 190 | 3.8 |
| 9.06 | 470 | 1-Ethoxy-1-pentoxyethane (Acetaldehyde ethyl amyl acetal) | - | 167 | - | 48 | - | 130 | 290 | 4.2 |
| 9.75 | 484/485 | 2-Methylbutyl acetate/3-Methylbutyl acetate (Isoamyl acetate) | 60,000 | 19,000 | 48,000 | 27,000 | 44,000 | 18,000 | 19,000 | 6.6 |
| 10.11 | 500 | Ethyl pentanoate (Ethyl valerate) | - | - | tr. | tr. | - | - | 39 | 3.1 |
| 10.29 | 504 | δ-3-Carene | tr. | 14 | tr. | tr. | tr. | 11 | tr. | 8.3 |
| 10.56 | 510 | 1-Butanol | tr. | tr. | 15 | 12 | 26 | 14 | 25 | 5.1 |
| 11.12 | 523 | Isobutyl butyrate | 12 | tr. | tr. | tr. | tr. | tr. | tr. | 7.3 |
| 11.25 | 525 | Ethyl-2-butenoate (Ethyl crotonate) | - | - | 37 | 29 | 37 | 17 | - | 6.9 |
| 11.25 | 525/527 | Ethyl-2-butenoate (Ethyl crotonate)/β-Myrcene | 45 | 42 | - | - | - | - | 83 | 5.1 |
| 11.62 | 535 | α-Terpinene | tr. | 21 | tr. | 16 | tr. | tr. | 12 | 5.8 |
| 11.73 | 538 | n-Amyl acetate | 38 | 16 | 36 | 28 | 35 | 16 | 31 | 1.4 |
| 11.97 | 543 | 2-Heptanone | 11 | 17 | 13 | 30 | 15 | tr. | 41 | 2.3 |
| 12.10 | 546 | Heptanal | - | - | - | - | - | - | tr. | - |
| 12.27 | 550 | Methyl hexanoate | 45 | 53 | 18 | 23 | 45 | 25 | 33 | 8.6 |
| 12.42 | 554 | Limonene | 200 | 100 | 55 | 50 | 56 | 110 | 230 | 8.8 |
| 12.64 | 559 | Isoamyl isobutyrate/1,8-Cineol | 35 | 19 | - | - | - | - | 35 | 7.9 |
| 12.64 | 559 | Isoamyl isobutyrate | - | - | 17 | 16 | 27 | 87 | - | 7.6 |
| 13.26 | 573 | 2-Methyl-1-butanol/3-Methyl-1-butanol (Isoamyl alcohol) | 10,700 | 8600 | 10,200 | 7800 | 18,000 | 21,000 | 22,000 | 5.5 |
| 14.53 | 600 | Ethyl hexanoate | 86,000 | 91,000 | 59,000 | 68,000 | 67,000 | 43,000 | 97,000 | 5.5 |
| 14.69 | 604 | γ-Terpinene | 54 | 48 | 31 | 37 | 29 | 32 | 154 | 3.3 |
| 15.09 | 616 | (E)-β-Ocimene | tr. | 16 | tr. | 12 | tr. | tr. | 81 | 1.6 |
| 15.35 | 622 | 1-(1-Ethoxyethoxy) hexane (Acetaldehyde ethyl hexyl acetal) | - | 11 | - | - | - | - | 20 | 3.3 |
| 15.69 | 630/631 | p-Cymene/Isoamyl butyrate | 156 | 150 | 79 | 111 | 88 | 116 | 238 | 3.1 |
| 16.18 | 641 | Hexyl acetate | 39,000 | 13,700 | 22,000 | 12,900 | 18,200 | 3500 | 6400 | 5.0 |
| 16.22 | 642 | α-Terpinolene | 13 | 39 | tr. | 13 | 12 | 12 | 71 | 4.8 |
| 16.26 | 643 | 3-Methylbutyl-2-methyl butyrate (Isoamyl-2-methyl butyrate) | - | - | - | - | tr. | 15 | - | 5.7 |
| 16.67 | 653 | Octanal | tr. | tr. | - | - | - | - | tr. | - |
| 17.36 | 669 | Ethyl-(Z)-3-hexenoate | 38 | 36 | - | 19 | - | 108 | - | 5.0 |
| 17.56 | 673 | Ethyl-(E)-3-hexenoate | 17 | 16 | 14 | 22 | tr. | tr. | 20 | 7.2 |
| 17.67 | - | (E)-3-Hexenyl acetate* | 200 | 80 | 148 | 156 | 44 | 18 | 133 | 5.0 |
| 18.01 | 682/685 | (Z)-3-Hexenyl acetate/Propyl hexanoate | 400 | 227 | 176 | 149 | 760 | 198 | 157 | 3.7 |
| 18.71 | 700 | Ethyl heptanoate | 138 | 122 | 51 | 59 | 68 | 99 | 310 | 5.0 |
| 18.78 | 702 | 6-Methyl-5-hepten-2-one | tr. | tr. | - | - | 21 | tr. | 21 | 7.2 |
| 18.78 | 702 | 6-Methyl-5-hepten-2-one/(E)-2-Hexenyl acetate | - | - | 21 | 16 | - | - | - | 6.7 |
| 19.13 | 710 | Ethyl-(E)-2-hexenoate | 42 | 103 | 330 | 228 | 168 | 225 | 360 | 3.5 |
| 19.17 | 711 | Ethyl lactate | 122 | 126 | 28 | 28 | 76 | 105 | 52 | 4.2 |
| 19.23 | 712 | (Z)-Rose oxide | tr. | tr. | 30 | 28 | 75 | 100 | 260 | 7.8 |
| 19.52 | 719 | Isobutyl hexanoate | 19 | tr. | 34 | 37 | 36 | 42 | - | 8.6 |
| 19.59 | 721 | 1-Hexanol | 2400 | 2100 | 1320 | 1350 | 1700 | 1140 | 3400 | 6.4 |
| 20.04 | 731 | (E)-3-Hexenol | 26 | 23 | 17 | 24 | 13 | 14 | 36 | 8.3 |
| 20.45 | 741 | Heptyl acetate | 70 | 71 | 54 | 28 | 50 | tr. | - | 3.0 |
| 20.89 | 752 | (Z)-3-Hexenol | 34 | 32 | - | 48 | 138 | 123 | 82 | 2.0 |
| 20.96 | 753 | 2-Nonanone (Methyl heptyl ketone) | 70 | 112 | 172 | 96 | 87 | 40 | 230 | 4.1 |
| 21.10 | 757 | Methyl octanoate | 600 | 630 | 240 | 250 | 540 | 230 | 330 | 5.1 |
| 21.39 | 763 | 3-Octanol | - | - | - | - | - | - | tr. | - |
| 23.64 | 800 | Ethyl octanoate | 370,000 | 370,000 | 274,000 | 310,000 | 310,000 | 206,000 | 360,000 | 3.4 |
| 23.88 | 822 | 1-Octen-3-ol | tr. | tr. | - | - | tr. | tr. | tr. | - |
| 23.94 | 824 | Acetic acid | 450 | 118 | 278 | 360 | 244 | 273 | 350 | 2.9 |
| 24.01 | 825 | 1-Heptanol | 11 | - | 13 | tr. | 15 | 11 | - | 6.2 |
| 24.12 | 828 | Isoamyl hexanoate | 710 | 910 | 570 | 610 | 610 | 630 | 900 | 3.4 |
| 24.26 | 831 | Furfural | tr. | tr. | tr. | 14 | 13 | tr. | 12 | 5.9 |
| 24.41 | 835 | (E)-Linalool oxide (Furanoid)/Nerol oxide | - | - | - | - | - | - | 592 | 0.6 |
| 24.42 | - | Nerol oxide * | 168 | 289 | 216 | 214 | 167 | 164 | - | 0.7 |
| 24.72 | 842 | Octyl acetate | 76 | 166 | 83 | 27 | 92 | 67 | - | 3.0 |
| 25.29 | 856 | (E)-Theaspirane | - | - | - | - | - | 39 | - | 5.3 |
| 25.39 | 858 | 2-Ethyl-1-hexanol | - | - | - | - | 17 | - | - | 5.3 |
| 25.59 | 863 | Decanal | 11 | - | tr. | tr. | 1.1 | |||
| 25.75 | 867 | Camphor | 16 | tr. | - | - | - | - | tr. | 8.7 |
| 26.16 | - | Geranyl ethyl ether * | 183 | 310 | 142 | 185 | 90 | 100 | 450 | 3.5 |
| 26.32 | 880 | Benzaldehyde/Vitispirane (Unknown isomer) * | 118 | 320 | - | - | 53 | 115 | - | 4.9 |
| 26.32 | 880 | Benzaldehyde | - | - | 90 | 129 | - | - | 2100 | 4.9 |
| 26.33 | - | Vitispirane (Unknown isomer) * | - | - | - | - | - | 70 | - | 4.6 |
| 26.49 | 884 | Propyl octanoate | 410 | 660 | 269 | 255 | 305 | 70 | 283 | 2.8 |
| 26.59 | 886 | 2-Nonanol | 14 | 25 | 22 | 66 | 36 | 33 | 19 | 3.1 |
| 26.87 | 893 | (Z)-Theaspirane | 24 | 41 | - | - | 17 | 26 | - | 7.8 |
| 27.16 | 900 | Ethyl nonanoate | 330 | 280 | 204 | 280 | 200 | 250 | 390 | 4.0 |
| 27.28 | 903 | Propionic acid | tr. | tr. | tr. | tr. | tr. | tr. | - | - |
| 27.49 | - | Ethyl-2-hydroxyhexanoate * | 29 | 51 | 20 | 18 | 26 | 60 | 11 | 6.4 |
| 27.76 | 913/917 | Linalool/Isobutyl octanoate | 690 | 690 | 446 | 535 | 580 | 446 | 5050 | 1.6 |
| 28.05 | 922 | 1-Octanol | 226 | 121 | 62 | 83 | 108 | 293 | 75 | 2.6 |
| 29.27 | 948 | Isobutyric acid | - | - | 17 | 24 | 26 | 25 | - | 5.6 |
| 29.44 | 958 | Methyl decanoate/2-Undecanone | 435 | 570 | - | - | - | - | - | 2.8 |
| 29.44 | 958 | Methyl decanoate | - | - | 264 | 344 | 470 | 241 | 340 | 2.7 |
| 29.48 | 956 | 4-Terpineol | - | - | 13 | - | - | - | - | 5.2 |
| 29.97 | 967/973 | β-Cyclocitral/Hexyl hexanoate | 37 | 40 | 37 | 22 | 41 | 38 | - | 4.4 |
| 30.15 | 973 | Hotrienol | 40 | 246 | 226 | 268 | 83 | 123 | 383 | 0.4 |
| 30.29 | 976 | γ-Butyrolactone | 37 | 29 | 36 | 40 | 27 | 30 | 17 | 6.5 |
| 30.50 | 981 | Ethyl-2-furoate | 18 | 57 | 12 | 19 | 16 | 37 | 23 | 1.0 |
| 31.57 | 1000 | Ethyl decanoate | 260,000 | 310,000 | 230,000 | 290,000 | 260,000 | 176,000 | 310,000 | 4.7 |
| 31.96 | 1024 | Isoamyl octanoate | 2700 | 3500 | 2000 | 2300 | 2300 | 2300 | 3300 | 6.3 |
| 31.98 | 1024 | 1-Nonanol | - | - | - | 43 | - | - | - | 0.2 |
| 32.06 | 1026 | Citronellyl acetate | 194 | 40 | 217 | 40 | 190 | 81 | 50 | 3.1 |
| 32.23 | 1031 | (E)-β-Farnesene | 80 | 91 | 123 | 83 | 102 | 73 | 121 | 5.3 |
| 32.62 | 1039 | Diethyl succinate | 990 | 1670 | 551 | 1020 | 549 | 3420 | 704 | 1.0 |
| 33.10 | 1053 | Ethyl-9-decenoate | 240 | 2050 | 6800 | 7600 | 20,200 | 9400 | 410 | 4.3 |
| 33.18 | 1055 | α-Terpineol | 97 | 130 | 80 | 105 | 16 | 58 | 274 | 2.0 |
| 33.91 | 1074 | 3-(Methylthio)-1-propanol (Methionol) | 14 | tr. | - | - | tr. | 15 | 15 | 11.1 |
| 34.26 | 1083 | Propyl decanoate | 280 | 450 | 206 | 230 | 210 | 129 | 260 | 4.2 |
| 34.36 | 1086 | Neryl acetate | 39 | 14 | 44 | 26 | 53 | 30 | - | 1.9 |
| 34.60 | - | 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) * | 48 | 101 | 34 | 23 | 93 | 164 | 74 | 1.6 |
| 34.83 | 1098 | Geranyl nitrile | - | - | - | - | - | - | - | 5.9 |
| 34.91 | 1100 | Ethyl undecanoate | 61 | 74 | 56 | 78 | 23 | 33 | 190 | 6.8 |
| 35.00 | 1102 | Ethyl geranate | - | - | - | - | 78 | 63 | - | 3.2 |
| 35.16 | 1107 | (E,E)-α-Farnesene | 33 | 12 | 36 | 13 | 23 | 24 | 78 | 8.3 |
| 35.41 | 1113 | Isobutyl decanoate | 190 | 160 | 114 | 140 | 160 | 180 | 270 | 6.7 |
| 35.47 | 1115 | Geranyl acetate | 76 | - | 118 | 14 | 63 | - | - | 5.4 |
| 35.55 | 1117 | Ethyl-(E)-2-decenoate | 36 | 46 | tr. | 11 | 63 | 26 | 16 | 2.3 |
| 35.73 | 1122 | 1-Decanol | 541 | 491 | 962 | 2220 | 656 | 818 | 672 | 0.6 |
| 35.88 | 1126 | β-Citronellol | 108 | 86 | 133 | 169 | 158 | 220 | 660 | 5.5 |
| 36.33 | 1138 | Diethyl pentanedioate (Diethyl glutarate) | 18 | 46 | 36 | 41 | 20 | 34 | - | 4.3 |
| 36.40 | 1140 | Ethyl phenylacetate | 93 | 94 | 21 | 48 | 33 | 172 | 34 | 4.1 |
| 37.06 | 1158 | Nerol | 44 | 33 | 25 | - | - | - | - | 4.0 |
| 37.06 | 1158 | Nerol/Methyl dodecanoate (Methyl laurate) | - | - | - | - | 76 | 50 | 274 | 2.0 |
| 37.14 | 1160 | Ethyl nicotinate | - | - | - | - | 36 | 14 | - | 4.7 |
| 37.14 | 1160/1161 | Ethyl nicotinate/β-Damascone | - | - | 37 | - | - | 44 | - | 5.2 |
| 37.41 | 1167 | 2-Phenylethyl acetate | 17,000 | - | 15,800 | 6800 | 19,300 | 6000 | - | 3.3 |
| 37.41 | 1167/1169 | 2-Phenylethyl acetate/(E)-β-Damascenone | - | 4470 | - | - | - | - | 3080 | 1.2 |
| 38.56 | 1197 | Hexanoic acid | 1660 | 2880 | 1910 | 2450 | 2140 | 1200 | 910 | 3.5 |
| 38.63 | 1200 | Ethyl dodecanoate (Ethyl laurate) | 32,000 | 43,000 | 21,000 | 42,000 | 25,000 | 20,000 | 112,000 | 7.3 |
| 38.71 | 1202 | Geraniol | 123 | 140 | 134 | 163 | 149 | 79 | - | 1.4 |
| 38.81 | 1205 | Geranyl acetone | 86 | 87 | 16 | 39 | 79 | 94 | 161 | 4.1 |
| 39.17 | 1216 | Isoamyl decanoate | 1800 | 2300 | 1100 | 1500 | 1100 | 1800 | 5000 | 8.7 |
| 39.53 | 1226 | Benzyl alcohol | 190 | 200 | 27 | 37 | 35 | 50 | 113 | 5.1 |
| 39.59 | 1227 | (Z)-β-Methyl-γ-octalactone (cis-Oak lactone) | - | 39 | - | 46 | - | 108 | - | 3.0 |
| 39.73 | 1232 | Ethyl-3-phenylpropionate | 26 | 48 | 27 | 33 | 22 | 58 | 68 | 3.0 |
| 40.09 | - | Ethyl-3-hydroxyoctanoate * | 39 | 92 | 63 | 66 | 58 | 54 | 45 | 1.8 |
| 40.52 | - | Ethyl-3-methylbutyl butanedioate * | 730 | 960 | 220 | 290 | 330 | 2100 | 420 | 5.4 |
| 40.68 | 1260 | 2-Phenylethanol | 12,400 | 6580 | 6800 | 5030 | 10,800 | 17,400 | 9400 | 1.2 |
| 41.32 | 1278 | β-Ionone | 34 | 41 | tr. | 14 | 36 | 60 | 34 | 2.5 |
| 41.43 | 1282 | Propyl dodecanoate | - | - | - | - | - | - | 53 | 6.8 |
| 41.73 | 1291 | Benzothiazole | 13 | 13 | 13 | 23 | - | - | 21 | 6.9 |
| 41.90 | 1293 | (E)-β-Methyl-γ-octalactone (trans-Oak lactone) | - | 115 | - | 153 | - | 152 | - | |
| 42.22 | - | 3,7-Dimethyl-1,5-octadiene-3,7-diol (Terpendiol) * | tr. | - | 24 | 34 | - | - | - | 5.3 |
| 42.35 | 1309 | 2-Phenylethyl butyrate | 130 | 31 | 87 | 73 | 100 | 107 | 35 | 7.9 |
| 42.77 | 1321 | 1-Dodecanol | 200 | 170 | 290 | 910 | 170 | 280 | 910 | 5.6 |
| 43.76 | 1351 | Diphenyl oxide | 24 | tr. | 12 | 18 | 14 | 42 | 20 | 11.0 |
| 43.90 | 1354 | (Z)-Nerolidol | 109 | 46 | 45 | - | 36 | 36 | 12 | 5.6 |
| 44.24 | 1365 | γ-Nonalactone | 80 | 32 | - | - | - | - | 85 | 5.8 |
| 45.13 | 1391 | Diethyl malate | 98 | 214 | - | - | - | - | - | 4.0 |
| 45.20 | 1393 | (E)-Nerolidol | 2000 | 2200 | 1800 | 2100 | 1600 | 2500 | 2300 | 9.0 |
| 45.43 | 1400 | Ethyl tetradecanoate (Ethyl myristate) | 900 | 1700 | 410 | 1200 | 450 | 800 | 4500 | 13.3 |
| 45.81 | 1412 | (E)-Methyl cinnamate | 40 | 51 | 17 | 27 | - | - | 56 | 10.2 |
| 46.07 | 1421 | Isoamyl laurate | 12 | - | 36 | 46 | 39 | 55 | 100 | 10.6 |
| 46.08 | 1421 | 1-Tridecanol | - | - | - | - | - | - | - | 6.3 |
| 46.10 | 1422 | Octanoic acid | 59,000 | 66,000 | 37,000 | 43,000 | 49,000 | 28,000 | 52,000 | 6.2 |
| 46.95 | - | Ethyl-3-hydroxydecanoate * | 1200 | 1900 | 620 | 450 | 610 | 380 | 310 | 9.0 |
| 47.09 | 1454 | Diethyl suberate | 100 | 90 | 12 | 30 | 15 | 60 | 460 | 15.1 |
| 47.58 | 1469 | (E)-Ethyl cinnamate | 220 | 360 | 250 | 360 | 140 | 170 | 560 | 8.4 |
| 48.06 | 1485 | 2-Phenoxyethanol (Rose ether) | 12 | tr. | 11 | 21 | tr. | 12 | - | 7.9 |
| 48.53 | 1500 | Ethyl pentadecanoate | 24 | 31 | 25 | 24 | 16 | 12 | 131 | 5.6 |
| 48.82 | 1509 | 2-Phenylethyl hexanoate | 390 | 270 | 143 | 200 | 150 | 290 | 240 | 6.2 |
| 49.18 | 1520 | Nonanoic acid | 82 | 123 | 58 | 78 | 61 | 38 | 126 | 6.2 |
| 49.23 | 1522 | 1-Tetradecanol | - | - | - | - | - | - | 1800 | 7.8 |
| 49.24 | 1522 | 1-Tetradecanol/δ-Decalactone | 127 | 150 | 118 | 400 | 130 | 320 | - | 7.5 |
| 49.74 | 1538 | 2-Methoxy-4-vinylphenol | 300 | 250 | 380 | 390 | 450 | 110 | 83 | 5.0 |
| 51.71 | 1600 | Ethyl hexadecanoate (Ethyl palmitate) | 1300 | 7700 | 2000 | 3700 | 1600 | 1500 | 8100 | 8.3 |
| 51.95 | - | Ethyl-2-hydroxy-3-phenyl propionate * | 240 | 163 | - | - | 137 | 400 | 225 | 2.4 |
| 52.12 | - | 2,3-Dihydrofarnesol * | 400 | 300 | 760 | 1050 | 500 | 1600 | 1600 | 5.5 |
| 52.45 | - | Ethyl-9-hexadecenoate | - | - | 450 | 570 | - | 920 | - | 2.8 |
| 52.60 | 1632 | Decanoic acid | 87,000 | 113,000 | 54,000 | 68,000 | 72,000 | 41,000 | 101,000 | 5.8 |
| 53.25 | - | Ethyl-3-hydroxydodecanoate * | 1300 | 1500 | 410 | 340 | 190 | 108 | 190 | 8.0 |
| 53.71 | - | (Z,E)-Farnesol * | 113 | 31 | 30 | 27 | - | 2.5 | ||
| 54.26 | 1690 | α-Hexylcinnamic aldehyde | tr. | 21 | tr. | tr.- | - | - | - | 9.4 |
| 54.31 | 1692 | Geranic acid | - | - | - | - | - | - | 1120 | 4.8 |
| 54.45 | 1697 | (E,E)-Farnesol | 2700 | 1600 | 2200 | 3200 | 3700 | 3100 | 8300 | 5.4 |
| 54.62 | 1703 | γ-Dodecalactone | 200 | 300 | 450 | 270 | 380 | 182 | 450 | 4.6 |
| 54.95 | 1715 | 2-Phenylethyl octanoate | 530 | 310 | 212 | 207 | 253 | 266 | 398 | 2.3 |
| 55.15 | 1722 | 1-Hexadecanol | 81 | 64 | 135 | 135 | 60 | 20 | 370 | 4.6 |
| 55.57 | 1737 | para-Vinylphenol | 82 | 112 | 550 | 510 | 250 | 250 | 142 | 4.7 |
| 55.91 | 1749 | δ-Dodecalactone | 260 | 440 | 188 | 191 | 220 | 175 | 230 | 4.7 |
| 57.15 | - | 3,4-Dihydro-8-hydroxy-3-methyl-1H-2-benzopyran-1-one (Ochracin) * | - | - | - | - | - | - | 29 | 5.5 |
| 57.19 | - | 3,4-Dihydro-8-hydroxy-3-methyl-1H-2-benzopyran-1-one (Ochracin) */3a,4,5,7a-Tetrahydro-3,6-dimethylbenzofuran-2(3H)-one (Wine lactone) * | 47 | 49 | 171 | 220 | 27 | 34 | - | 4.5 |
| 57.35 | 1800 | Ethyl octadecanoate (Ethyl stearate) | 125 | 530 | 150 | 200 | 210 | 200 | 1000 | 7.1 |
| 58.47 | 1841 | Dodecanoic acid (Lauric acid) | 12,000 | 12,000 | 14,000 | 11,000 | 19,000 | 13,000 | 31,000 | 8.3 |
| 59.07 | 1861/1863 | Ethyl linoleate/Diethyl dodecanedioate | 420 | 1100 | 800 | 1500 | 900 | 1300 | - | 11.3 |
| 59.10 | 1865 | Ethyl linoleate | - | 270 | - | 320 | - | 330 | 1700 | 8.2 |
| 60.80 | - | Ethyl linoleolate * | 113 | 270 | 230 | 320 | 160 | 330 | 500 | 7.3 |
| 61.30 | 1946 | Benzyl benzoate | 50 | 78 | - | 56 | 28 | 22 | - | 6.5 |
| 62.02 | 1973 | δ-Tetradecalactone | 270 | 380 | 320 | 370 | 280 | 270 | 330 | 6.9 |
| 63.94 | 2044 | Tetradecanoic acid (Myristic acid) | 1300 | 1040 | 254 | 420 | 277 | 188 | 3500 | 3.2 |
| 66.52 | 2140 | Pentadecanoic acid | 212 | 191 | 21 | 27 | 16 | 16 | - | 4.2 |
| 69.00 | 2230 | Hexadecanoic acid (Palmitic acid) | 4700 | 4200 | 670 | 2000 | 560 | 820 | 3900 | 5.5 |
| 71.41 | 2320 | Heptadecanoic acid | 450 | 250 | - | - | - | - | 22 | 4.5 |
| 73.96 | 2414 | Octadecanoic acid (Stearic acid) | 650 | 690 | 65 | 71 | 33 | 35 | 105 | 5.6 |
| 74.74 | 2443 | 9-Octadecenoic acid (Oleic acid) | 1500 | 1700 | - | 145 | - | - | - | 6.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caven-Quantrill, D.J.; Buglass, A.J. Analysis of Volatile Components of Varietal English Wines Using Stir Bar Sorptive Extraction/Gas Chromatography-Mass Spectrometry. Beverages 2017, 3, 62. https://doi.org/10.3390/beverages3040062
Caven-Quantrill DJ, Buglass AJ. Analysis of Volatile Components of Varietal English Wines Using Stir Bar Sorptive Extraction/Gas Chromatography-Mass Spectrometry. Beverages. 2017; 3(4):62. https://doi.org/10.3390/beverages3040062
Chicago/Turabian StyleCaven-Quantrill, Darren J., and Alan J. Buglass. 2017. "Analysis of Volatile Components of Varietal English Wines Using Stir Bar Sorptive Extraction/Gas Chromatography-Mass Spectrometry" Beverages 3, no. 4: 62. https://doi.org/10.3390/beverages3040062