Dissecting the Role of the Extracellular Matrix in Heart Disease: Lessons from the Drosophila Genetic Model
Abstract
:1. Introduction
2. ECM Regulation and Cardiac Dysfunction
3. The Drosophila Model
3.1. Simplicity and Homology
3.2. Life History
3.3. Generating Genetic Mosaics
3.4. In Vivo Imaging
4. The Drosophila Heart
4.1. Early Morphogenesis
4.2. Embryonic and Larval Heart
4.3. Adult Heart
5. The Drosophila Heart ECM
5.1. Form and Function
5.2. Basement Membrane Constituents
5.3. Structural Proteins
5.4. Receptors
6. ECM Regulation and Turnover
6.1. MMPs and TIMPs
6.2. ECM Remodelling in Vertebrates
6.3. ECM Remodelling in Drosophila
7. Cardiac Aging and ECM Disruption
7.1. Altered Expression or Deposition of Structural Proteins
7.2. Mis-Regulation of Receptors and Linker Proteins
7.3. Mis-Expression of MMPs
8. Conclusions
Note Added in Proof
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bogatan, S.; Cevik, D.; Demidov, V.; Vanderploeg, J.; Panchbhaya, A.; Vitkin, A.; Jacobs, J.R. Talin is required continuously for cardiomyocyte remodeling during heart growth in Drosophila. PLoS ONE 2015, 10, e0131238. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Serano, J.; Sante, J.M.; Rubin, G.M. Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev. Cell 2003, 4, 95–106. [Google Scholar] [CrossRef]
- Lehmacher, C.; Abeln, B.; Paululat, A. The ultrastructure of Drosophila heart cells. Arthropod Struct. Dev. 2012, 41, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Louzao-Martinez, L.; Vink, A.; Harakalova, M.; Asselbergs, F.W.; Verhaar, M.C.; Cheng, C. Characteristic adaptations of the extracellular matrix in dilated cardiomyopathy. Int. J. Cardiol. 2016, 220, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Matricellular proteins in cardiac inflammation and fibrosis. Physiol. Rev. 2012, 92, 635–688. [Google Scholar] [CrossRef] [PubMed]
- Łój, M.; Garncarz, M.; Jank, M. Genomic and genetic aspects of heart failure in dogs—A review. Acta Vet. Hung. 2012, 60, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Schenke-Layland, K.; Stock, U.A.; Nsair, A.; Xie, J.; Angelis, E.; Fonseca, C.G.; Larbig, R.; Mahajan, A.; Shivkumar, K.; Fishbein, M.C.; et al. Cardiomyopathy is associated with structural remodelling of heart valve extracellular matrix. Eur. Heart J. 2009, 30, 2254–2265. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.T.; Liu, S.K.; Liu, M.; Chen, S.P.; Lin, Y.H.; Mao, S.J.T.; Chu, R. Characterization and quantitation of extracellular collagen matrix in myocardium of pigs with spontaneously occurring hypertrophic cardiomyopathy. Cardiovasc. Pathol. 1999, 8, 169–175. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, M.; Schmidt, A.C.; Meyer, H.; Paululat, A. The Conserved ADAMTS-like protein lonely heart mediates matrix formation and cardiac tissue integrity. PLoS Genet. 2013, 9, e1003616. [Google Scholar] [CrossRef] [PubMed]
- Berk, B.C.; Fujiwara, K.; Lehoux, S. ECM remodeling in hypertensive heart disease. J. Clin. Investig. 2007, 117, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.L.; Smaill, B.H.; LeGrice, I.J. Structural remodeling and mechanical function in heart failure. Microsc. Microanal. 2012, 18, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Takawale, A.; Lee, J.; Kassiri, Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenes. Tissue Repair 2012, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Bayomy, A.F.; Bauer, M.; Qiu, Y.; Liao, R. Regeneration in heart disease—Is ECM the key? Life Sci. 2012, 91, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Tidholm, A. Retrospective study of congenital heart defects in 151 dogs. J. Small Anim. Pract. 1997, 38, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linde, D.; Konings, E.E.M.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.M.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Pierpont, M.E.; Basson, C.T.; Benson, D.W.; Gelb, B.D.; Giglia, T.M.; Goldmuntz, E.; McGee, G.; Sable, C.A.; Srivastava, D.; Webb, C.L. Genetic basis for congenital heart defects: Current knowledge. Circulation 2007, 115, 3015–3038. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, R.L. Geriatric heart diseases in dogs. Vet. Clin. Small Anim. Pract. 2005, 35, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.G.; Meurs, K.M.; Ostrander, E.A. Finding cardiovascular disease genes in the dog. J. Vet. Cardiol. 2006, 8, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Schoenebeck, J.J.; Ostrander, E.A. Insights into morphology and disease from the dog genome project. Annu. Rev. Cell Dev. Biol. 2014, 30, 535–560. [Google Scholar]
- Falk, V.; Garbade, J.; Walther, T. Experimental Models of Heart Failure. In Practical Methods in Cardiovascular Research; Dhein, S., Mohr, F.W., Delmar, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 83–110. [Google Scholar]
- Steudemann, C.; Bauersachs, S.; Weber, K.; Wess, G. Detection and comparison of microRNA expression in the serum of Doberman Pinschers with dilated cardiomyopathy and healthy controls. BMC Vet. Res. 2013, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Meurs, K.M.; Fox, P.R.; Norgard, M.; Spier, A.W.; Lamb, A.; Koplitz, S.L.; Baumwart, R.D. A prospective genetic evaluation of familial dilated cardiomyopathy in the Doberman pinscher. J. Vet. Intern. Med. 2007, 21, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, H.M.; Fonfara, S.; López-Alvarez, J.; Cripps, P.; Dukes-McEwan, J. Screening for Dilated Cardiomyopathy in Great Danes in the United Kingdom. J. Vet. Intern. Med. 2012, 26, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Meurs, K.M.; Stern, J.A.; Sisson, D.D.; Kittleson, M.D.; Cunningham, S.M.; Ames, M.K.; Atkins, C.E.; Defrancesco, T.; Hodge, T.E.; Keene, B.W.; et al. Association of Dilated Cardiomyopathy with the Striatin Mutation Genotype in Boxer Dogs. J. Vet. Intern. Med. 2013, 27, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Palermo, V.; Stafford Johnson, M.J.; Sala, E.; Brambilla, P.G.; Martin, M.W.S. Cardiomyopathy in Boxer dogs: A retrospective study of the clinical presentation, diagnostic findings and survival. J. Vet. Cardiol. 2011, 13, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, A. The prevalence of cardiomyopathy in the Irish wolfhound: A clinical study of 500 dogs. J. Am. Anim. Hosp. Assoc. 2000, 36, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Reist-Marti, S.B.; Dolf, G.; Leeb, T.; Kottmann, S.; Kietzmann, S.; Butenhoff, K.; Rieder, S. Genetic evidence of subaortic stenosis in the Newfoundland dog. Vet. Rec. 2012, 170, 597. [Google Scholar] [CrossRef] [PubMed]
- Pyle, R.L.; Patterson, D.F.; Chacko, S. The genetics and pathology of discrete subaortic stenosis in the Newfoundland dog. Am. Heart J. 1976, 92, 324–334. [Google Scholar] [CrossRef]
- Stern, J.A.; Meurs, K.M.; Nelson, O.L.; Lahmers, S.M.; Lehmkuhl, L.B. Familial subvalvular aortic stenosis in golden retrievers: Inheritance and echocardiographic findings. J. Small Anim. Pract. 2012, 53, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, R. Heart Development and Its Relationship in Drosophila to Vertebrates. Trends Cardiovasc. Med. 1995, 5, 21–28. [Google Scholar] [CrossRef]
- Bier, E.; Bodmer, R. Drosophila, an emerging model for cardiac disease. Gene 2004, 342, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bier, E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 2005, 6, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Ocorr, K.; Bodmer, R.; Cartry, J. Drosophila as a model to study cardiac aging. Exp. Gerontol. 2011, 46, 326–330. [Google Scholar] [CrossRef] [PubMed]
- del Valle Rodríguez, A.; Didiano, D.; Desplan, C. Power tools for gene expression and clonal analysis in Drosophila. Nat. Methods 2012, 9, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bellen, H.J.; Levis, R.W.; He, Y.; Carlson, J.W.; Evans-Holm, M.; Bae, E.; Kim, J.; Metaxakis, A.; Savakis, C.; Schulze, K.L.; et al. The Drosophila gene disruption project: Progress using transposons with distinctive site specificities. Genetics 2011, 188, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Dietzl, G.; Chen, D.; Schnorrer, F.; Su, K.-C.; Barinova, Y.; Fellner, M.; Gasser, B.; Kinsey, K.; Oppel, S.; Scheiblauer, S.; et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 2007, 448, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhou, R.; Czech, B.; Liu, L.-P.; Holderbaum, L.; Yang-Zhou, D.; Shim, S.; Handler, D.; Karpowicz, P.; Binari, R.; et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 2011, 8, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.; Werb, Z. How Matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A. Remodeling the model organism: Matrix metalloproteinase functions in invertebrates. Semin. Cell Dev. Biol. 2008, 19, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.B.; Boley, N.; Eisman, R.; May, G.E.; Stoiber, M.H.; Duff, M.O.; Booth, B.W.; Wen, J.; Park, S.; Suzuki, A.M.; et al. Diversity and dynamics of the Drosophila transcriptome. Nature 2014, 512, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guenet, J.L. The mouse genome. Genome Res. 2005, 15, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Ezkurdia, I.; Juan, D.; Rodriguez, J.M.; Frankish, A.; Diekhans, M.; Harrow, J.; Vazquez, J.; Valencia, A.; Tress, M.L. Multiple evidence strands suggest that theremay be as few as 19,000 human protein-coding genes. Hum. Mol. Genet. 2014, 23, 5866–5878. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.H.; Gregory, S.L.; Rickoll, W.L.; Fessler, L.I.; Prout, M.; White, R.A.H.; Fristrom, J.W. Talin is essential for integrin function in Drosophila. Dev. Cell 2002, 3, 569–579. [Google Scholar] [CrossRef]
- Chang, H.C.; Newmyer, S.L.; Hull, M.J.; Ebersold, M.; Schmid, S.L.; Mellman, I. Hsc70 is required for endocytosis and clathrin function in Drosophila. J. Cell Biol. 2002, 159, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.J.; Amrein, H.; Izatt, J.A.; Choma, M.A.; Reedy, M.C.; Rockman, H.A. Drosophila as a model for the identification of genes causing adult human heart disease. PNAS 2006, 103, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Cammarato, A.; Dambacher, C.M.; Knowles, A.F.; Kronert, W.A.; Bodmer, R.; Ocorr, K.; Bernstein, S.I. Myosin Transducer Mutations Differentially Affect Motor Function, Myofibril Structure, and the Performance of Skeletal and Cardiac Muscles. Mol. Biol. Cell 2008, 19, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, R.; Venkatesh, T.V. Heart development in Drosophila and vertebrates: Conservation of molecular mechanisms. Dev. Genet. 1998, 22, 181–186. [Google Scholar] [CrossRef]
- Zeitouni, B.; Sénatore, S.; Séverac, D.; Aknin, C.; Sémériva, M.; Perrin, L. Signalling pathways involved in adult heart formation revealed by gene expression profiling in Drosophila. PLoS Genet. 2007, 3, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Vogler, G.; Bodmer, R. Cellular Mechanisms of Drosophila Heart Morphogenesis. J. Cardiovasc. Dev. Dis. 2015, 2, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, K.; Fossett, N.; Molkentin, J.D.; Schulz, R.A. The zinc finger proteins Pannier and GATA4 function as cardiogenic factors in Drosophila. Development 1999, 126, 5679–5688. [Google Scholar] [PubMed]
- Park, M.; Lewis, C.; Turbay, D.; Chung, A.; Chen, J.N.; Evans, S.; Breitbart, R.E.; Fishman, M.C.; Izumo, S.; Bodmer, R. Differential rescue of visceral and cardiac defects in Drosophila by vertebrate tinman-related genes. Proc. Natl. Acad. Sci. USA 1998, 95, 9366–9371. [Google Scholar] [CrossRef] [PubMed]
- Ranganayakulu, G.; Elliott, D.A.; Harvey, R.P.; Olson, E.N. Divergent roles for NK-2 class homeobox genes in cardiogenesis in flies and mice. Development 1998, 125, 3037–3048. [Google Scholar] [PubMed]
- Harvey, R.P. NK-2 homeobox genes and heart development. Dev. Biol. 1996, 178, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Azpiazu, N.; Frasch, M. Tinman and bagpipe: Two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993, 7, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Klinedinst, S.L.; Bodmer, R. Gata factor Pannier is required to establish competence for heart progenitor formation. Development 2003, 130, 3027–3038. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Bodmer, R.; Abmayr, S.M.; McDermott, J.C.; Spoerel, N.A. D-mef2: A Drosophila mesoderm-specific MADS box-containing gene with a biphasic expression profile during embryogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 7520–7524. [Google Scholar] [CrossRef] [PubMed]
- Bour, B.A.; O’Brien, M.A.; Lockwood, W.L.; Goldstein, E.S.; Bodmer, R.; Taghert, P.H.; Abmayr, S.M.; Nguyen, H.T. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 1995, 9, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Sellin, J.; Albrecht, S.; Kölsch, V.; Paululat, A. Dynamics of heart differentiation, visualized utilizing heart enhancer elements of the Drosophila melanogaster bHLH transcription factor Hand. Gene Expr. Patterns 2006, 6, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Hallier, B.; Hoffmann, J.; Roeder, T.; Tögel, M.; Meyer, H.; Paululat, A. The bHLH Transcription Factor Hand Regulates the Expression of Genes Critical to Heart and Muscle Function in Drosophila melanogaster. PLoS ONE 2015, 10, e0134204. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Yi, P.; Li, X.; Olson, E.N. Hand, an evolutionarily conserved bHLH transcription factor required for Drosophila cardiogenesis and hematopoiesis. Development 2006, 133, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.C.H.; Frasch, M. A role for the COUP-TF-related gene seven-up in the diversification of cardioblast identities in the dorsal vessel of Drosophila. Mech. Dev. 2001, 104, 49–60. [Google Scholar] [CrossRef]
- Mann, T.; Bodmer, R.; Pandur, P. The Drosophila homolog of vertebrate Islet1 is a key component in early cardiogenesis. Development 2009, 136, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kooij, V.; Venkatraman, V.; Tra, J.; Kirk, J.A.; Rowell, J.; Blice-Baum, A.; Cammarato, A.; Van Eyk, J.E. Sizing up models of heart failure: Proteomics from flies to humans. Proteom. Clin. Appl. 2014, 8, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Cammarato, A.; Ahrens, C.H.; Alayari, N.N.; Qeli, E.; Rucker, J.; Reedy, M.C.; Zmasek, C.M.; Gucek, M.; Cole, R.N.; Van Eyk, J.E.; et al. A mighty small heart: The cardiac proteome of adult Drosophila melanogaster. PLoS ONE 2011, 6, e18497. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.C.; Kaushik, G.; Engler, A.J.; Lehman, W.; Cammarato, A. A Drosophila melanogaster model of diastolic dysfunction and cardiomyopathy based on impaired troponin-T function. Circ. Res. 2014, 114, e6–e17. [Google Scholar] [CrossRef] [PubMed]
- Ocorr, K.; Perrin, L.; Lim, H.-Y.Y.; Qian, L.; Wu, X.; Bodmer, R. Genetic control of heart function and aging in Drosophila. Trends Cardiovasc. Med. 2007, 17, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Taghli-Lamallem, O.; Akasaka, T.; Hogg, G.; Nudel, U.; Yaffe, D.; Chamberlain, J.S.; Ocorr, K.; Bodmer, R. Dystrophin deficiency in Drosophila reduces lifespan and causes a dilated cardiomyopathy phenotype. Aging Cell 2008, 7, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Medioni, C.; Sénatore, S.; Salmand, P.A.; Lalevée, N.; Perrin, L.; Sémériva, M. The fabulous destiny of the Drosophila heart. Curr. Opin. Genet. Dev. 2009, 19, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Bassett, A.R.; Tibbit, C.; Ponting, C.P.; Liu, J.L. Highly Efficient Targeted Mutagenesis of Drosophila with the CRISPR/Cas9 System. Cell Rep. 2013, 4, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Bassett, A.R.; Liu, J.-L. CRISPR/Cas9 and genome editing in Drosophila. J. Genet. Genom. 2014, 41, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Bellen, H.J.; Kane, C.J.O.; Wilson, C.; Grossniklaus, U.; Pearson, R.K.; Gehring, W.J. P-element-mediated enhancer detection: A versatile method to study development in Drosophila. Genes Dev. 1989, 3, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Perrimon, N.; Noll, E.; McCall, K.; Brand, A. Generating lineage-specific markers to study Drosophila development. Dev. Genet. 1991, 12, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Venken, K.J.T.; Sarrion-Perdigones, A.; Vandeventer, P.J.; Abel, N.S.; Christiansen, A.E.; Hoffman, K.L. Genome engineering: Drosophila melanogaster and beyond. WIREs Dev. Biol. 2015, 5, 233–267. [Google Scholar] [CrossRef] [PubMed]
- Venken, K.J.T.; Bellen, H.J. Emerging technologies for gene manipulation in Drosophila melanogaster. Nat. Rev. Genet. 2005, 6, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Golic, K.G.; Lindquist, S. The FLP recombinase of yeast catalyzes site-specific recombination in the drosophila genome. Cell 1989, 59, 499–509. [Google Scholar] [CrossRef]
- Blair, S.S. Genetic mosaic techniques for studying Drosophila development. Development 2003, 130, 5065–5072. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.J.; Rockman, H.A. Drosophila, Genetic Screens, and Cardiac Function. Circ. Res. 2011, 109, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [PubMed]
- Duffy, J.B. GAL4 system in Drosophila: A fly geneticist’s Swiss army knife. Genesis 2002, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.; Gustafson, K.; Boulianne, G.L. Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc. Natl. Acad. Sci. USA 1995, 92, 7036–7040. [Google Scholar] [CrossRef] [PubMed]
- Suster, M.L.; Seugnet, L.; Bate, M.; Sokolowski, M.B. Refining GAL4-driven transgene expression in Drosophila with a GAL80 enhancer-trap. Genesis 2004, 39, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Morin, X.; Daneman, R.; Zavortink, M.; Chia, W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. PNAS 2001, 98, 15050–15055. [Google Scholar] [CrossRef] [PubMed]
- Clyne, P.J.; Brotman, J.S.; Sweeney, S.T.; Davis, G. Green Fluorescent Protein Tagging Drosophila Proteins at Their Native Genomic Loci with Small P Elements. Genetics 2003, 165, 1433–1441. [Google Scholar] [PubMed]
- Sarov, M.; Barz, C.; Jambor, H.; Hein, M.Y.; Schmied, C.; Suchold, D.; Stender, B.; Janosch, S.; Vinay Vikas, K.J.; Krishnan, R.T.; et al. A genome-wide resource for the analysis of protein localisation in Drosophila. Elife 2016, 5, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Raza, Q.; Jacobs, J.R. Guidance signalling regulates Leading Edge behaviour during collective cell migration of cardiac cells in Drosophila. Dev. Biol. 2016, 419, 285–297. [Google Scholar] [CrossRef] [PubMed]
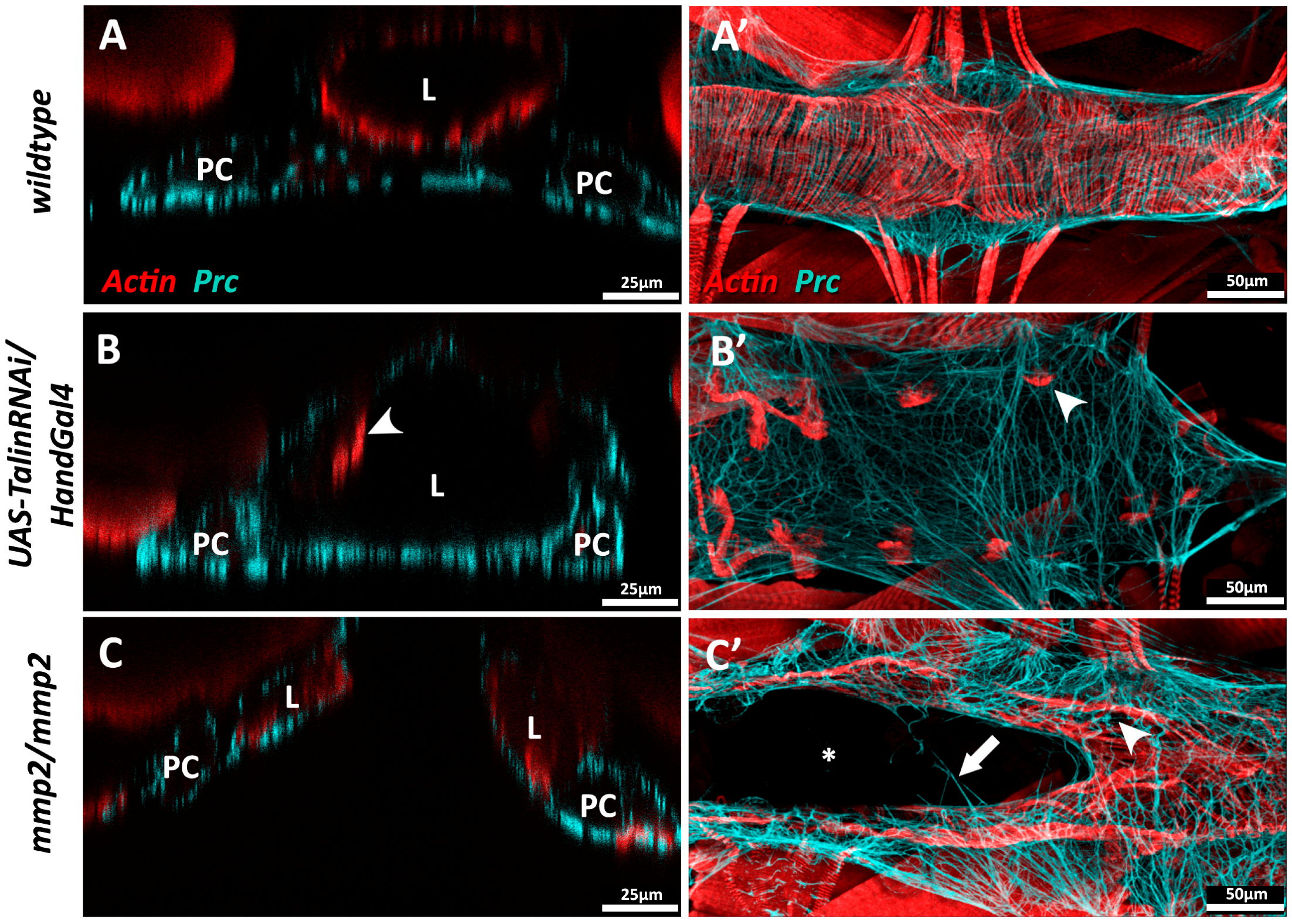
- Vanderploeg, J.; Jacobs, J.R. Talin is required to position and expand the luminal domain of the Drosophila heart tube. Dev. Biol. 2015, 405, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Vanderploeg, J.; Jacobs, J.R. Mapping heart development in flies: Src42A acts non-autonomously to promote heart tube formation in Drosophila. Vet. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Ocorr, K.; Vogler, G.; Bodmer, R. Methods to assess Drosophila heart development, function and aging. Methods 2014, 68, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Bradu, A.; Ma, L.; Bloor, J.W.; Podoleanu, A. Dual optical coherence tomography/fluorescence microscopy for monitoring of Drosophila melanogaster larval heart. J. Biophotonics 2009, 2, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, B.; Paululat, A. On the Morphology of the Drosophila Heart. J. Cardiovasc. Dev. Dis. 2016, 3, 15. [Google Scholar] [CrossRef]
- Reim, I.; Frasch, M. The Dorsocross T-box genes are key components of the regulatory network controlling early cardiogenesis in Drosophila. Development 2005, 132, 4911–4925. [Google Scholar] [CrossRef] [PubMed]
- Bryantsev, A.L.; Cripps, R.M. Cardiac gene regulatory networks in Drosophila. Biochim. Biophys. Acta Gene Regul. Mech. 2009, 1789, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Haack, T.; Schneider, M.; Schwendele, B.; Renault, A.D. Drosophila heart cell movement to the midline occurs through both cell autonomous migration and dorsal closure. Dev. Biol. 2014, 396, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Schulz, R.A. Heart development in Drosophila. Semin. Cell Dev. Biol. 2007, 18, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Denholm, B.; Skaer, H. Bringing together components of the fly renal system. Curr. Opin. Genet. Dev. 2009, 19, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Weavers, H.; Prieto-Sánchez, S.; Grawe, F.; Garcia-López, A.; Artero, R.; Wilsch-Bräuninger, M.; Ruiz-Gómez, M.; Skaer, H.; Denholm, B. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 2009, 457, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Chartier, A.; Zaffran, S.; Astier, M.; Sémériva, M.; Gratecos, D. Pericardin, a Drosophila type IV collagen-like protein is involved in the morphogenesis and maintenance of the heart epithelium during dorsal ectoderm closure. Development 2002, 129, 3241–3253. [Google Scholar] [PubMed]
- Molina, M.R.; Cripps, R.M. Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech. Dev. 2001, 109, 51–59. [Google Scholar] [CrossRef]
- Lo, P.C.H.; Frasch, M. Establishing A-P polarity in the embryonic heart tube: A conserved function of Hox genes in Drosophila and vertebrates? Trends Cardiovasc. Med. 2003, 13, 182–187. [Google Scholar] [CrossRef]
- Ivy, J.R.; Drechsler, M.; Catterson, J.H.; Bodmer, R.; Ocorr, K.; Paululat, A.; Hartley, P.S. Klf15 is critical for the development and differentiation of drosophila nephrocytes. PLoS ONE 2015, 10, e0134620. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.P.; Nongthomba, U.; Kelly Tanaka, K.K.; Denton, M.L.B.; Meadows, S.M.; Bancroft, N.; Molina, M.R.; Cripps, R.M. Cardiac remodeling in Drosophila arises from changes in actin gene expression and from a contribution of lymph gland-like cells to the heart musculature. Mech. Dev. 2011, 128, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Martínez, E.; Soplop, N.H.; Patel, R.; Kramer, S.G. Repulsion by Slit and Roundabout prevents Shotgun/E-cadherin-mediated cell adhesion during Drosophila heart tube lumen formation. J. Cell Biol. 2008, 182, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Heeneman, S.; Cleutjens, J.P.; Faber, B.C.; Creemers, E.E.; Van Suylen, R.; Lutgens, E.; Cleutjens, K.B.; Daemen, M.J. The dynamic extracellular matrix: Intervention strategies during heart failure and atherosclerosis. J. Pathol. 2003, 200, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.A.; Trafford, A.W. Aging and the cardiac collagen matrix: Novel mediators of fibrotic remodelling. J. Mol. Cell. Cardiol. 2016, 93, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: A family of cell surface receptors. Cell 1987, 48, 549–554. [Google Scholar] [CrossRef]
- Vanderploeg, J.; Vazquez Paz, L.L.; MacMullin, A.; Jacobs, J.R. Integrins are required for cardioblast polarisation in Drosophila. BMC Dev. Biol. 2012, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.A.; Henry, M.D.; Daniels, K.J.; Hrstka, R.F.; Lee, J.C.; Sunada, Y.; Ibraghimov-Beskrovnaya, O.; Campbell, K.P. Dystroglycan is essential for early embryonic development: Disruption of Reichert’s membrane in Dag1-null mice. Hum. Mol. Genet. 1997, 6, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.-M.; Schneider, M.; Frock, R.; Castillejo-Lopez, C.; Gaman, E.A.; Baumgartner, S.; Ruohola-Baker, H. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development 2003, 130, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Xian, X.; Gopal, S.; Couchman, J.R. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 2010, 339, 31–46. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Macdonald, B.; Kalluri, R. Structure and function of basement membranes. Exp. Biol. Med. 2007, 232, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Isabella, A.J.; Horne-Badovinac, S. Building from the Ground Up; Elsevier Ltd: Amsterdam, The Netherlands, 2015; Volume 76. [Google Scholar]
- Yurchenco, P.D. Basement Membranes: Cell Scaffoldings and Signaling Platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef] [PubMed]
- Haag, T.A.; Haag, N.P.; Lekven, A.C.; Hartenstein, V. The role of cell adhesion molecules in Drosophila heart morphogenesis: Faint sausage, shotgun/DE-cadherin, and laminin A are required for discrete stages in heart development. Dev. Biol. 1999, 208, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, N.; Ordan, E.; Ocorr, K.; Bodmer, R.; Volk, T. Multiplexin Promotes Heart but Not Aorta Morphogenesis by Polarized Enhancement of Slit/Robo Activity at the Heart Lumen. PLoS Genet. 2013, 9, e1003597. [Google Scholar] [CrossRef] [PubMed]
- Hollfelder, D.; Frasch, M.; Reim, I. Distinct functions of the laminin β LN domain and collagen IV during cardiac extracellular matrix formation and stabilization of alary muscle attachments revealed by EMS mutagenesis in Drosophila. BMC Dev. Biol. 2014, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Medioni, C.; Noselli, S. Dynamics of the basement membrane in invasive epithelial clusters in Drosophila. Development 2005, 132, 3069–3077. [Google Scholar] [CrossRef] [PubMed]
- Sessions, A.O.; Kaushik, G.; Parker, S.; Raedschelders, K.; Bodmer, R.; Van Eyk, J.E.; Engler, A.J. Extracellular matrix downregulation in the Drosophila heart preserves contractile function and improves lifespan. Matrix Biol. 2016, in press. [Google Scholar]
- Morishita, N.; Kusachi, S.; Yamasaki, S.; Kondo, J.; Tsuji, T. Sequential Changes in Laminin and Type IV Collagen in the Infarct Zone. Jpn. Circ. J. 1996, 60, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kusachi, S.; Yamanishi, A.; Kumashiro, H.; Nunoyama, H.; Sano, I.; Nakahama, M.; Murakami, T.; Naito, I.; Ninomiya, Y.; et al. Localization of type IV collagen alpha chain in the myocardium of dilated and hypertrophic cardiomyopathy. Jpn. Heart J. 1998, 39, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, A.; Kusachi, S.; Nakahama, M.; Ninomiya, Y.; Watanabe, T.; Kumashiro, H.; Nunoyama, H.; Kondo, J.; Naito, I.; Tsuji, T. Sequential changes in the localization of the type IV collagen alpha chain in the infarct zone: Immunohistochemical study of experimental myocardial infarction in the rat. Pathol. Res. Pr. 1998, 194, 413–422. [Google Scholar] [CrossRef]
- Rasi, K.; Piuhola, J.; Czabanka, M.; Sormunen, R.; Ilves, M.; Leskinen, H.; Rysä, J.; Kerkelä, R.; Janmey, P.; Heljasvaara, R.; et al. Collagen XV is necessary for modeling of the extracellular matrix and its deficiency predisposes to cardiomyopathy. Circ. Res. 2010, 107, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Utriainen, A.; Sormunen, R.; Kettunen, M.; Carvalhaes, L.S.; Sajanti, E.; Eklund, L.; Kauppinen, R.; Kitten, G.T.; Pihlajaniemi, T. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum. Mol. Genet. 2004, 13, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Isobe, K.; Kuba, K.; Maejima, Y.; Suzuki, J.; Kubota, S.; Isobe, M. Inhibition of endostatin/collagen XVIII deteriorates left ventricular remodeling and heart failure in rat myocardial infarction model. Circ. J. 2010, 74, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Urbano, J.M.; Torgler, C.N.; Molnar, C.; Tepass, U.; López-varea, A.; Brown, N.H.; De Celis, J.F.; Martín-bermudo, M.D. Drosophila laminins act as key regulators of basement membrane assembly and morphogenesis. Development 2009, 136, 4165–4176. [Google Scholar] [CrossRef] [PubMed]
- Wolfstetter, G.; Holz, A. The role of LamininB2 (LanB2) during mesoderm differentiation in Drosophila. Cell. Mol. Life Sci. 2012, 69, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Galchev, V.I.; Kim, J.Y.; Misek, S.A.; Stevenson, T.K.; Campbell, M.D.; Pagani, F.D.; Day, S.M.; Johnson, T.C.; Washburn, J.G.; et al. Differential protein expression and basal lamina remodeling in human heart failure. Proteom. Clin. Appl. 2016, 10, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hoshijima, M.; Lam, J.; Zhou, Z.; Jokiel, A.; Dalton, N.D.; Hultenby, K.; Ruiz-Lozano, P.; Ross, J.; Tryggvason, K.; et al. Cardiomyopathy associated with microcirculation dysfunction in laminin a4 chain-deficient mice. J. Biol. Chem. 2006, 281, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Martinek, N.; Shahab, J.; Saathoff, M.; Ringuette, M. Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J. Cell Sci. 2008, 121, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Hartley, P.S.; Motamedchaboki, K.; Bodmer, R.; Ocorr, K. SPARC-dependent cardiomyopathy in drosophila. Circ. Cardiovasc. Genet. 2016, 9, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.D. The role of secreted protein acidic and rich in cysteine (SPARC) in cardiac repair and fibrosis: Does expression of SPARC by macrophages influence outcomes? J. Mol. Cell. Cardiol. 2016, 93, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Chanana, B.; Graf, R.; Koledachkina, T.; Pflanz, R.; Vorbrüggen, G. αPS2 integrin-mediated muscle attachment in Drosophila requires the ECM protein Thrombospondin. Mech. Dev. 2007, 124, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Bunch, T.; Wayburn, B.; Volk, T. Thrombospondin-mediated adhesion is essential for the formation of the myotendinous junction in. Development 2007, 1278, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Ren, G.; Dewald, O.; Zymek, P.; Haudek, S.; Koerting, A.; Winkelmann, K.; Michael, L.H.; Lawler, J.; Entman, M.L. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation 2005, 111, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, E.; Aro, J.; Puhakka, J.; Ilves, M.; Soini, Y.; Leskinen, H.; Ruskoaho, H.; Rysä, J. Thrombospondin-4 expression is rapidly upregulated by cardiac overload. Biochem. Biophys. Res. Commun. 2008, 373, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Frolova, E.G.; Sopko, N.; Blech, L.; Popovic, Z.B.; Li, J.; Vasanji, A.; Drumm, C.; Krukovets, I.; Jain, M.K.; Penn, M.S.; et al. Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload. FASEB J. 2012, 26, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K.; Manabe, R.I.; Yamada, T.; Nakano, I.; Oguri, Y.; Keene, D.R.; Sengle, G.; Sakai, L.Y.; Sekiguchi, K. ADAMTSL-6 is a novel extracellular matrix protein that binds to fibrillin-1 and promotes fibrillin-1 fibril formation. J. Biol. Chem. 2010, 285, 4870–4882. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Kumsta, C.; Kaushik, G.; Diop, S.B.; Ding, Y.; Bisharat-Kernizan, J.; Catan, H.; Cammarato, A.; Ross, R.S.; Engler, A.J.; et al. A dual role for integrin-linked kinase and β1-integrin in modulating cardiac aging. Aging Cell 2014, 13, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zhu, Y.; Sun, Z.; Trzeciakowski, J.P.; Gansner, M.; Depre, C.; Resuello, R.R.G.; Natividad, F.F.; Hunter, W.C.; Genin, G.M.; et al. Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ. Res. 2010, 107, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Shai, S.Y.; Harpf, A.E.; Babbitt, C.J.; Jordan, M.C.; Fishbein, M.C.; Chen, J.; Omura, M.; Leil, T.A.; Becker, K.D.; Jiang, M.; et al. Cardiac myocyte-specific excision of the β1 integrin gene results in myocardial fibrosis and cardiac failure. Circ. Res. 2002, 90, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Shcherbata, H.R.; Yatsenko, A.S.; Patterson, L.; Sood, V.D.; Nudel, U.; Yaffe, D.; Baker, D.; Ruohola-Baker, H. Dissecting muscle and neuronal disorders in a Drosophila model of muscular dystrophy. EMBO J. 2007, 26, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Lefeber, D.J.; de Brouwer, A.P.M.; Morava, E.; Riemersma, M.; Schuurs-Hoeijmakers, J.H.M.; Absmanner, B.; Verrijp, K.; van den Akker, W.M.R.; Huijben, K.; Steenbergen, G.; et al. Autosomal recessive dilated cardiomyopathy due to DOLK mutations results from abnormal dystroglycan O-mannosylation. PLoS Genet. 2011, 7, e1002427. [Google Scholar] [CrossRef] [PubMed]
- Grossman, T.R.; Gamliel, A.; Wessells, R.J.; Taghli-Lamallem, O.; Jepsen, K.; Ocorr, K.; Korenberg, J.R.; Peterson, K.L.; Rosenfeld, M.G.; Bodmer, R.; et al. Over-expression of DSCAM and COL6A2 cooperatively generates congenital heart defects. PLoS Genet. 2011, 7, e1002344. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.L.; Clemens, J.C.; Neves, G.; Hattori, D.; Flanagan, J.J.; Hummel, T.; Vasconcelos, M.L.; Chess, A.; Zipursky, S.L. Analysis of Dscam diversity in regulating axon guidance in Drosophila mushroom bodies. Neuron 2004, 43, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; You, X.; Gogol-Dö ring, A.; He, H.; Kise, Y.; Sohn, M.; Chen, T.; Klebes, A.; Schmucker, D.; Chen, W. Ultra-deep profiling of alternatively spliced Drosophila Dscam isoforms by circularization- assisted multi-segment sequencing. EMBO J. 2013, 32, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Barlow, G.M.; Chen, X.N.; Shi, Z.Y.; Lyons, G.E.; Kurnit, D.M.; Celle, L.; Spinner, N.B.; Zackai, E.; Pettenati, M.J.; Van Riper, A.J.; et al. Down syndrome congenital heart disease: A narrowed region and a candidate gene. Genet. Med. 2001, 3, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.; Moyer, K.; Yacoub, N.; Soldaat, C.; Komosa, M.; Vassilieva, K.; Wilk, R.; Hu, J.; de Vazquez Paz, L.L.; Syed, Q.; et al. Syndecan contributes to heart cell specification and lumen formation during Drosophila cardiogenesis. Dev. Biol. 2011, 356, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Lunde, I.G.; Herum, K.M.; Carlson, C.C.; Christensen, G. Syndecans in heart fibrosis. Cell Tissue Res. 2016, 365, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Liu, J.; Bodmer, R. Slit and Robo control cardiac cell polarity and morphogenesis. Curr. Biol. 2005, 15, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Medioni, C.; Astier, M.; Zmojdzian, M.; Jagla, K.; Sémériva, M. Genetic control of cell morphogenesis during Drosophila melanogaster cardiac tube formation. J. Cell Biol. 2008, 182, 249–261. [Google Scholar] [CrossRef] [PubMed]
- MacMullin, A.; Jacobs, J.R. Slit coordinates cardiac morphogenesis in Drosophila. Dev. Biol. 2006, 293, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, M.T.M.; Andrews, W.D.; Ypsilanti, A.R.; Zelina, P.; Yeh, M.L.; Norden, J.; Kispert, A.; Chédotal, A.; Christoffels, V.M.; Parnavelas, J.G. Slit-roundabout signaling regulates the development of the cardiac systemic venous return and pericardium. Circ. Res. 2013, 112, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, M.T.M.; Yeh, M.L.; Parnavelas, J.G.; Andrews, W.D. Disrupted Slit-Robo signalling results in membranous ventricular septum defects and bicuspid aortic valves. Cardiovasc. Res. 2015, 106, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Macabenta, F.D.; Jensen, A.G.; Cheng, Y.S.; Kramer, J.J.; Kramer, S.G. Frazzled/DCC facilitates cardiac cell outgrowth and attachment during Drosophila dorsal vessel formation. Dev. Biol. 2013, 380, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, P.; Ye, K.; Cai, H. Central role of SIAH inhibition in DCC-dependent cardioprotection provoked by netrin-1/NO. Proc. Natl. Acad. Sci. USA 2015, 112, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Raza, Q.S.; Vanderploeg, J.L.; Jacobs, J.R. Matrix Metalloproteinases are required for membrane motility and lumenogenesis during Drosophila heart development. PLoS ONE 2017, 12, e0171905. [Google Scholar] [CrossRef] [PubMed]
- Raza, Q.; Vanderploeg, J.; Jacobs, J.R. Transmembrane and Secreted MMPs are Required for Heart Morphogenesis. In Proceedings of the Drosophila Research Conference, Chicago, IN, USA, 4–8 March 2015. [Google Scholar]
- Fonfara, S.; Hetzel, U.; Tew, S.R.; Cripps, P.; Dukes-McEwan, J.; Clegg, P.D. Expression of matrix metalloproteinases, their inhibitors, and lysyl oxidase in myocardial samples from dogs with end-stage systemic and cardiac diseases. Am. J. Vet. Res. 2013, 74, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, A.; Frantz, S.; Aikawa, M.; Rabkin, E.; Lindsey, M.; Rohde, L.E.; Schoen, F.J.; Kelly, R.A.; Werb, Z.; Libby, P.; et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J. Clin. Investig. 2000, 106, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hayashidani, S.; Tsutsui, H.; Ikeuchi, M.; Shiomi, T.; Matsusaka, H.; Kubota, T.; Imanaka-Yoshida, K.; Itoh, T.; Takeshita, A. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1229–H1235. [Google Scholar] [CrossRef] [PubMed]
- Etoh, T.; Joffs, C.; Deschamps, A.M.; Davis, J.; Dowdy, K.; Hendrick, J.; Baicu, S.; Mukherjee, R.; Manhaini, M.; Spinale, F.G. Myocardial and interstitial matrix metalloproteinase activity after acute myocardial infarction in pigs. Am. J. Physiol. Circ. Physiol. 2001, 281, H987–H994. [Google Scholar]
- Tian, H.; Cimini, M.; Fedak, P.W.M.; Altamentova, S.; Fazel, S.; Huang, M.L.; Weisel, R.D.; Li, R.K. TIMP-3 deficiency accelerates cardiac remodeling after myocardial infarction. J. Mol. Cell. Cardiol. 2007, 43, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Kandalam, V.; Basu, R.; Abraham, T.; Wang, X.; Soloway, P.D.; Jaworski, D.M.; Oudit, G.Y.; Kassiri, Z. TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ. Res. 2010, 106, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.J.M.; Davis, J.N.; Parkhurst, A.M.; Leenders, P.; Dowdy, K.B.; Hapke, E.; Hauet, A.M.; Escobar, P.G.; Cleutjens, J.P.M.; Smits, J.F.M.; et al. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H364–H371. [Google Scholar] [CrossRef] [PubMed]
- Fedak, P.W.M.; Smookler, D.S.; Kassiri, Z.; Ohno, N.; Leco, K.J.; Verma, S.; Mickle, D.A.G.; Watson, K.L.; Hojilla, C.V.; Cruz, W.; et al. TIMP-3 deficiency leads to dilated cardiomyopathy. Circulation 2004, 110, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G. Myocardial Matrix Remodeling and the Matrix Metalloproteinases: Influence on Cardiac Form and Function. Physiol. Rev. 2007, 87, 1285–1342. [Google Scholar] [CrossRef] [PubMed]
- Myllyharju, J.; Kivirikko, K.I. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004, 20, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Volk, T.; Wang, S.; Rotstein, B.; Paululat, A. Matricellular proteins in development: Perspectives from the Drosophila heart. Matrix Biol. 2014, 37, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Moussian, B. Drosophila multiplexin (Dmp) modulates motor axon pathfinding accuracy. Dev. Growth Differ. 2009, 51, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Price, R.L.; Nakagawa, M.; Terracio, L.; Borg, T.K. Ultrastructural localization of laminin on in vivo embryonic, neonatal, and adult rat cardiac myocytes and in early rat embryos raised in whole-embryo culture. J. Histochem. Cytochem. 1992, 40, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.A.; Ogle, R.C.; Little, C.D. Embryonic heart mesenchymal cell migration in laminin. Dev. Biol. 1989, 133, 37–43. [Google Scholar] [CrossRef]
- Colognato, H.; Yurchenco, P.D. Form and function: The laminin family of heterotrimers. Dev. Dyn. 2000, 218, 213–234. [Google Scholar] [CrossRef]
- Adams, J.C. Thrombospondins: Multifunctional Regulators of Cell Interactions. Annu. Rev. Cell Dev. Biol. 2001, 17, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Manso, A.M.; Elsherif, L.; Kang, S.M.; Ross, R.S. Integrins, membrane-type matrix metalloproteinases and ADAMs: Potential implications for cardiac remodeling. Cardiovasc. Res. 2006, 69, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Lapidos, K.A.; Kakkar, R.; McNally, E.M. The Dystrophin Glycoprotein Complex: Signaling Strength and Integrity for the Sarcolemma. Circ. Res. 2004, 94, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Kreipke, R.E.; Kwon, Y.V.; Shcherbata, H.R.; Ruohola-Baker, H. Drosophila melanogaster as a Model of Muscle Degeneration Disorders. Curr. Top. Dev. Biol. 2017, 121, 83–109. [Google Scholar] [PubMed]
- Allikian, M.J.; Bhabha, G.; Dospoy, P.; Heydemann, A.; Ryder, P.; Earley, J.U.; Wolf, M.J.; Rockman, H.A.; McNally, E.M. Reduced life span with heart and muscle dysfunction in Drosophila sarcoglycan mutants. Hum. Mol. Genet. 2007, 16, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- Durbeej, M.; Larsson, E.; Ibraghimov-Beskrovnaya, O.; Roberds, S.L.; Campbell, K.P.; Ekblom, P. Non-muscle α-dystroglycan is involved in epithelial development. J. Cell Biol. 1995, 130, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Ibraghimov-Beskrovnaya, O.; Ervasti, J.M.; Leveille, C.J.; Slaughter, C.A.; Sernett, S.W.; Campbell, K.P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 1992, 355, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Tögel, M.; Meyer, H.; Lehmacher, C.; Heinisch, J.J.; Pass, G.; Paululat, A. The bHLH transcription factor hand is required for proper wing heart formation in Drosophila. Dev. Biol. 2013, 381, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; Coker, M.L.; Thomas, C.V.; Walker, J.D.; Mukherjee, R.; Hebbar, L. Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: Relation to ventricular and myocyte function. Circ. Res. 1998, 82, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Coker, M.L.; Thomas, C.V.; Clair, M.J.; Hendrick, J.W.; Krombach, R.S.; Galis, Z.S.; Spinale, F.G. Myocardial matrix metalloproteinase activity and abundance with congestive heart failure. Am. J. Physiol. 1998, 274, H1516–H1523. [Google Scholar] [PubMed]
- Reinhardt, D.; Sigusch, H.H.; Henβe, J.; Tyagi, S.C.; Körfer, R.; Figulla, H.R. Cardiac remodelling in end stage heart failure: Upregulation of matrix metalloproteinase (MMP) irrespective of the underlying disease, and evidence for a direct inhibitory effect of ACE inhibitors on MMP. Heart 2002, 88, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Fan, D.; Basu, R.; Kandalam, V.; Kassiri, Z. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail. Rev. 2012, 17, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Fondard, O.; Detaint, D.; Iung, B.; Choqueux, C.; Adle-biassette, H.; Jarraya, M.; Hvass, U.; Couetil, J.; Henin, D.; Michel, J.; et al. Extracellular matrix remodelling in human aortic valve disease: The role of matrix metalloproteinases and their tissue inhibitors. Eur. Heart J. 2005, 26, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Yamamoto, K.; Mano, T.; Nishikawa, N.; Yoshida, J.; Hori, M.; Miwa, T.; Masuyama, T. Activation of Matrix Metalloproteinases Precedes Left Ventricular Remodeling in Hypertensive Heart Failure Rats: Its Inhibition as a Primary Effect of Angiotensin-Converting Enzyme Inhibitor. Circulation 2004, 109, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Van Wart, H.E.; Birkedal-Hansen, H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA 1990, 87, 5578–5582. [Google Scholar] [CrossRef] [PubMed]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44-46, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Lemaître, V.; D’Armiento, J. Matrix metalloproteinases in development and disease. Birth Defects Res. 2006, 78, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Knorr, E.; Schmidtberg, H.; Vilcinskas, A.; Altincicek, B. MMPs Regulate both Development and Immunity in the Tribolium Model Insect. PLoS ONE 2009, 4, e4751. [Google Scholar] [CrossRef] [PubMed]
- Ra, H.J.; Parks, W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007, 26, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Parks, W.C.; Wilson, C.L.; Lopez-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation. Nat. Rev. Immunol. 2004, 4, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G. Matrix metalloproteinases: Regulation and dysregulation in the failing heart. Circ. Res. 2002, 90, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; McTiernan, C.F.; Feldman, A.M. Proinflammatory cytokines regulate tissue inhibitors of metalloproteinases and disintegrin metalloproteinase in cardiac cells. Cardiovasc. Res. 1999, 42, 162–172. [Google Scholar] [CrossRef]
- Koskivirta, I.; Kassiri, Z.; Rahkonen, O.; Kiviranta, R.; Oudit, G.Y.; McKee, T.D.; Kytö, V.; Saraste, A.; Jokinen, E.; Liu, P.P.; et al. Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J. Biol. Chem. 2010, 285, 24487–24493. [Google Scholar] [CrossRef] [PubMed]
- Moe, G.W.; Laurent, G.; Doumanovskaia, L.; Konig, A.; Hu, X.; Dorian, P. Matrix Metalloproteinase Inhibition Attenuates Atrial Remodeling and Vulnerability to Atrial Fibrillation in a Canine Model of Heart Failure. J. Card. Fail. 2008, 14, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Müller, P.; Nurzynska, D.; Casarsa, C.; Torella, D.; Nascimbene, A.; Castaldo, C.; Cascapera, S.; Böhm, M.; Quaini, F.; et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc. Natl. Acad. Sci. USA 2005, 102, 8966–8971. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Llano, E.; Pendas, A.M.; Aza-Blanc, P.; Kornberg, T.B.; Lopez-Otin, C. Dm1-MMP, a matrix metalloproteinase from Drosophila with a potential role in extracellular matrix remodeling during neural development. J. Biol. Chem. 2000, 275, 35978–35985. [Google Scholar] [CrossRef] [PubMed]
- Llano, E.; Adam, G.; Pendás, A.M.; Quesada, V.; Sánchez, L.M.; Santamaría, I.; Noselli, S.; López-Otín, C.; De Oviedo, U. Structural and enzymatic characterization of Drosophila Dm2-MMP, a membrane-bound matrix metalloproteinase with tissue-specific expression. J. Biol. Chem. 2002, 277, 23321–23329. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.M.; Page-McCaw, A.; Broihier, H.T. Matrix metalloproteinases promote motor axon fasciculation in the Drosophila embryo. Development 2008, 135, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.A. Cardiac Physiology of Aging: Extracellular Considerations. Compr. Physiol. 2015, 5, 1069–1121. [Google Scholar] [PubMed]
- Brancaccio, M.; Hirsch, E.; Notte, A.; Selvetella, G.; Lembo, G.; Tarone, G. Integrin signalling: The tug-of-war in heart hypertrophy. Cardiovasc. Res. 2006, 70, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Sessions, A.O.; Engler, A.J. Mechanical Regulation of Cardiac Aging in Model Systems. Circ. Res. 2016, 118, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Eghbali, M.; Czaja, M.J.; Zeydel, M.; Weiner, F.R.; Zern, M.A.; Seifter, S.; Blumenfeld, O.O. Collagen chain mRNAs in isolated heart cells from young and adult rats. J. Mol. Cell. Cardiol. 1988, 20, 267–276. [Google Scholar] [CrossRef]
- Li, D.; Fareh, S.; Leung, T.K.; Nattel, S. Promotion of atrial fibrillation by heart failure in dogs: Atrial remodeling of a different sort. Circulation 1999, 100, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.A.; Copeland, C.R.; Reich, D.H.; Tung, L. Mechanical Coupling Between Myofibroblasts and Cardiomyocytes Slows Electrical Conduction in Fibrotic Cell Monolayers. Circulation 2012, 123, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Burstein, B.; Nattel, S. Atrial Fibrosis: Mechanisms and Clinical Relevance in Atrial Fibrillation. J. Am. Coll. Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Mandel, W.J.; Kobayashi, Y.; Karagueuzian, H.S. Cardiac fibrosis as a determinant of ventricular tachyarrhythmias. J. Arrhythm. 2014, 30, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.H.; Brooks, W.W.; Hayes, J.A.; Sen, S.; Robinson, K.G.; Bing, O.H.L. Myocardial Fibrosis and Stiffness With Hypertrophy and Heart Failure in the Spontaneously Hypertensive Rat. Circulation 1995, 91, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.M.; Frazier, O.H.; Buja, L.M. Fibrosis and heart failure. Heart Fail. Rev. 2014, 19, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Anversa, P.; Hiller, B.; Ricci, R.; Guideri, G.; Olivetti, G. Myocyte cell loss and myocyte hypertrophy in the aging rat heart. J. Am. Coll. Cardiol. 1986, 8, 1441–1448. [Google Scholar] [CrossRef]
- Kapelko, V.I. Extracellular matrix alterations in cardiomyopathy: The possible crucial role in the dilative form. Exp. Clin. Cardiol. 2001, 6, 41–49. [Google Scholar] [PubMed]
- Brooks, A.; Schinde, V.; Bateman, A.C.; Gallagher, P.J. Interstitial fibrosis in the dilated non-ischaemic myocardium. Heart 2003, 89, 1255–1256. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Frangogiannis, N.G. Aging and Cardiac Fibrosis. Aging Dis. 2011, 2, 158–173. [Google Scholar] [PubMed]
- Burgess, M.L.; Mccrea, J.C.; Hedrick, H.L. Age-associated changes in cardiac matrix and integrins. Mech. Ageing Dev. 2001, 122, 1739–1756. [Google Scholar]
- de Castro Brás, L.E.; Toba, H.; Baicu, C.F.; Zile, M.R.; Weintraub, S.T.; Lindsey, M.L.; Bradshaw, A.D. Age and SPARC change the extracellular matrix composition of the left ventricle. Biomed Res. Int. 2014, 2014, 810562. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.A.; Graham, H.K.; Richards, M.A.; Clarke, J.D.; Greensmith, D.J.; Briston, S.J.; Hall, M.C.S.; Dibb, K.M.; Trafford, A.W. Age-related divergent remodeling of the cardiac extracellular matrix in heart failure: Collagen accumulation in the young and loss in the aged. J. Mol. Cell. Cardiol. 2012, 53, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Musselman, L.P.; Pendse, J.; Baranski, T.J.; Bodmer, R.; Ocorr, K.; Cagan, R. A Drosophila Model of High Sugar Diet-Induced Cardiomyopathy. PLoS Genet. 2013, 9, e1003175. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Z.; Feng, Z.; Wang, J.; Ouyang, C.; Liu, W.; Fu, B.; Cai, G.; Wu, C.; Wei, R.; et al. Integrin-linked kinase induces both senescence-associated alterations and extracellular fibronectin assembly in aging cardiac fibroblasts. J. Gerontol. Biol. Sci. 2006, 61A, 1232–1245. [Google Scholar] [CrossRef]
- White, D.E.; Coutu, P.; Shi, Y.F.; Tardif, J.C.; Nattel, S.; St. Arnaud, R.; Dedhar, S.; Muller, W.J. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006, 20, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Zambon, A.C.; Fuhrmann, A.; Bernstein, S.I.; Bodmer, R.; Engler, A.J.; Cammarato, A. Measuring passive myocardial stiffness in Drosophila melanogaster to investigate diastolic dysfunction. J. Cell. Mol. Med. 2012, 16, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Spenlehauer, A.; Sessions, A.O.; Trujillo, A.S.; Fuhrmann, A.; Fu, Z.; Venkatraman, V.; Pohl, D.; Tuler, J.; Wang, M.; et al. Vinculin network-mediated cytoskeletal remodeling regulates contractile function in the aging heart. Sci. Transl. Med. 2015, 7, 292ra99. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Stöllberger, C. The heart in human dystrophinopathies. Cardiology 2003, 99, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Monkley, S.J.; Pritchard, C.A.; Critchley, D.R. Analysis of the Mammalian Talin2 Gene TLN2. Biochem. Biophys. Res. Commun. 2001, 286, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Bonnema, D.D.; Webb, C.S.; Pennington, W.R.; Stroud, R.E.; Leonardi, A.E.; Clark, L.L.; Mcclure, C.D.; Spinale, F.G.; Zile, M.R. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J. Card. Fail. 2007, 13, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Apple, K.A.; Yarbrough, W.M.; Mukherjee, R.; Deschamps, A.M.; Escobar, P.G.; Mingoia, J.T.; Sample, J.A.; Hendrick, J.W.; Dowdy, K.B.; McLean, J.E.; et al. Selective targeting of matrix metalloproteinase inhibition in post-infarction myocardial remodeling. J. Cardiovasc. Pharmacol. 2006, 47, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; Coker, M.L.; Krombach, S.R.; Mukherjee, R.; Hallak, H.; Houck, W.V.; Clair, M.J.; Kribbs, S.B.; Johnson, L.L.; Peterson, J.T.; et al. Matrix metalloproteinase inhibition during the development of congestive heart failure: Effects on left ventricular dimensions and function. Circ. Res. 1999, 85, 364–376. [Google Scholar] [CrossRef] [PubMed]
- LaFever, K. S.; Wang, X.; Page-McCaw, P.; Bhave, G.; Page-McCaw, A. Both Drosophila matrix metalloproteinases have released and membrane-tethered forms but have different substrates. Sci. Rep. 2017, 7, 44560. [Google Scholar] [CrossRef] [PubMed]
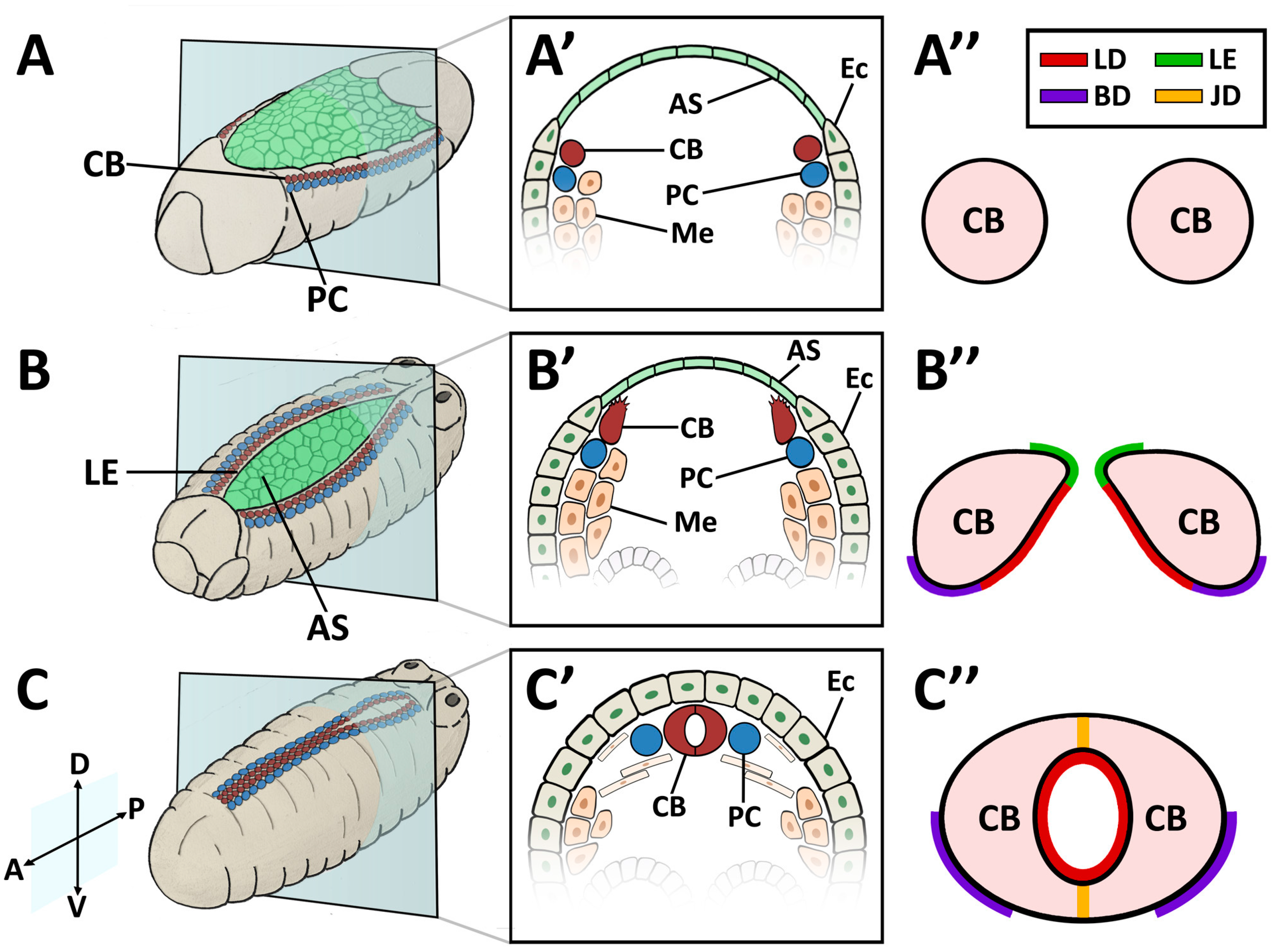
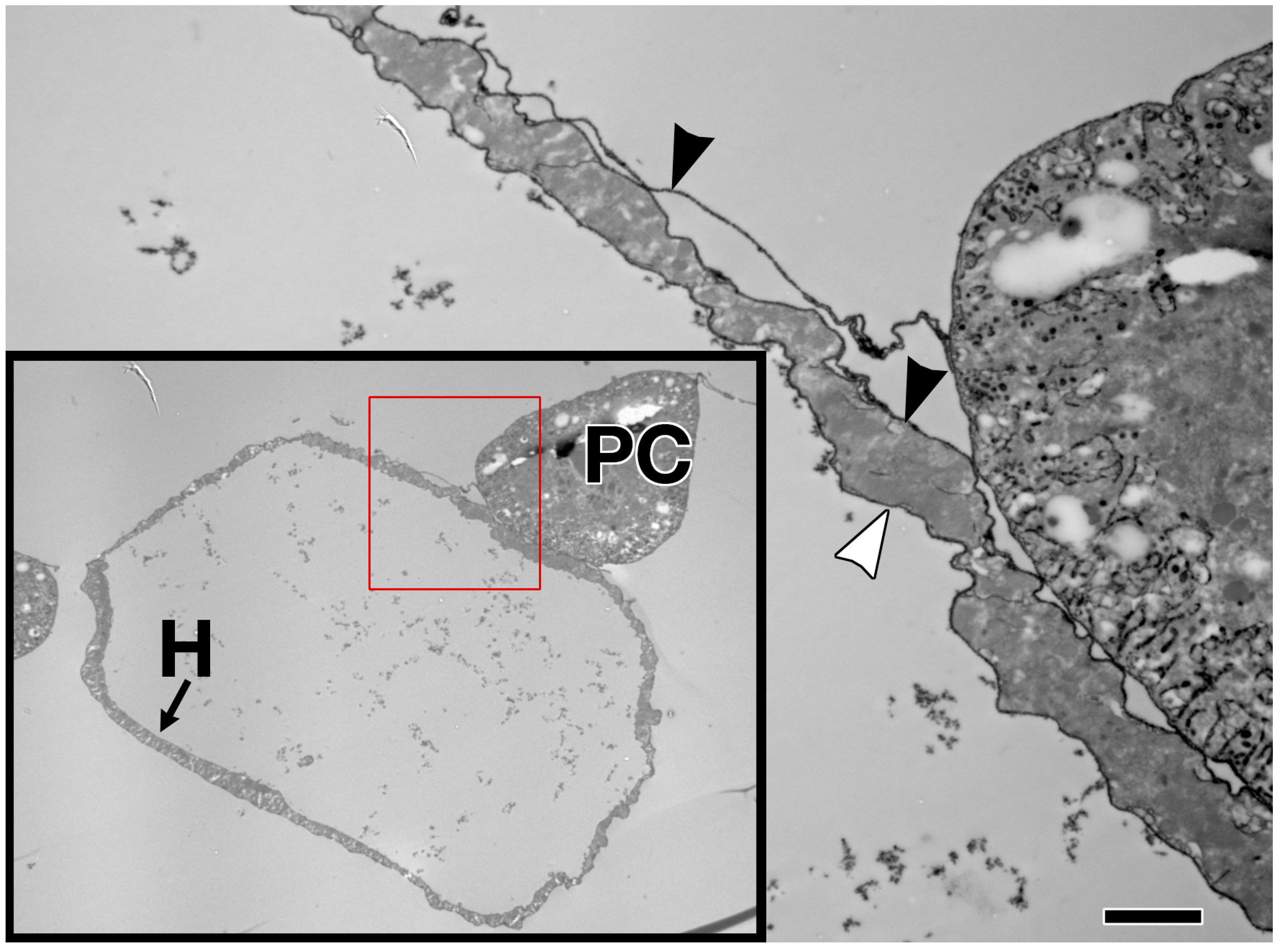
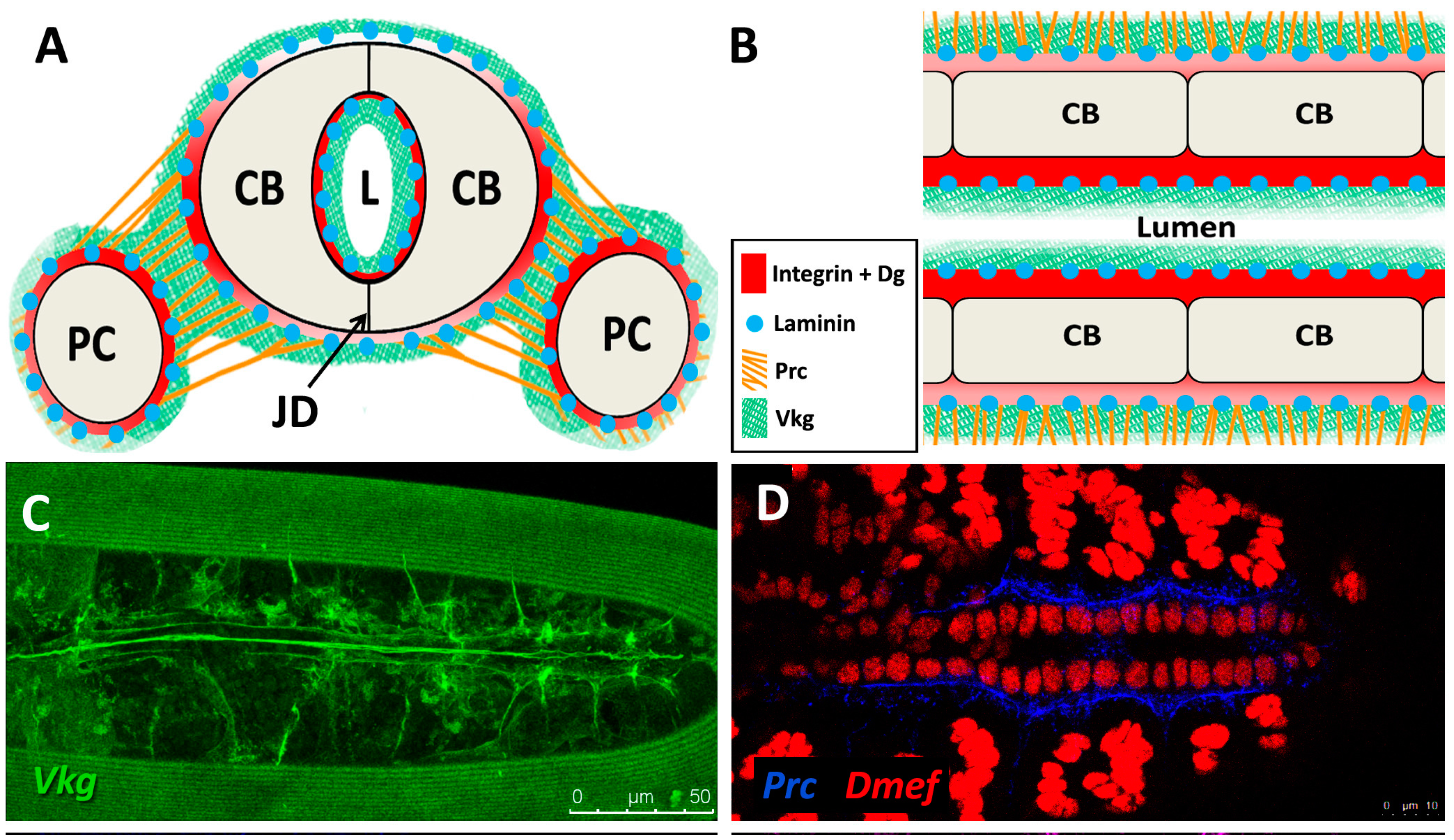

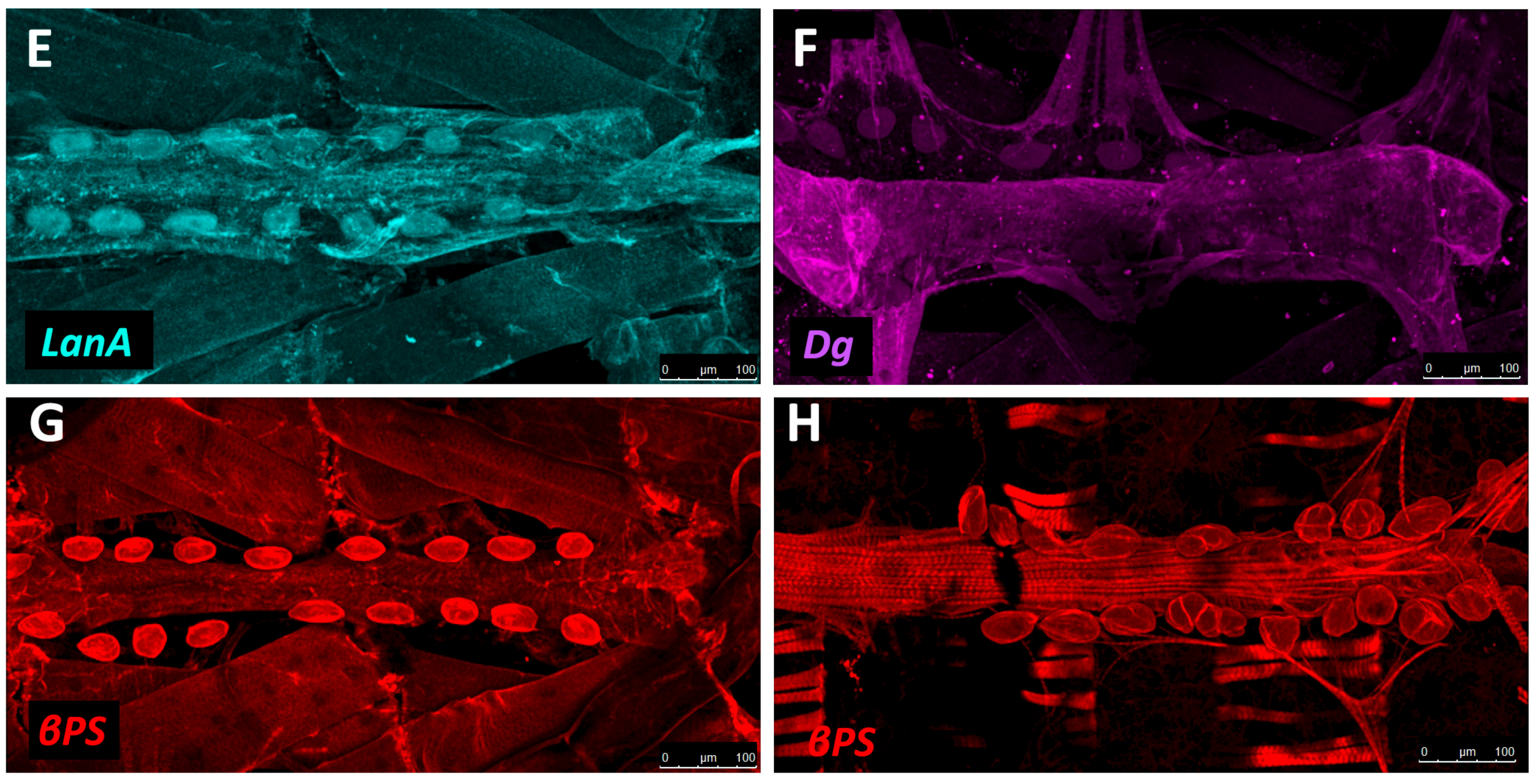
| Collagen-IV | Collagen-IV | Cell adhesion, Basal Lamina | Mutants exhibit alary muscle and PC detachment, and accumulation of Perlecan within hemocytes [119]. RNAi knock-down in the heart mitigates age-related decline in fractional shortening and increases longevity [121]. | Up-regulated post-infarction during repair in rats [122]. Not restricted to the BM during DCM in humans or myocardial infarction in rats; found in fibrotic lesions [123,124]. |
| Prc | N/A (Collagen-IV α-like) | Cell adhesion | Mutants exhibit cardiomyocytes that do not properly polarise and fail to align, detachment of PCs and alary muscles from the heart tube, and reduced lifespan [11,101]. RNAi knock-down in the heart mitigates age-related decline in fractional shortening and increases longevity [121]. | N/A (see Collagen-IV) |
| Mp | Collagen-XV/XVIII | Lumen expansion | Loss of function results in a small lumen and diminished fractional shortening; over-expression results in increased lumen size or development of ectopic lumens [118]. | Collagen-XV deficiency in mice causes disorganised fibrillar Collagen, increased left ventricle (LV) myocardial stiffness, cardiac hypotrophy, aberrant cardiomyocyte structure, and cardiomyopathy [125]. Collagen-XVIII deficiency in rats results in extended cardiac BM, adverse remodelling, and heart failure post-infarction [126,127]. Up-regulated after hypoxia [127]. |
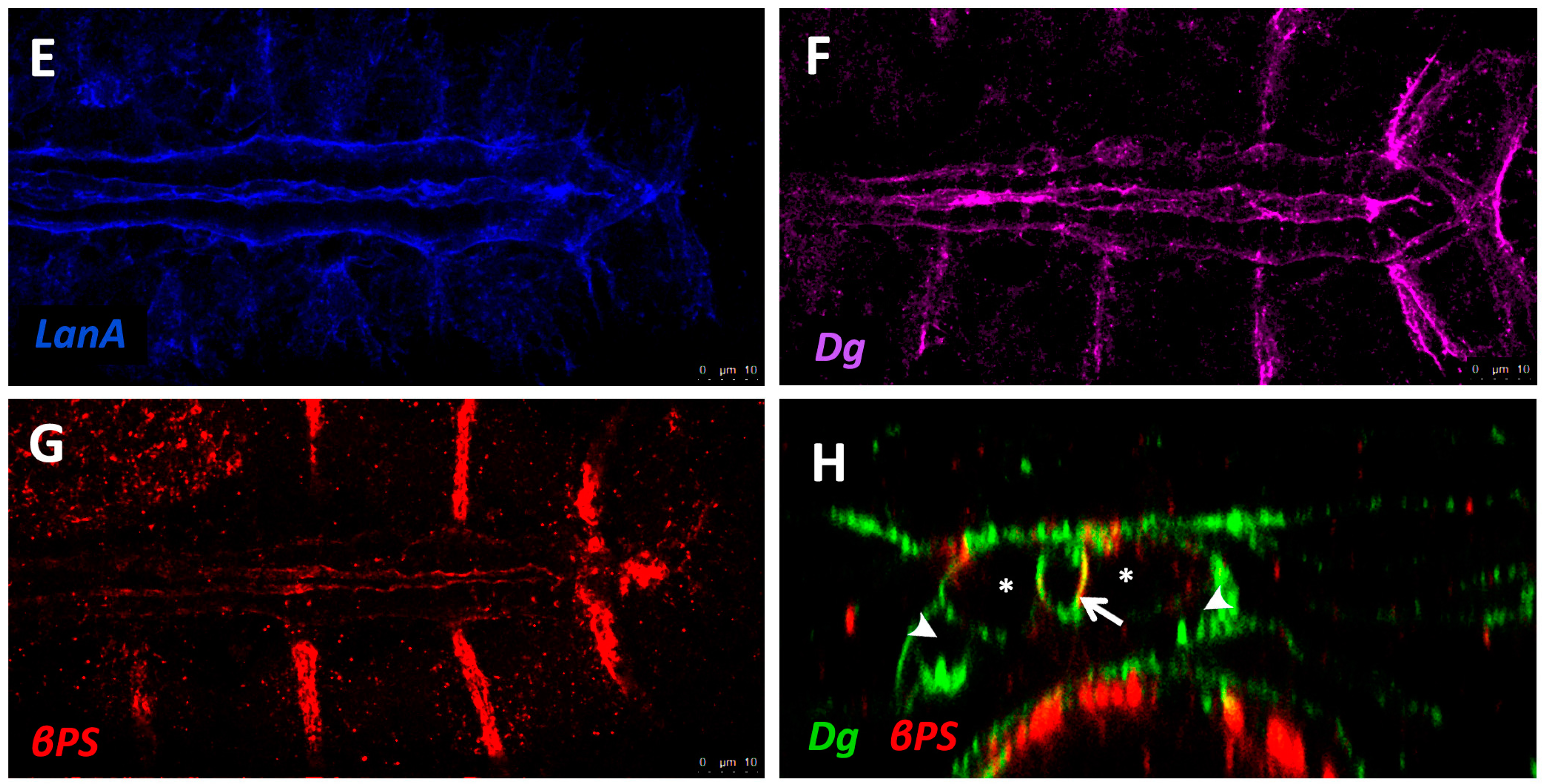
| Laminin | Laminin | Cell adhesion, Collagen assembly | Mutants show failed accumulation of Perlecan, Collagen-IV, and Prc [128], gaps between CBs, breaks in the cardiac tube, a small lumen, muscle attachment defects [119], and dissociation of PCs from heart tube [129]. RNAi knock-down in the heart mitigates age-related decline in fractional shortening and increases longevity [121]. | Decreased expression during ischemic heart failure [130]. Mutant mice develop cardiomyopathy and cardiac hypertrophy [131]. |
| SPARC | SPARC | Collagen assembly | Knock-down causes disorganisation of Laminin, failed Collagen-IV assembly, and reduced heart contractility [132,133]. | Up-regulated in older mice, causing ventricular stiffness [134]. Up-regulated in infarcted mouse and canine hearts (protective role) [5,14]. |
| TSP | TSP-3/4 | Cell adhesion | Mutants exhibit detachment of muscle cells from tendons [135,136]. | Canine TSP-1 is up-regulated post-infarction [137]. TSP-3/4 up-regulated during remodelling and pressure overload [5,138]. Mice mutant for TSP-1 exhibit increased fibrosis, and detrimental LV remodelling post-infarction [137]. Mice mutant for TSP-2 are more prone to cardiac rupture post-infarction and exhibit DCM and fibrosis with aging [5]. Mice mutant for TSP-4 deposit more ECM , leading to fibrosis and decreased contractility [139]. |
| Loh | ADAMTSL6 | Cell adhesion | In mutants, Prc does not localise between PCs and heart tube; PCs and alary muscles detach from the heart tube, and lifespan is reduced [11]. | Over-expression results in the accumulation of Fibrillin-1-containing ECM microfibrils in mice [140]. |
| β-Integrin | β-Integrin | Cell-ECM linker, adhesion signalling | Age-dependent up-regulation results in increased myocardial stiffness [141]. Mutants exhibit reduced CB Leading Edge activity, mis-aligned CBs, failed localisation of βPS-Integrin to CB surface, ectopic Prc and Slit, and lack luminal domains [110]. | Age-dependent up-regulation results in increased aortic stiffness in monkeys [142]. Knock-down mice causes fibrosis, reduced LV contractility, and DCM [143]. |
| Dg | Dg | Cell-ECM linker | Mutants exhibit mis-expression of epithelial cell apical markers in basal domain and loss of anterior-posterior polarity, as well as age-related muscular degeneration [112,144]. | In humans, reduced Dg glycosylation weakens ECM attachment, and causes DCM [145]. |
| Dscam | Dscam | Cell adhesion and signalling | Mutants exhibit reduced CB Leading Edge velocity, disrupted (non-continuous) heart lumen [89]. Concomitant over-expression with Collagen-VI reduces heart rate and causes arhythmia/asystole [146]. The large number of DSCAM isoforms constrains extension by homology to vertebrates [147,148]. | Candidate Down syndrome congenital heart defect (CHD) gene in humans [149]. Mouse over-expression causes atrial-septal defects and LV thickening (CHD, hypertrophic cardiomyopathy) [146]. |
| Sdc | Sdc | Cell adhesion and signalling | Mutants and RNAi knock-downs exhibit gaps between CBs and between PCs, mis-localisation of Prc, and failure of CBs to polarise (no apicalisation of Robo/Slit) [150]. | Contributes to post-infarction fibrosis ECM stiffness and cardiac hypertrophy (reviewed in [151]). |
| Robo | Robo | Morphogen receptor | Mutants exhibit gaps between CBs at the midline, and small, intermittent, or no lumen [106,152,153], and reduced CB migration velocity [154]. | Mutant mice exhibit cardiac valve and septum morphogenesis, ectopic pericardial cavities, and caval vein malformation [155,156]. |
| Slit | Slit | Secreted morphogen | Mutants exhibit CB mis-alignment and gaps between CBs at the midline, resulting in small, intermittent, or no lumen [152,153], as well as cardiac tube lesions and reduced CB migration velocity [154]. Over-expression causes the formation of ectopic lumens [106]. | Mutant mice exhibit reduced angiogenesis, cardiac valve and septum morphogenesis, ectopic pericardial cavities, and caval vein malformation [155,156]. |
| Fra | DCC | Morphogen receptor | Mutants exhibit CB mis-alignment at the midline, and defective contralateral attachments between CBs results in an open or enlarged lumen, or no lumen [157]. | Cardioprotective role in elevating nitric oxide production [158]. |
| MMP1/2 | MMP1-28 | ECM protease | MMP1 mutants have a reduced heart lumen, and embryonic CB migration is less organised; in MMP2 mutants embryonic CBs do not form cell junctions, resulting in reduced or absent heart lumen; surviving larvae show cardia bifida [159,160]. | MMP1/2/3/7/8/9/14 show increased protein expression in DCM (reviewed in [4]). MMP1/2/3/9/13 are up-regulated in canines exhibiting cardiac abnormalities [161]. MMP2/9 are up-regulated in infarcted mouse and pig hearts, with depletion resulting in decreased odds of cardiac rupture [162,163,164]. |
| TIMP | TIMP1-4 | Inhibitor of ECM proteases | Ectopic ectodermal expression of TIMP inhibits heart lumen formation [159]. | Mouse TIMP mutants experience adverse remodelling / increased ECM degradation post-infarction [165,166]; TIMP1 mutants develop cardiac hypertrophy [167] and TIMP3 mutants develop DCM [168]. TIMP1/2/3/4 are up-regulated in canines exhibiting cardiac abnormalities [169]. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, C.J.R.; Jacobs, J.R. Dissecting the Role of the Extracellular Matrix in Heart Disease: Lessons from the Drosophila Genetic Model. Vet. Sci. 2017, 4, 24. https://doi.org/10.3390/vetsci4020024
Hughes CJR, Jacobs JR. Dissecting the Role of the Extracellular Matrix in Heart Disease: Lessons from the Drosophila Genetic Model. Veterinary Sciences. 2017; 4(2):24. https://doi.org/10.3390/vetsci4020024
Chicago/Turabian StyleHughes, Chris J. R., and J. Roger Jacobs. 2017. "Dissecting the Role of the Extracellular Matrix in Heart Disease: Lessons from the Drosophila Genetic Model" Veterinary Sciences 4, no. 2: 24. https://doi.org/10.3390/vetsci4020024






