Tick-Borne Relapsing Fever Spirochetes in the Americas
Abstract
:1. Introduction
2. Clinical Manifestation of Disease
3. Diagnosis of Exposure to RF Spirochetes
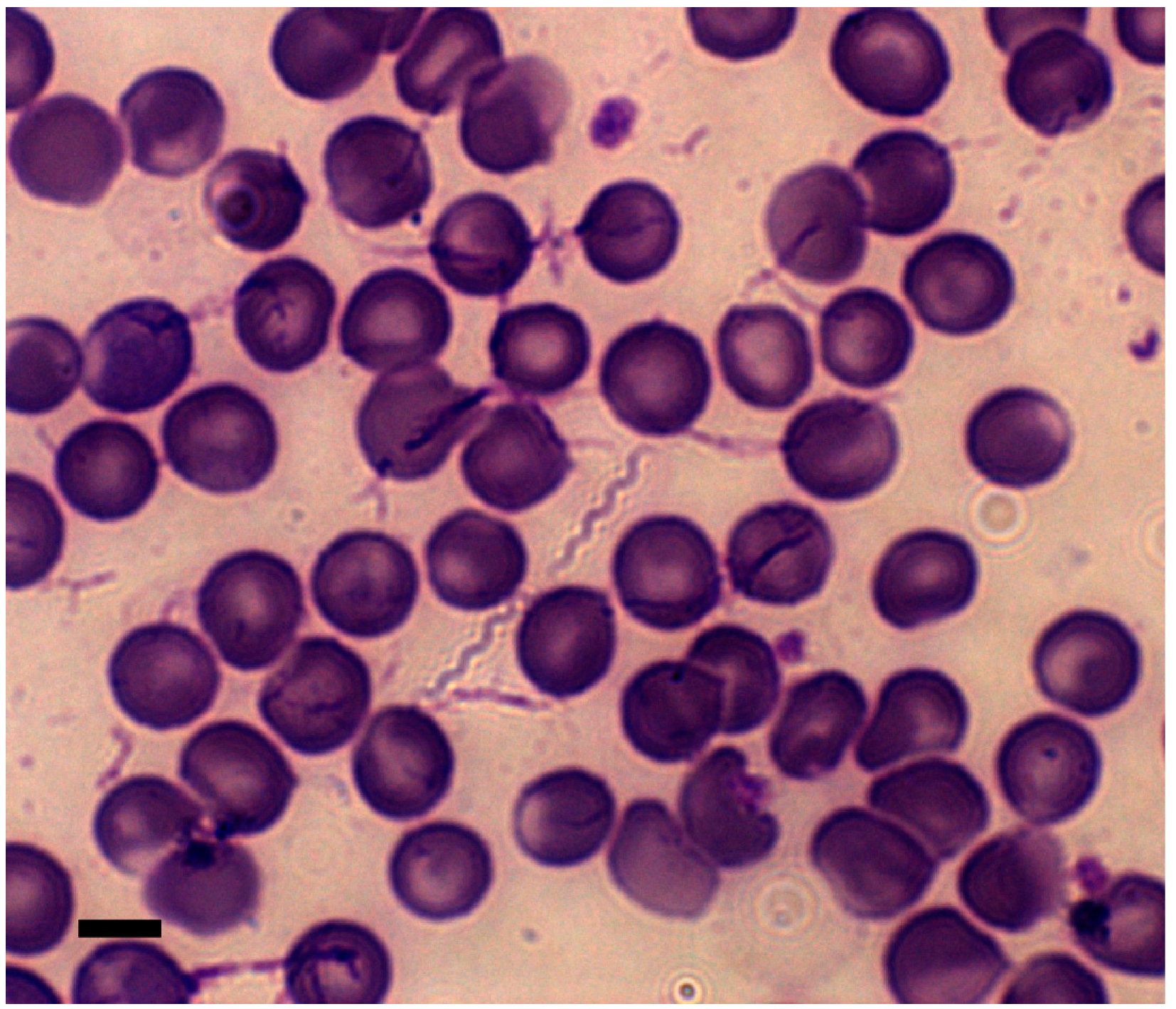
4. The Life Cycle of ABRF Spirochetes in the Mammal
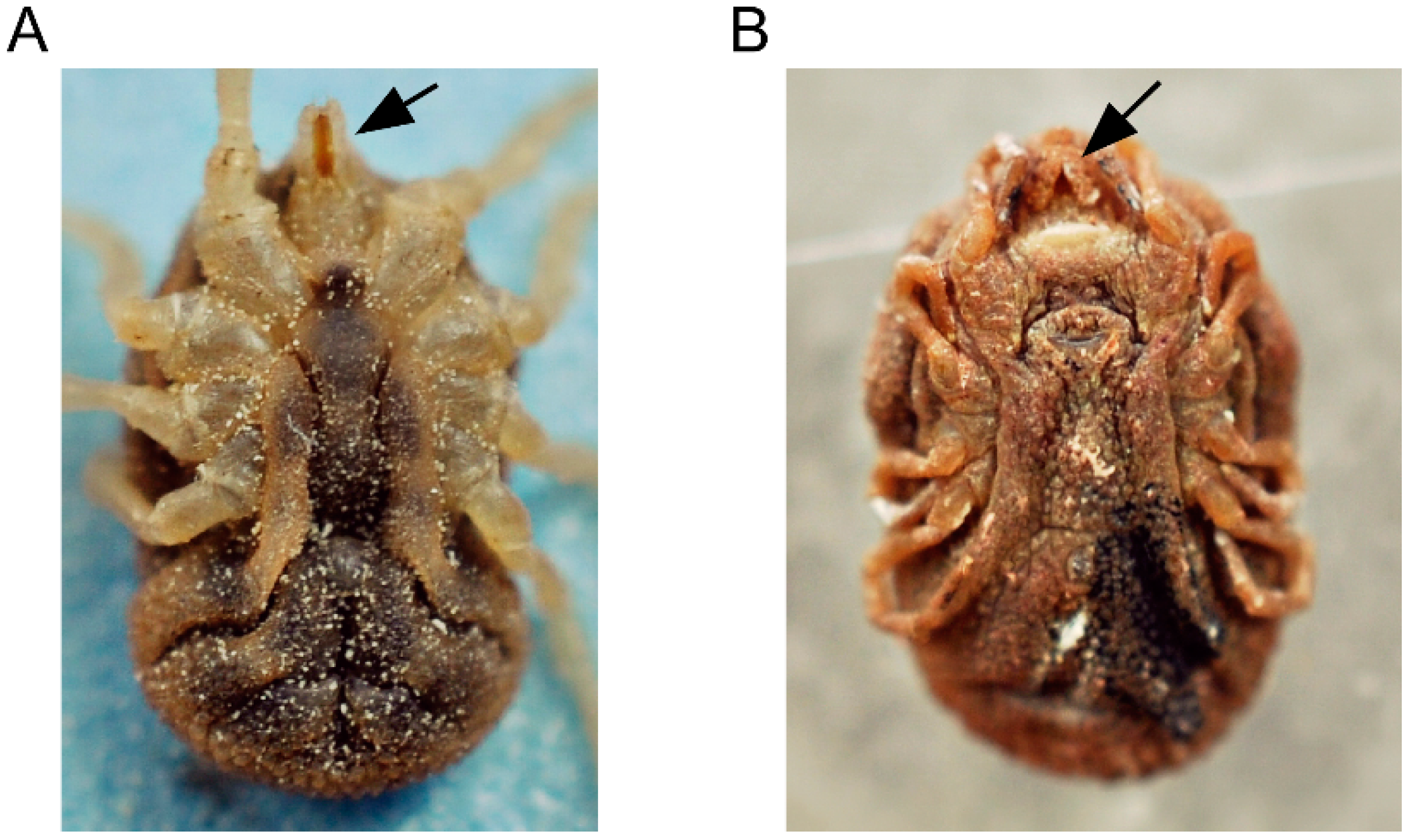
5. The Life Cycle of ABRF Spirochetes in the Tick Vector
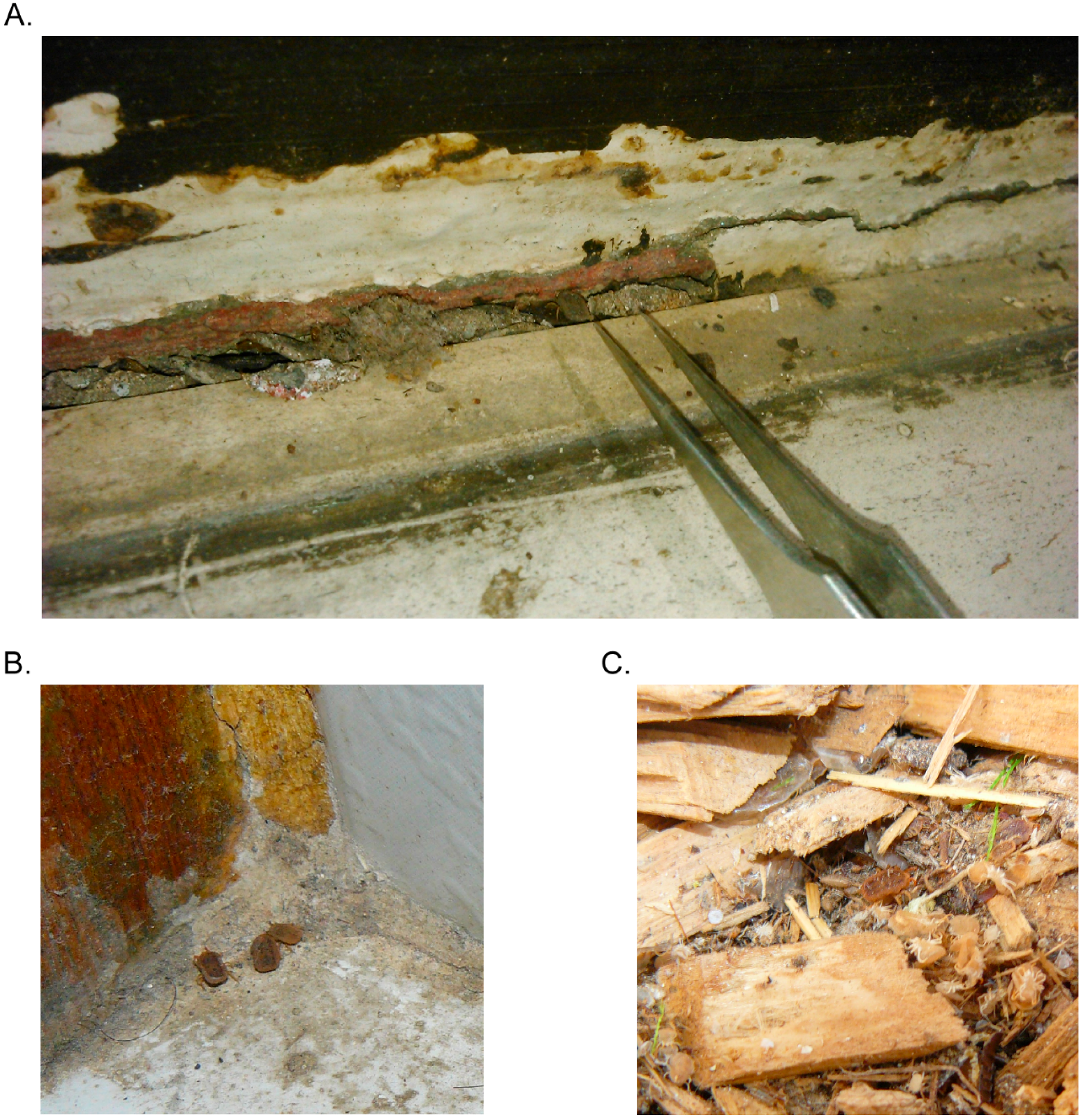
6. Ecology of ABRF in North America
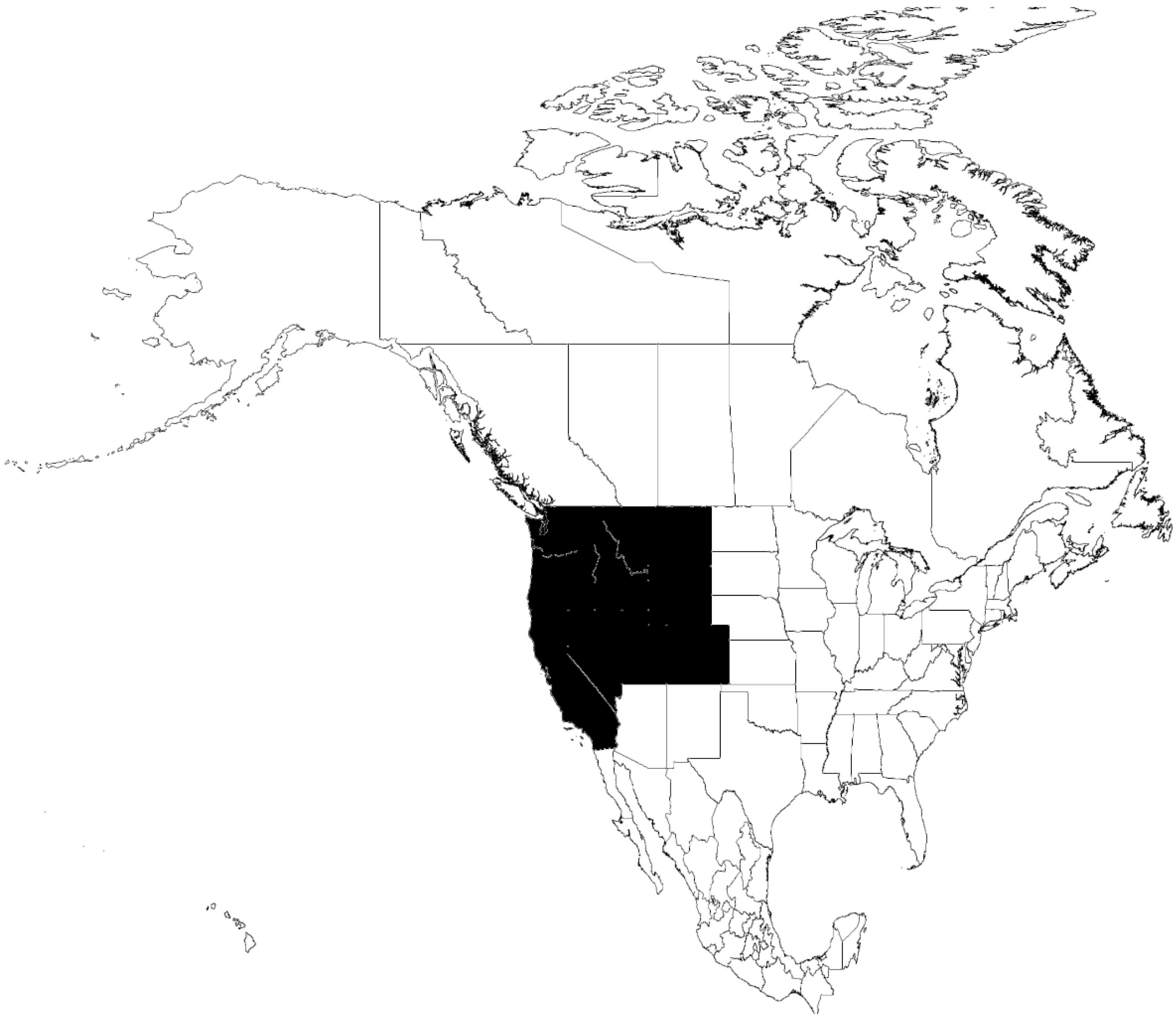
6.1. O. parkeri-B. parkeri
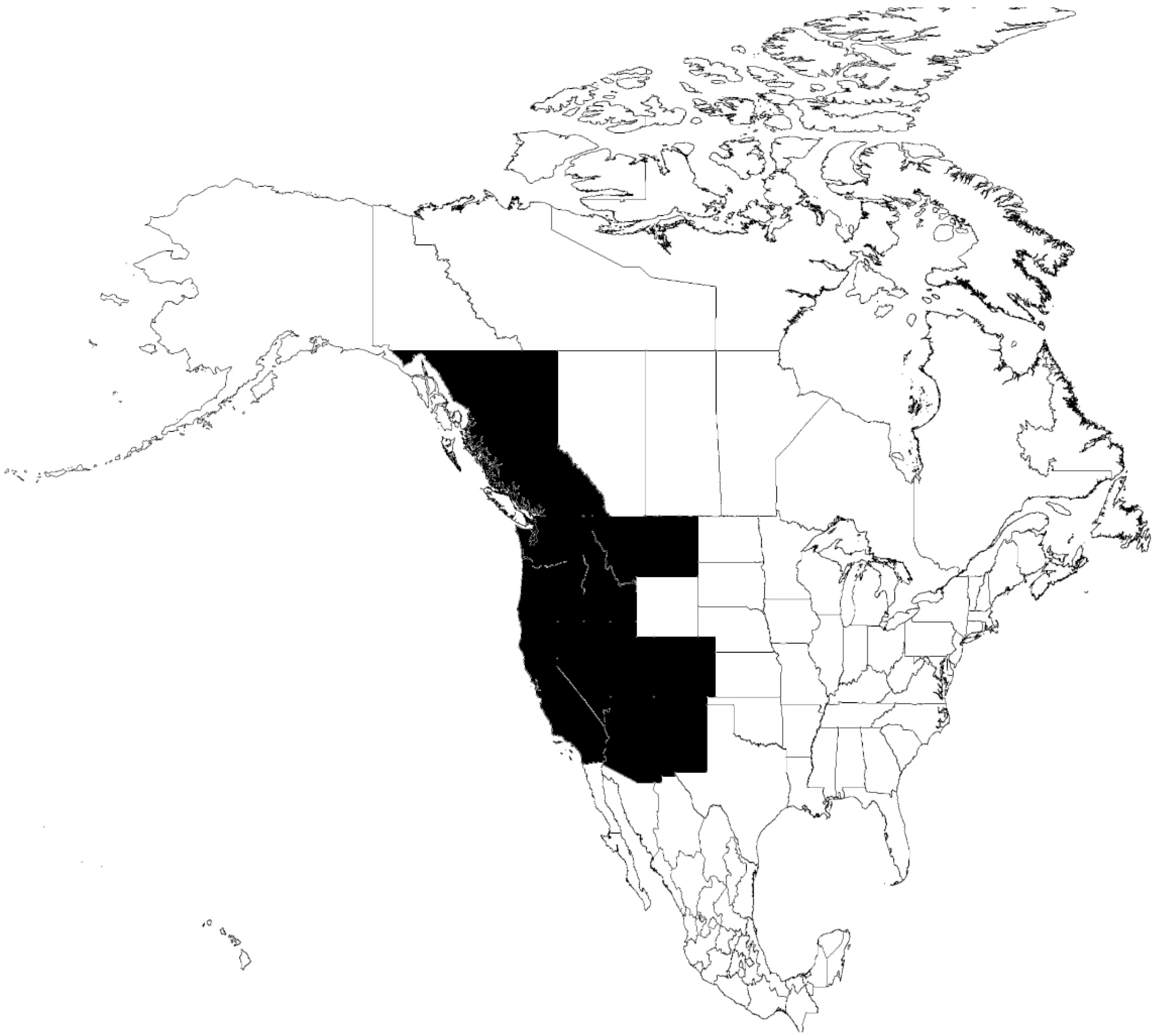
6.2. O. hermsi-B. hermsii
6.3. O. turicata-B. turicatae
6.4. O. talaje-B. mazzottii
7. ABRF in Central America
8. ABRF in South America
9. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Varma, M.G.R. Transmission of relapsing fever spirochetes by ticks. In Proceedings of the Symposia of the Zoological Society of London, Regent’s Park, London, UK, 8 March 1962.
- Davis, G.E. Ticks and relapsing fever in the United States. Public Health Rep. 1940, 55, 2347–2351. [Google Scholar] [CrossRef]
- Larsson, C.; Andersson, M.; Bergstrom, S. Current issues in relapsing fever. Curr. Opin. Infect. Dis. 2009, 22, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S. Spirochaetes: Past lessons to future directions. Clin. Microbiol. Infect. 2011, 17, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.J. Possibilities for relapsing fever reemergence. Emerg. Inf. Dis. 2006, 12, 369–374. [Google Scholar] [CrossRef]
- Nordstrand, A.; Bunikis, I.; Larsson, C.; Tsogbe, K.; Schwan, T.G.; Nilsson, M.; Bergström, S. Tickborne relapsing fever diagnosis obscured by malaria, Togo. Emerg. Infect. Dis. 2007, 13, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Socolovschi, C.; Bassene, H.; Diatta, G.; Ratmanov, P.; Fenollar, F.; Sokhna, C.; Raoult, D. Borrelia crocidurae infection in acutely febrile patients, Senegal. Emerg. Infect. Dis. 2014, 20, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Trape, J.F.; Duplantier, J.M.; Bouganali, H.; Godeluck, B.; Legros, F.; Cornet, J.P.; Camicas, J.L. Tick-borne borreliosis in West Africa. Lancet 1991, 337, 473–475. [Google Scholar] [CrossRef]
- Trape, J.F.; Godeluck, B.; Diatta, G.; Rogier, C.; Legros, F.; Albergel, J.; Pepin, Y.; Duplantier, J.M. Tick-borne borreliosis in West Africa: Recent epidemiological studies. Rocz. Akad. Med. Bialymst. 1996, 41, 136–141. [Google Scholar] [CrossRef]
- Schwan, T.G.; Anderson, J.M.; Lopez, J.E.; Fischer, R.J.; Raffel, S.J.; McCoy, B.N.; Safronetz, D.; Sogoba, N.; Maiga, O.; Traore, S.F. Endemic foci of the tick-borne relapsing fever spirochete Borrelia crocidurae in Mali, West Africa, and the potential for human infection. PLoS Negl. Trop. Dis. 2012, 6, e1924. [Google Scholar] [CrossRef] [PubMed]
- Diatta, G.; Souidi, Y.; Granjon, L.; Arnathau, C.; Durand, P.; Chauvancy, G.; Mane, Y.; Sarih, M.; Belghyti, D.; Renaud, F.; et al. Epidemiology of tick-borne borreliosis in Morocco. PLoS Negl. Trop. Dis. 2012, 6, e1810. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, M.S.; Anderson, D.E., Jr.; Schwan, T.G.; Shoemaker, P.C.; Banerjee, S.N.; Kassen, B.O.; Burgdorfer, W. Tick-borne relapsing fever in the Northwestern United States and Southwestern Canada. Clin. Infect. Dis. 1998, 26, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, M.S.; Schwan, T.G.; Anderson, D.E. Tick-borne relapsing fever in North America. Med. Clin. N. Am. 2002, 86, 417–433. [Google Scholar] [CrossRef]
- Dworkin, M.S.; Schwan, T.G.; Anderson, D.E., Jr.; Borchardt, S.M. Tick-borne relapsing fever. Infect. Dis. Clin. N. Am. 2008, 22, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, M.S.; Shoemaker, P.C.; Fritz, C.L.; Dowell, M.E.; Anderson, D.E., Jr. The epidemiology of tick-borne relapsing fever in the United States. Am. J. Trop. Med. Hyg. 2002, 66, 753–758. [Google Scholar] [PubMed]
- Krause, P.J.; Narasimhan, S.; Wormser, G.P.; Rollend, L.; Fikrig, E.; Lepore, T.; Barbour, A.; Fish, D. Human Borrelia miyamotoi infection in the United States. N. Engl. J. Med. 2013, 368, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Southern, P.M.; Sanford, J.P. Relapsing fever: A clinical and microbiological review. Medicine 1969, 48, 129–149. [Google Scholar] [CrossRef]
- Davis, G.E. Relapsing fever: The tick Ornithodoros turicata as a spirochetal reservoir. Public Health Rep. 1968, 58, 839–842. [Google Scholar] [CrossRef]
- Barbour, A.G.; Dai, Q.; Restrepo, B.I.; Stoenner, H.G.; Frank, S.A. Pathogen escape from host immunity by a genome program for antigenic variation. Proc. Natl. Acad. Sci. USA 2006, 103, 18290–18295. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Restrepo, B.I.; Porcella, S.F.; Raffel, S.J.; Schwan, T.G.; Barbour, A.G. Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids. Mol. Microbiol. 2006, 60, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.T.; Simon, M.I.; Barbour, A.G. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell 1985, 41, 403–409. [Google Scholar] [CrossRef]
- Davis, R.D.; Burke, J.P.; Wright, L.J. Relapsing fever associated with ARDS in a parturient woman. A case report and review of the literature. Chest 1992, 102, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Fihn, S.; Larson, E.B. Tick-borne relapsing fever in the Pacific Northwest: An underdiagnosed illness? West. J. Med. 1980, 133, 203–209. [Google Scholar] [PubMed]
- Badger, M.S. Tick talk: Unusually severe case of tick-borne relapsing fever with acute respiratory distress syndrome—Case report and review of the literature. Wilderness Environ. Med. 2008, 19, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.C.; Oyama, A.A. Neonatal relapsing fever due to transplacental transmission of Borrelia. JAMA 1969, 208, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Bryceson, A.D. Clinical pathology of the Jarisch-Herxheimer reaction. J. Infect. Dis. 1976, 133, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Vidal, V.; Scragg, I.G.; Cutler, S.J.; Rockett, K.A.; Fekade, D.; Warrell, D.A.; Wright, D.J.; Kwiatkowski, D. Variable major lipoprotein is a principal TNF-inducing factor of louse-borne relapsing fever. Nat. Med. 1998, 4, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Hovius, J.W.; de Wever, B.; Sohne, M.; Brouwer, M.C.; Coumou, J.; Wagemakers, A.; Oei, A.; Knol, H.; Narasimhan, S.; Hodiamont, C.J.; et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 2013, 382, 658. [Google Scholar] [CrossRef]
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V.V.; Fish, D.; Krause, P.J. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Molloy, P.J.; Telford, S.R., 3rd; Chowdri, H.R.; Lepore, T.J.; Gugliotta, J.L.; Weeks, K.E.; Hewins, M.E.; Goethert, H.K.; Berardi, V.P. Borrelia miyamotoi disease in the Northeastern United States: A case series. Ann. Intern. Med. 2015, 163, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Telford, S.R., 3rd; Goethert, H.K.; Molloy, P.J.; Berardi, V.P.; Chowdri, H.R.; Gugliotta, J.L.; Lepore, T.J. Borrelia miyamotoi disease: Neither Lyme disease nor relapsing fever. Clin. Lab. Med. 2015, 35, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G. Multiple and Diverse vsp and vlp Sequences in Borrelia miyamotoi, a hard tick-borne zoonotic pathogen. PLoS ONE 2016, 11, e0146283. [Google Scholar] [CrossRef] [PubMed]
- McCoy, B.N.; Raffel, S.J.; Lopez, J.E.; Schwan, T.G. Bloodmeal size and spirochete acquisition of Ornithodoros hermsi (Acari: Argasidae) during feeding. J. Med. Entomol. 2010, 47, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Schwan, T.G.; Schrumpf, M.E.; Hinnebusch, B.J.; Anderson, D.E.; Konkel, M.E. GlpQ: An antigen for serological discrimination between relapsing fever and Lyme borreliosis. J. Clin. Microbiol. 1996, 34, 2483–2492. [Google Scholar] [PubMed]
- Lopez, J.E.; Porcella, S.F.; Schrumpf, M.E.; Raffel, S.J.; Hammer, C.H.; Zhao, M.; Robinson, M.A.; Schwan, T.G. Identification of conserved antigens for early serodiagnosis of relapsing fever Borrelia. Microbiology 2009, 155, 2641–2651. [Google Scholar] [CrossRef] [PubMed]
- Wilder, H.K.; Wozniak, E.; Huddleston, E.; Tata, S.R.; Fitzkee, N.C.; Lopez, J.E. Case report: A retrospective serological analysis indicating human exposure to tick-borne relapsing fever spirochetes in Texas. PLoS Negl. Trop. Dis. 2015, 9, e0003617. [Google Scholar] [CrossRef] [PubMed]
- Porcella, S.F.; Raffel, S.J.; Schrumpf, M.E.; Schriefer, M.E.; Dennis, D.T.; Schwan, T.G. Serodiagnosis of louse-borne relapsing fever with glycerophosphodiester phosphodiesterase (GlpQ) from Borrelia recurrentis. J. Clin. Microbiol. 2000, 38, 3561–3571. [Google Scholar] [PubMed]
- Lopez, J.E.; Schrumpf, M.E.; Nagarajan, V.; Raffel, S.J.; McCoy, B.N.; Schwan, T.G. A novel surface antigen of relapsing fever spirochetes can discriminate between relapsing fever and Lyme borreliosis. Clin. Vaccine Immunol. 2010, 17, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.E.; Wilder, H.K.; Boyle, W.; Drumheller, L.B.; Thornton, J.A.; Willeford, B.; Morgan, T.W.; Varela-Stokes, A. Sequence analysis and serological responses against Borrelia turicatae BipA, a putative species-specific antigen. PLoS Negl. Trop. Dis. 2013, 7, e2454. [Google Scholar] [CrossRef] [PubMed]
- Cooley, R.A.; Kohls, G.M. The Agarasidae of North America, Central America, and Cuba; The University Press: Notre Dame, IN, USA, 1944; pp. 1–152. [Google Scholar]
- Balashov, Y.S. Bloodsucking ticks (Ixodoidea)-vectors of diseases of man and animals. Misc. Publ. Entomol. Soc. Am. 1972, 8, 161–376. [Google Scholar]
- Boyle, W.K.; Wilder, H.K.; Lawrence, A.M.; Lopez, J.E. Transmission dynamics of Borrelia turicatae from the arthropod vector. PLoS Negl. Trop. Dis. 2014, 8, e2767. [Google Scholar] [CrossRef] [PubMed]
- Alugupalli, K.R.; Gerstein, R.M.; Chen, J.; Szomolanyi-Tsuda, E.; Woodland, R.T.; Leong, J.M. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J. Immunol. 2003, 170, 3819–3827. [Google Scholar] [CrossRef] [PubMed]
- Woodman, M.E.; Cooley, A.E.; Avdiushko, R.; Bowman, A.; Botto, M.; Wooten, R.M.; van Rooijen, N.; Cohen, D.A.; Stevenson, B. Roles for phagocytic cells and complement in controlling relapsing fever infection. J. Leukoc. Biol. 2009, 86, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Schwan, T.G.; Hinnebusch, B.J. Bloodstream-versus tick-associated variants of a relapsing fever bacterium. Science 1998, 280, 1938–1940. [Google Scholar] [CrossRef] [PubMed]
- Raffel, S.J.; Battisti, J.M.; Fischer, R.J.; Schwan, T.G. Inactivation of genes for antigenic variation in the relapsing fever spirochete Borrelia hermsii reduces infectivity in mice and transmission by ticks. PLoS Pathog. 2014, 10, e1004056. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.E.; McCoy, B.N.; Krajacich, B.J.; Schwan, T.G. Acquisition and subsequent transmission of Borrelia hermsii by the soft tick Ornithodoros hermsi. J. Med. Entomol. 2011, 48, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Landguth, E.L.; Stone, E.F. Modeling relapsing disease dynamics in a host-vector community. PLoS Negl. Trop. Dis. 2016, 10, e0004428. [Google Scholar] [CrossRef] [PubMed]
- Schwan, T.G.; Policastro, P.F.; Miller, Z.; Thompson, R.L.; Damrow, T.; Keirans, J.E. Tick-borne relapsing fever caused by Borrelia hermsii, Montana. Emerg. Infect. Dis. 2003, 9, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Saido-Sakanaka, H.; Taylor, D.; Yamakawa, M. Up-regulated humoral immune response in the soft tick, Ornithodoros moubata (Acari: Argasidae). Parasitol. Res. 2003, 91, 476–481. [Google Scholar] [PubMed]
- Nakajima, Y.; Taylor, D.; Yamakawa, M. Involvement of antibacterial peptide defensin in tick midgut defense. Exp. Appl. Acarol. 2002, 28, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; van der Goes van Naters-Yasui, A.; Taylor, D.; Yamakawa, M. Antibacterial peptide defensin is involved in midgut immunity of the soft tick, Ornithodoros moubata. Insect Mol. Biol. 2002, 11, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Francischetti, I.M.; Mans, B.J.; Meng, Z.; Gudderra, N.; Veenstra, T.D.; Pham, V.M.; Ribeiro, J.M. An insight into the sialome of the soft tick, Ornithodorus parkeri. Insect Biochem. Mol. Biol. 2008, 38, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; Andersen, J.F.; Francischetti, I.M.; Valenzuela, J.G.; Schwan, T.G.; Pham, V.M.; Garfield, M.K.; Hammer, C.H.; Ribeiro, J.M. Comparative sialomics between hard and soft ticks: Implications for the evolution of blood-feeding behavior. Insect Biochem. Mol. Biol. 2008, 38, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Schwan, T.G.; Piesman, J. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg. Infect. Dis. 2002, 8, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E. Ornithodoros turicata: The male; feeding and copulation habits, fertility, span of life, and the transmission of relapsing fever spirochetes. Public Health Rep. 1941, 56, 1799–1802. [Google Scholar] [CrossRef]
- Wilder, H.K.; Raffel, S.J.; Barbour, A.G.; Porcella, S.F.; Sturdevant, D.E.; Vaisvil, B.; Kapatral, V.; Schmitt, D.P.; Schwan, T.G.; Lopez, J.E. Transcriptional profiling the 150 kb linear megaplasmid of Borrelia turicatae suggests a role in vector colonization and initiating mammalian infection. PLoS ONE 2016, 11, e0147707. [Google Scholar] [CrossRef] [PubMed]
- Brumpt, E. Étude du Spirochaeta turicatae, n. sp., agent de la fièvre récurrente sporadique des Etats-Unis trasmise par Ornithodoros turicata. C. R. Soc. Biol. 1933, 113, 1369. [Google Scholar]
- Davis, GE. A relapsing fever spirochete, Borrelia mazzottii (sp. nov.) from Ornithodoros talaje from Mexico. Am. J. Hyg. 1956, 63, 13–17. [Google Scholar] [PubMed]
- Schwan, T.G. Ticks and Borrelia: Model systems for investigating pathogen-arthropod interactions. Infect. Agents Dis. 1996, 5, 167–181. [Google Scholar] [PubMed]
- Fedorova, N.; Kleinjan, J.E.; James, D.; Hui, L.T.; Peeters, H.; Lane, R.S. Remarkable diversity of tick or mammalian-associated Borreliae in the metropolitan San Francisco Bay area, California. Ticks Tick Borne Dis. 2014, 5, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Scoles, G.A.; Papero, M.; Beati, L.; Fish, D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoon. Dis. 2001, 1, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Geographic Distribution of Ticks That Bite Humans. Avaiable online: http://www.cdc.gov/ticks/geographic_distribution.html (accessed on 15 August 2016).
- Reisen, W.K. Landscape epidemiology of vector-borne diseases. Annu. Rev. Entomol. 2010, 55, 461–483. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E. Ornithodoros parkeri: Distribution and host data; spontaneous infection with relapsing fever spirochetes. Public Health Rep. 1939, 54, 1345–1349. [Google Scholar] [CrossRef]
- Davis, G.E. Ornithodoros parkeri Cooley: Observations on the biology of this tick. J. Parasitol. 1940, 27, 425–433. [Google Scholar]
- Cooley, R.A. Ornithodoros parkeri, a new species on rodents. Public Health Rep. 1936, 51, 431–433. [Google Scholar] [CrossRef]
- Nieto, N.C.; Teglas, M.B. Relapsing fever group Borrelia in Southern California rodents. J. Med. Entomol. 2014, 51, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Nieto, N.C.; Teglas, M.B.; Stewart, K.M.; Wasley, T.; Wolff, P.L. Detection of relapsing fever spirochetes (Borrelia hermsii and Borrelia coriaceae) in free-ranging mule deer (Odocoileus hemionus) from Nevada, United States. Vector Borne Zoon. Dis. 2012, 12, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Fischer, R.J.; McCoy, B.N.; Raffel, S.J.; Schwan, T.G. Tickborne relapsing fever, Bitterroot Valley, Montana, USA. Emerg. Infect. Dis. 2015, 21, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Piesman, J.; Schwan, T.G. Ecology of Borreliae and Their Arthropod Vectors; Caister Academic Press: Norfolk, UK, 2010. [Google Scholar]
- Schwan, T.G.; Raffel, S.J.; Schrumpf, M.E.; Webster, L.S.; Marques, A.R.; Spano, R.; Rood, M.; Burns, J.; Hu, R. Tick-borne relapsing fever and Borrelia hermsii, Los Angeles County, California, USA. Emerg. Infect. Dis. 2009, 15, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Burgdorfer, W.; Mavros, A.J. Susceptibility of various species of rodents to the relapsing fever spirochete, Borrelia hermsii. Infect. Immun. 1970, 2, 256–259. [Google Scholar] [PubMed]
- Fritz, C.L.; Payne, J.R.; Schwan, T.G. Serologic evidence for Borrelia hermsii infection in rodents on federally owned recreational areas in California. Vector Borne Zoon. Dis. 2013, 13, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R. Cultivation of Borrelia hermsi. Science 1971, 173, 443–444. [Google Scholar] [CrossRef] [PubMed]
- Schwan, T.G.; Raffel, S.J.; Schrumpf, M.E.; Porcella, S.F. Diversity and distribution of Borrelia hermsii. Emerg. Infect. Dis. 2007, 13, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.J.; Bunikis, J.; Barbour, A.G.; Wolcott, M.J. Fatal spirochetosis due to a relapsing fever-like Borrelia sp. in a northern spotted owl. J. Wildl. Dis. 2002, 38, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Bunikis, J.; Tsao, J.; Garpmo, U.; Berglund, J.; Fish, D.; Barbour, A.G. Typing of Borrelia relapsing fever group strains. Emerg. Infect. Dis. 2004, 10, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.L.; Raffel, S.J.; Fischer, R.J.; Bellinghausen, M.; Stevenson, C.; Schwan, T.G. First isolation of the relapsing fever spirochete, Borrelia hermsii, from a domestic dog. Ticks Tick Borne Dis. 2014, 5, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Felsenfeld, O. The problem of relapsing fever in the Americas. IMS Ind. Med. Surg. 1973, 42, 7–10. [Google Scholar] [PubMed]
- Donaldson, T.G.; Perez de Leon, A.A.; Li, A.I.; Castro-Arellano, I.; Wozniak, E.; Boyle, W.K.; Hargrove, R.; Wilder, H.K.; Kim, H.J.; Teel, P.D.; et al. Assessment of the geographic distribution of Ornithodoros turicata (Argasidae): Climate variation and host diversity. PLoS Negl. Trop. Dis. 2016, 10, e0004383. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, O.A.; Butler, J.F. Population structure and seasonal intra-burrow movement of Ornithodoros turicata (Acari: Argasidae) in gopher tortoise burrows. J. Med. Entomol. 1989, 26, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, O.A.; Butler, J.F. Field evaluation of carbon dioxide baits for sampling Ornithodoros turicata (Acari: Argasidae) in gopher tortoise burrows. J. Med. Entomol. 1991, 28, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Largo, P.K. A survey of arthropods associated with gopher tortoise burrows in Mississippi. Entomol. News 1991, 102, 1–13. [Google Scholar]
- Beck, A.F.; Holscher, K.H.; Butler, J.F. Life cycle of Ornithodoros turicata americanus (Acari: Argasidae) in the laboratory. J. Med. Entomol. 1986, 23, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.D. Present distribution of relapsing fever in California. In Proceedings of the A Symposium on Relapsing Fever in the Americas; Moulton, F.R., Ed.; American Association for the Advancement of Science: Washington, DC, USA, 1942; pp. 20–25. [Google Scholar]
- Wheeler, C.M. The distribution of the spirochete of California relapsing fever within the body of the vector, Ornithodoros hermsi. In Proceedings of the A Symposium on Relapsing Fever in the Americas; Moulton, F.R., Ed.; American Association for the Advancement of Science: Washington, DC, USA, 1942; pp. 89–99. [Google Scholar]
- Breitschwerdt, E.B.; Nicholson, W.L.; Kiehl, A.R.; Steers, C.; Meuten, D.J.; Levine, J.F. Natural infections with Borrelia spirochetes in two dogs from Florida. J. Clin. Microbiol. 1994, 32, 352–357. [Google Scholar] [PubMed]
- Whitney, M.S.; Schwan, T.G.; Sultemeier, K.B.; McDonald, P.S.; Brillhart, M.N. Spirochetemia caused by Borrelia turicatae infection in 3 dogs in Texas. Vet. Clin. Pathol. 2007, 36, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Francis, E. Longevity of the tick Ornithodoros turicata and of Spirochaeta recurrentis with this tick. Public Health Rep. 1938, 53, 2220–2241. [Google Scholar] [CrossRef]
- Bates, L.B.; Dunn, L.H.; St. John, J.H. Relapsing fever in Panama. Am. J. Trop. Med. Hyg. 1921, 1, 183–210. [Google Scholar]
- Venzal, J.M.; Estrada-Pena, A.; Mangold, A.J.; Gonzalez-Acuna, D.; Guglielmone, A.A. The Ornithodoros (Alectorobius) talaje species group (Acari: Ixodida: Argasidae): Description of Ornithodoros (Alectorobius) rioplatensis n. sp. from southern South America. J. Med. Entomol. 2008, 45, 832–840. [Google Scholar] [PubMed]
- Hoogstraal, H. Argasid and nuttalliellid ticks as parasites and vectors. Adv. Parasitol. 1985, 24, 135–238. [Google Scholar] [PubMed]
- Bermudez, S.E.; Miranda, R.J.; Smith, D. Ticks species (Ixodida) in the Summit Municipal Park and adjacent areas, Panama City, Panama. Exp. Appl. Acarol. 2010, 52, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Need, J.T.; Butler, J.F. Sequential feedings by two species of argasid tick on laboratory mice: Effects on tick survival, weight gain, and attachment time. J. Med. Entomol. 1991, 28, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Dutton, J.E.; Todd, J.L. The nature of human tick-fever in the eastern part of the Congo Free State with notes on the distribution and bionomics of the tick. Liverp. Sch. Trop. Med. 1905, 17, 1–18. [Google Scholar]
- Darling, S.T. The relapsing fever of Panama. Arch. Intern. Med. 1909, 4, 150–185. [Google Scholar] [CrossRef]
- Dunn, L.H.; Clark, H.C. Notes on relapsing fever in Panama with special reference to animal hosts. Am. J. Trop. Med. Hyg. 1933, 13, 201–209. [Google Scholar]
- Heerdink, G.; Petit, P.L.; Hofwegen, H.; van Genderen, P.J. A patient with fever following a visit to the tropics: Tick-borne relapsing fever discovered in a thick blood smear preparation. Ned. Tijdschr. Geneeskd. 2006, 150, 2386–2389. [Google Scholar] [PubMed]
- Bermudez, S.E.; Miranda, R.; Cleghorn, J.; Venzal, J. Ornithodoros (Alectorobius) puertoricensis (Ixodida: Argasidae) parasitizing exotic reptiles pets in Panama. Rev. FAVE-Cienc. Vet. 2015, 14, 1–5. [Google Scholar] [CrossRef]
- Bermudez, S.E.; Miranda, R.; Kadoch, N. Reporte de larvas de Ornithodoros puertoricensis Fox 1947 (Ixodida: Argasidae) parasitando a Rhinella marina (L. 1758) (Anura: Bufonidae) en David, Chiriquí, Panamá. Puente Biol. 2013, 5, 81–85. [Google Scholar]
- Rangel, G.; Bermudez, S.E. Nota sobre un caso de parasitismo de Ornithodoros sp. (Ixodida: Argasidae) en una mujer proveniente de La Laja, Los Santos, Panamá. Rev. Méd. Panamá 2013, 34, 37–39. [Google Scholar]
- Pinto, C.; Primo, R. Contribuicão para a biologia dos Ixodidae so Estado do Rio Grande do Sul (Brazil). Rev. Med. Cir. Braz. 1931, 34, 5–6. [Google Scholar]
- Davis, G.E. Observations on the biology of the argasid tick, Ornithodoros brasiliensis Aragao, 1923; with the recovery of a spirochete, Borrelia brasiliensis, n. sp. J. Parasitol. 1952, 38, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Reck, J.; Marks, F.S.; Guimaraes, J.A.; Termignoni, C.; Martins, J.R. Epidemiology of Ornithodoros brasiliensis (mouro tick) in the southern Brazilian highlands and the description of human and animal retrospective cases of tick parasitism. Ticks Tick Borne Dis. 2013, 4, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.; Doyle, R.L.; Barros-Battesti, D.M.; Onofrio, V.C.; Guglielmone, A.A. Occurrence of Ornithodoros brasiliensis Aragao (Acari: Argasidae) in Sao Francisco de Paula, RS, Southern Brazil. Neotrop. Entomol. 2011, 40, 143–1444. [Google Scholar] [CrossRef] [PubMed]
- Reck, J.; Soares, J.F.; Termignoni, C.; Labruna, M.B.; Martins, J.R. Tick toxicosis in a dog bitten by Ornithodoros brasiliensis. Vet. Clin. Pathol. 2011, 40, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Reck, J.; Bandarra, P.; Pavarini, S.; Termignoni, C.; Driemeier, D.; Martins, J.R.; Guimaraes, J.A. Experimentally induced tick toxicosis in rats bitten by Ornithodoros brasiliensis (Chelicerata: Argasidae): A clinico-pathological characterization. Toxicon 2014, 88, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Reck, J.; Marks, F.S.; Termignoni, C.; Guimaraes, J.A.; Martins, J.R. Ornithodoros brasiliensis (mouro tick) salivary gland homogenates inhibit in vivo wound healing and in vitro endothelial cell proliferation. Parasitol. Res. 2013, 112, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Ryelandt, J.; Mangold, A.J.; Mediannikov, O.; Guglielmone, A.A.; Raoult, D. Relapsing fever Borrelia in Ornithodoros ticks from Bolivia. Ann. Trop. Med. Parasitol. 2011, 105, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Ciceroni, L.; Bartoloni, A.; Guglielmetti, P.; Paradisi, F.; Barahona, H.G.; Roselli, M.; Ciarrocchi, S.; Cacciapuoti, B. Prevalence of antibodies to Borrelia burgdorferi, Borrelia parkeri and Borrelia turicatae in human settlements of the Cordillera Province, Bolivia. J. Trop. Med. Hyg. 1994, 97, 13–17. [Google Scholar] [PubMed]
- Wagemakers, A.; Staarink, P.J.; Sprong, H.; Hovius, J.W. Borrelia miyamotoi: A widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015, 31, 260–269. [Google Scholar] [CrossRef] [PubMed]


| Biological Traits | Ixodes spp. | Ornithodoros spp. |
|---|---|---|
| Life span | 2–3 years | 5–20 years |
| Nymphal stages | 1 | >7 |
| Feeding strategy | Questing/Nidicolous A | Nidicolous |
| Feeding duration | 5–7 days | 5–60 min |
| Transmission | unknown | ~15 s B |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, J.E.; Krishnavahjala, A.; Garcia, M.N.; Bermudez, S. Tick-Borne Relapsing Fever Spirochetes in the Americas. Vet. Sci. 2016, 3, 16. https://doi.org/10.3390/vetsci3030016
Lopez JE, Krishnavahjala A, Garcia MN, Bermudez S. Tick-Borne Relapsing Fever Spirochetes in the Americas. Veterinary Sciences. 2016; 3(3):16. https://doi.org/10.3390/vetsci3030016
Chicago/Turabian StyleLopez, Job E., Aparna Krishnavahjala, Melissa N. Garcia, and Sergio Bermudez. 2016. "Tick-Borne Relapsing Fever Spirochetes in the Americas" Veterinary Sciences 3, no. 3: 16. https://doi.org/10.3390/vetsci3030016






