How Behavioral Changes Can Indicate Serious Cerebral Pathology: A Case Report of Concomitant Olfactory Neuroblastoma and Distemper Virus Encephalitis in a Swiss Shepherd Dog
Abstract
:1. Introduction
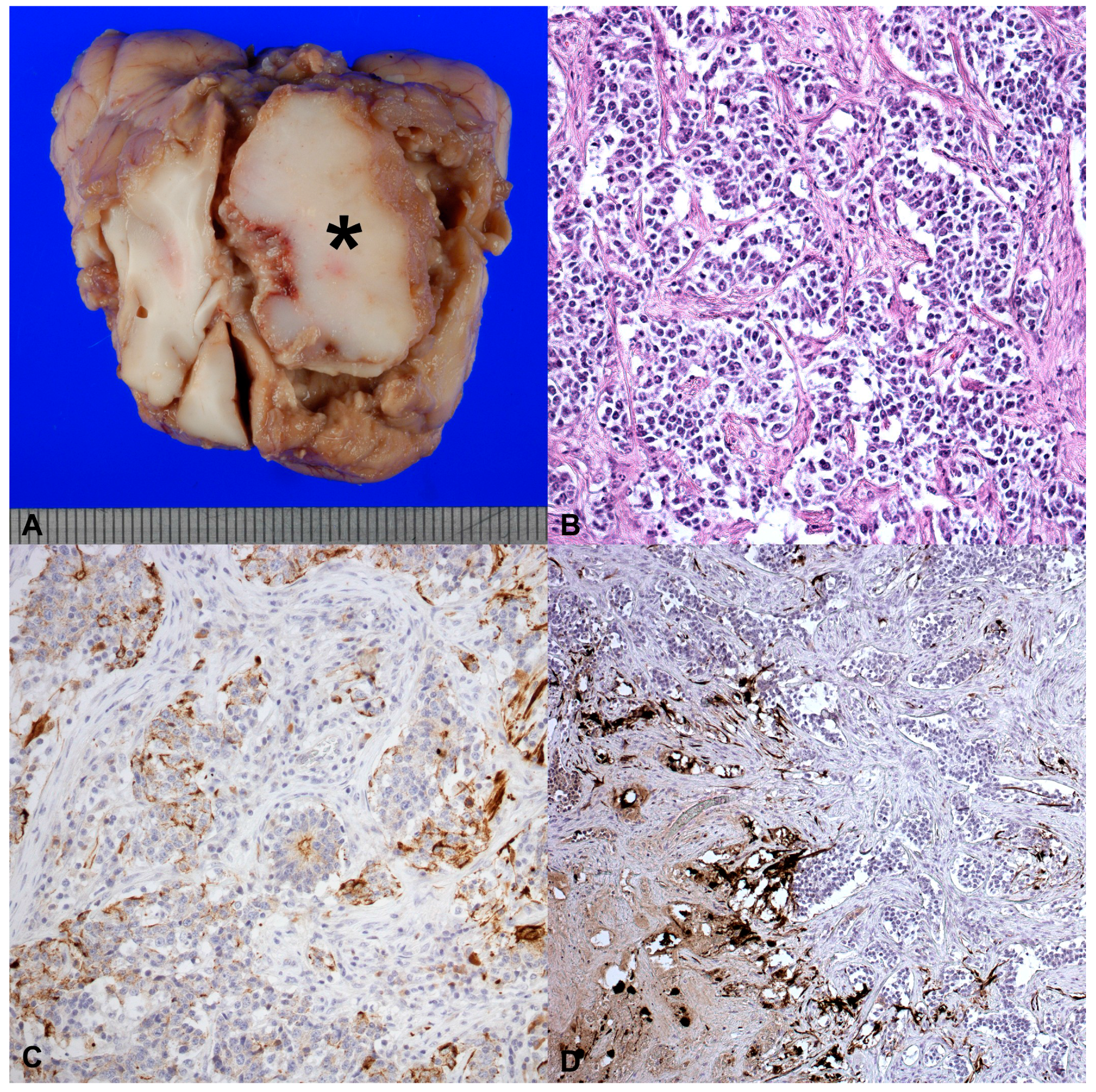
2. Case History
3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Lahunta, A.; Glass, E. Seizure disorders: Narcolepsy. In Veterinary Neuroanatomy and Clinical Neurology; De Lahunta, A., Glass, E., Eds.; Elsevier: Saunders, The Netherlands, 2009; pp. 454–459. ISBN 9780721667065. [Google Scholar]
- Maxie, G.; Caswell, J.L.; Williams, K.J. Respiratory system. In Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 5th ed.; Maxie, G., Ed.; Elsevier: Saunders, The Netherlands, 2007; pp. 635–636. ISBN 9780080569826. [Google Scholar]
- Wang, H.; Jia, X.; Yang, L.; Sun, L.; Wang, H.; Liu, W. Comparison of antiviral activity between FeIFN-omega and FeIFN-alpha. Sheng Wu Gong Cheng Xue Bao 2008, 24, 1556–1660. [Google Scholar] [PubMed]
- Bollo, E.; Zurbriggen, A.; Vandevelde, M.; Fankhauser, R. Canine distemper virus clearance in chronic inflammatory demyelination. Acta Neuropathol. 1986, 72, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgärtner, W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunopathol. 2009, 127, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zachary, J.F. Nervous System. In Pathologic Basis of Veterinary Disease; McGavin, M.D., Zachary, J.F., Eds.; Elsevier—Health Sciences Division: St. Louis, MI, USA, 2010; p. 934. [Google Scholar]
- Amude, A.M.; Headley, S.A.; Alfieri, A.A.; Beloni, S.N.; Alfieri, A.F. Atypical necrotizing encephalitis associated with systemic canine distemper virus infection in pups. J. Vet. Sci. 2011, 12, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Matiasek, K.; Pumarola, M.; Rosati, M.; Fernández-Flores, F.; Fischer, A.; Wagner, E.; Berendt, M.; Bhatti, S.F.; De Risio, L.; Farquhar, R.G.; et al. International veterinary epilepsy task force recommendations for systematic sampling and processing of brains from epileptic dogs and cats. BMC Vet. Res. 2015, 11, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, E.S.; Carrillo, J.M.; Patnaik, A.K. Clinical signs of tumors affecting the rostral cerebrum in 43 dogs. J. Vet. Int. Med. 1988, 2, 71–74. [Google Scholar] [CrossRef]
- Galán, A.; Gamito, A.; Carletti, B.E.; Guisado, A.; de las Mulas, J.M.; Pérez, J.; Martin, E.M. Uncommon acute neurologic presentation of canine distemper in 4 adult dogs. Can. Vet. J. 2014, 55, 373–378. [Google Scholar] [PubMed]
- Headley, S.A.; Alfieri, A.A.; Fritzen, J.T.; Garcia, J.L.; Weissenböck, H.; da Silva, A.P.; Bodnar, L.; Okano, W.; Alfieri, A.F. Concomitant canine distemper, infectious canine hepatitis, canine parvoviral enteritis, canine infectious tracheobronchitis, and toxoplasmosis in a puppy. J. Vet. Diagn. Investig. 2013, 25, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wenzlow, N.; Plattet, P.; Wittek, R.; Zurbriggen, A.; Gröne, A. Immunohistochemical demonstration of the putative canine distemper virus receptor CD150 in dogs with and without distemper. Vet. Pathol. 2007, 44, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Brosinski, K.; Janik, D.; Polkinghorne, A.; Von Bomhard, W.; Schmahl, W. Olfactory neuroblastoma in dogs and cats—A histological and immunohistochemical analysis. J. Comp. Pathol. 2012, 146, 152–159. [Google Scholar] [CrossRef]
- Kitagawa, M.; Okada, M.; Yamamura, H.; Kanayama, K.; Sakai, T. Diagnosis of olfactory neuroblastoma in a dog by magnetic resonance imaging. Vet. Rec. 2006, 159, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, L.M.; Drummond, K.J.; Andrewes, D.G. Emotional and personality changes following brain tumour resection. J. Clin. Neurosci. 2016, 29, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Efird, J.T.; Davies, S.W.; O’Neil, W.T.; Anderson, E.J. Animal viruses, bacteria and cancer: A brief commentary. Front. Public Health 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Reardon, T.R.; Murray, A.J.; Turi, G.F.; Wirblich, C.; Croce, K.R.; Schnell, M.J.; Jessell, T.M.; Losonczy, A. Rabies Virus CVS-N2c(ΔG) Strain Enhances Retrograde Synaptic Transfer and Neuronal Viability. Neuron 2016, 89, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Masemann, D.; Boergeling, Y.; Ludwig, S. Employing RNA viruses to fight cancer—Novel insights into oncolytic virotherapy. Biol. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Type/Company | Dilution | Tumour | Stroma |
|---|---|---|---|---|
| Vimentin | Monoclonal Mouse, Anti-Vimentin, Clone V9, Dako, Glostrup, Denmark | 1:200 | − | + |
| Cytokeratins | Monoclonal Mouse, Anti-Human Cytokeratins, Clones AE1/AE3, Dako, Glostrup, Denmark | 1:50 | + | − |
| Glial fibrillary acidic protein (GFAP) | Polyclonal Rabbit, Anti-Glial Fibrillary Acidic Protein, Dako, Glostrup, Denmark | 1:5000 | − | + |
| S-100 | Polyclonal Rabbit, Anti-Cow S-100, Z0311, Dako, Glostrup, Denmark | 1:200 | − | + |
| Neuron enolase (NSE) | Monoclonal Mouse, Anti-Human Neuron Specific Enolase, Clone BBS/NC/VI-H14, Dako, Glostrup, Denmark | 1:500 | + | − |
| Neurofilament (NF) | Monoclonal Mouse, Anti-Human Neurofilament Protein, Clone 2F11, Dako, Glostrup, Denmark | 1:10,000 | + | − |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candini, D.; Biasato, I.; Dell’Armelina Rocha, P.R.; Grego, E.; Capucchio, M.T.; Vercelli, C. How Behavioral Changes Can Indicate Serious Cerebral Pathology: A Case Report of Concomitant Olfactory Neuroblastoma and Distemper Virus Encephalitis in a Swiss Shepherd Dog. Vet. Sci. 2017, 4, 42. https://doi.org/10.3390/vetsci4030042
Candini D, Biasato I, Dell’Armelina Rocha PR, Grego E, Capucchio MT, Vercelli C. How Behavioral Changes Can Indicate Serious Cerebral Pathology: A Case Report of Concomitant Olfactory Neuroblastoma and Distemper Virus Encephalitis in a Swiss Shepherd Dog. Veterinary Sciences. 2017; 4(3):42. https://doi.org/10.3390/vetsci4030042
Chicago/Turabian StyleCandini, Dario, Ilaria Biasato, Paulo Ricardo Dell’Armelina Rocha, Elena Grego, Maria Teresa Capucchio, and Cristina Vercelli. 2017. "How Behavioral Changes Can Indicate Serious Cerebral Pathology: A Case Report of Concomitant Olfactory Neuroblastoma and Distemper Virus Encephalitis in a Swiss Shepherd Dog" Veterinary Sciences 4, no. 3: 42. https://doi.org/10.3390/vetsci4030042






