Histology of the Ovary of the Laying Hen (Gallus domesticus)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
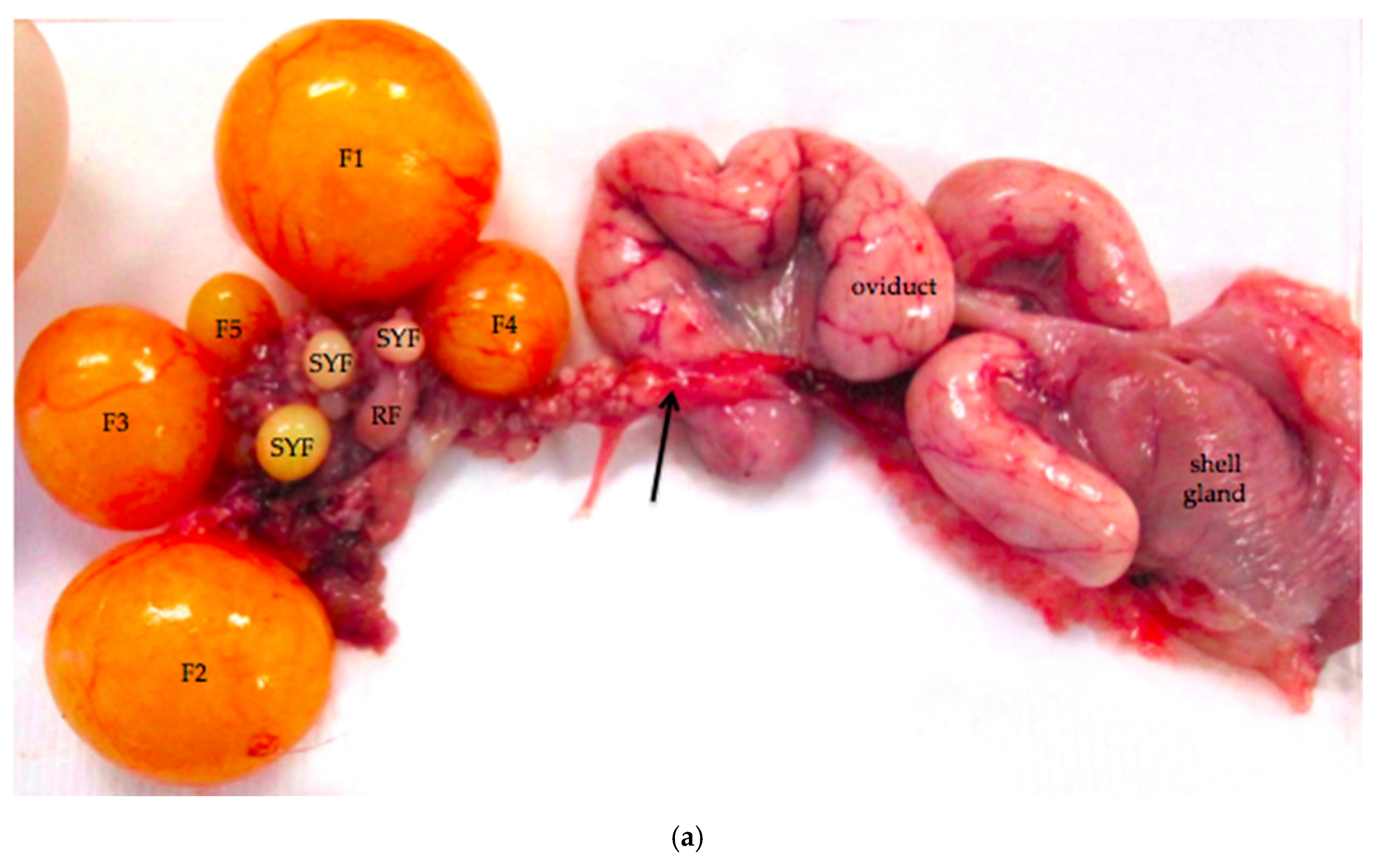
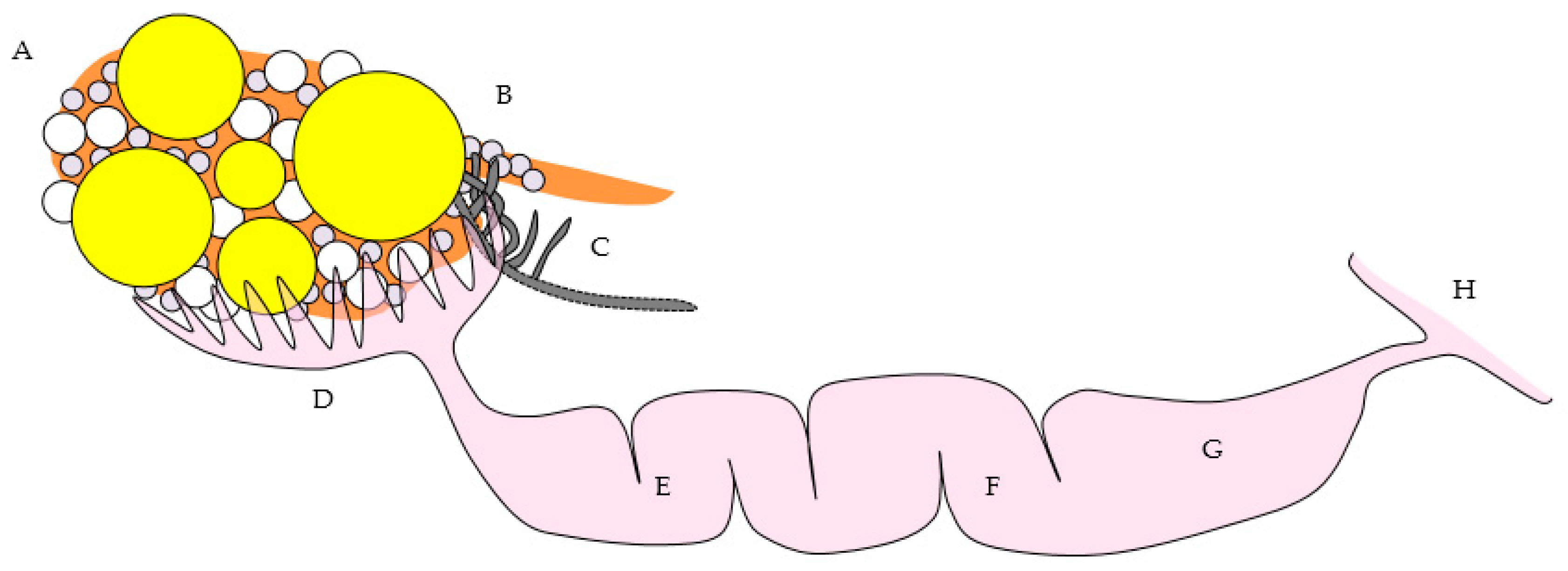
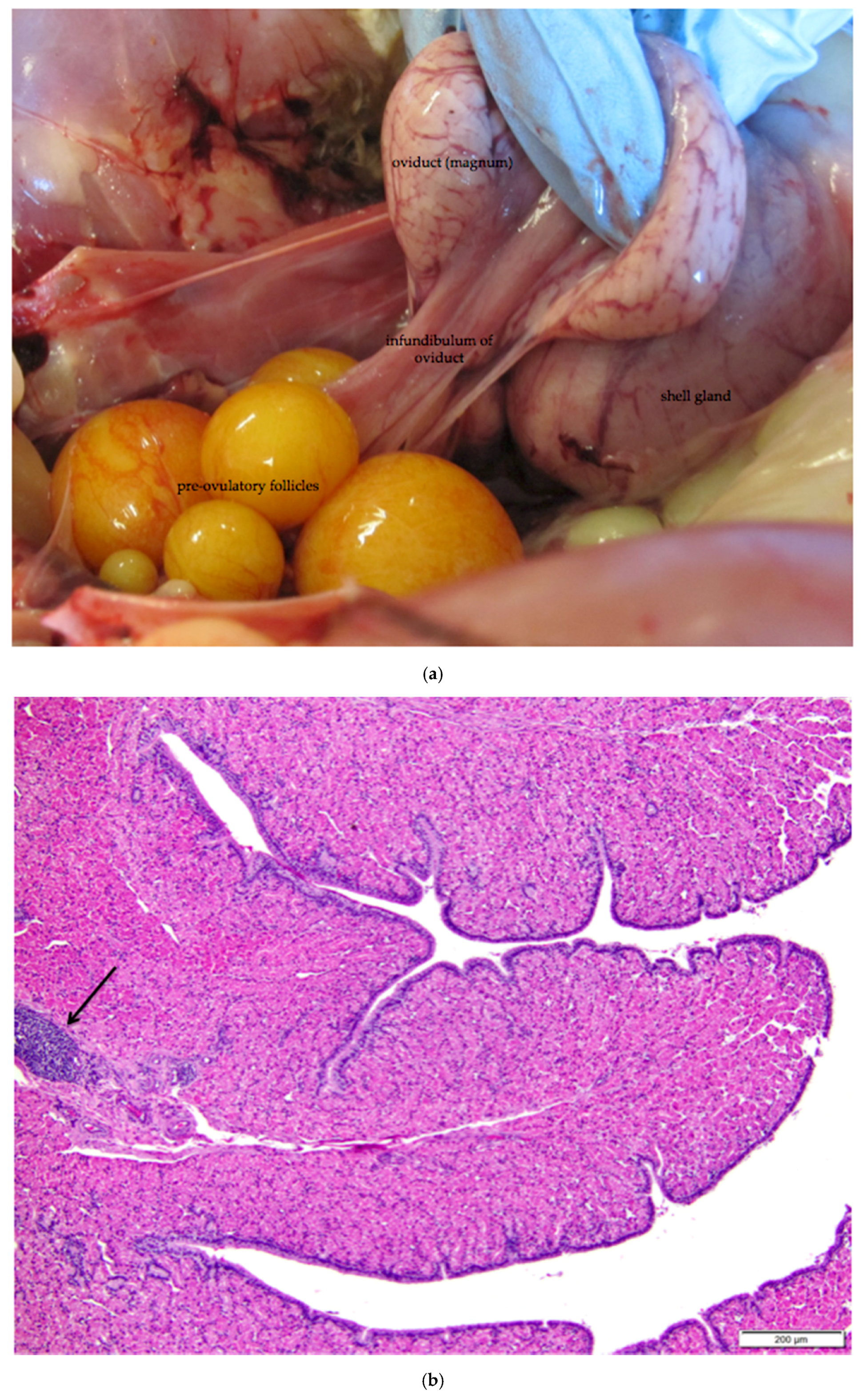
3.1. Overview of Gross and Microscopic Anatomy and Histology of the Laying Hen Ovary
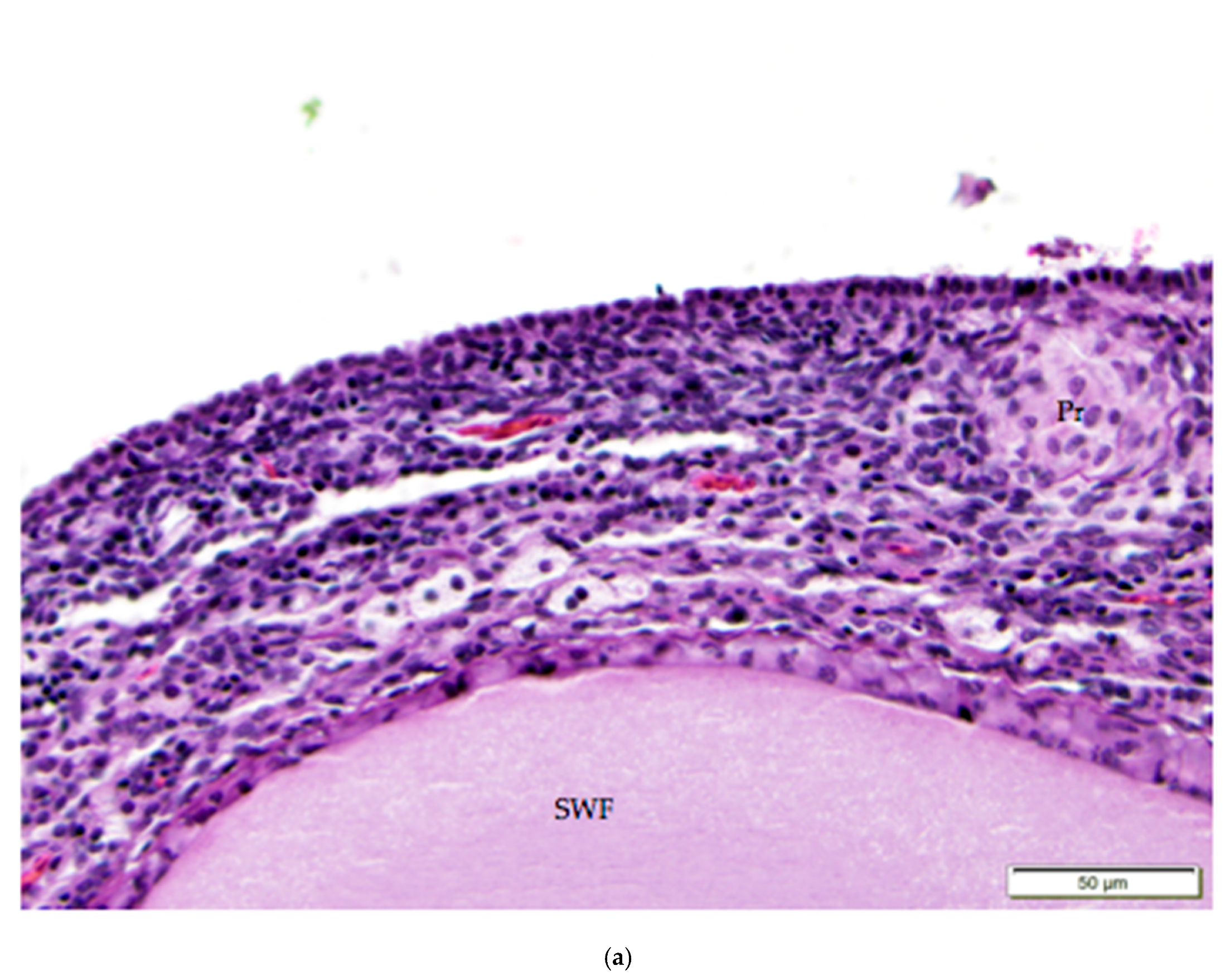
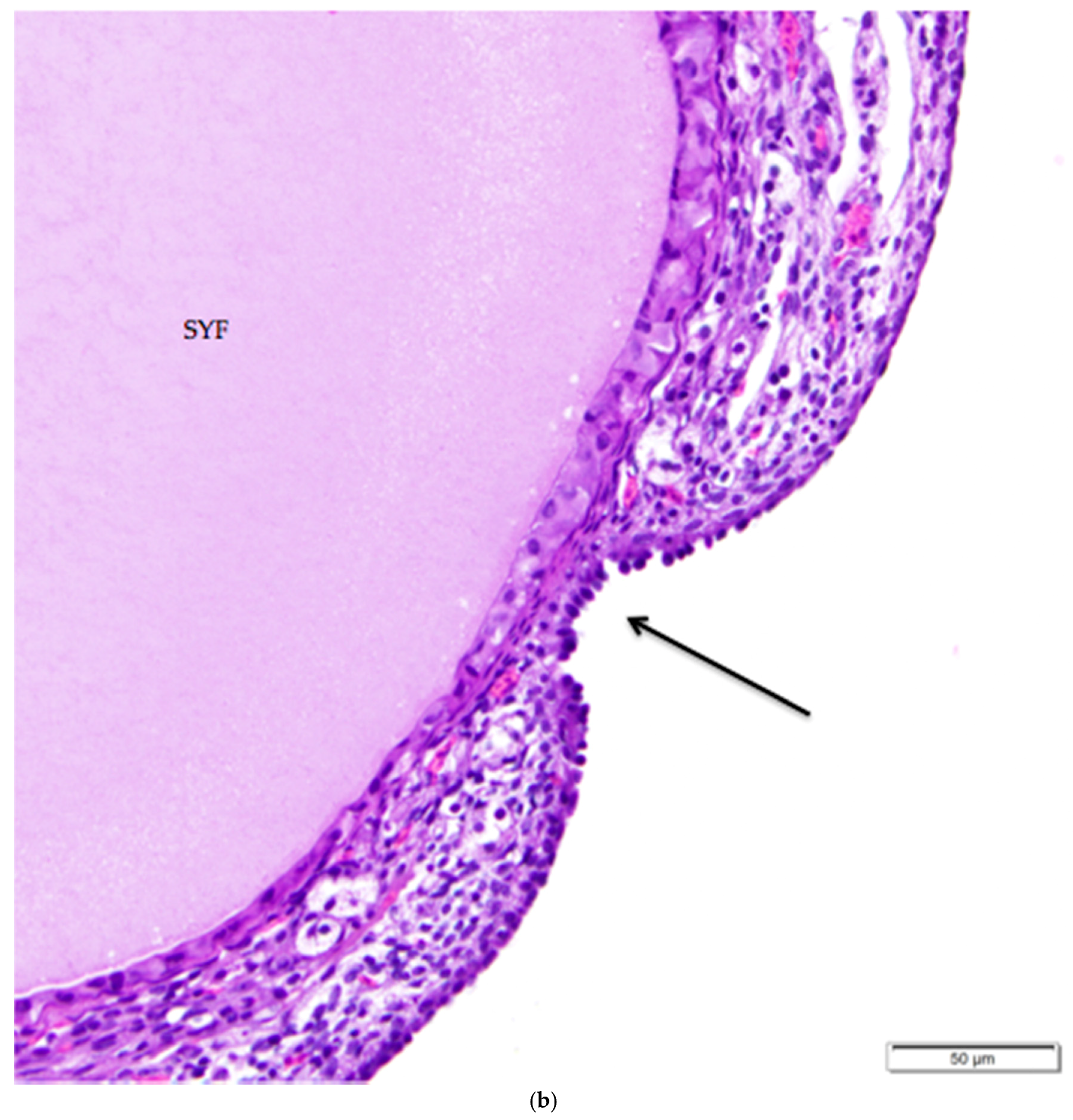
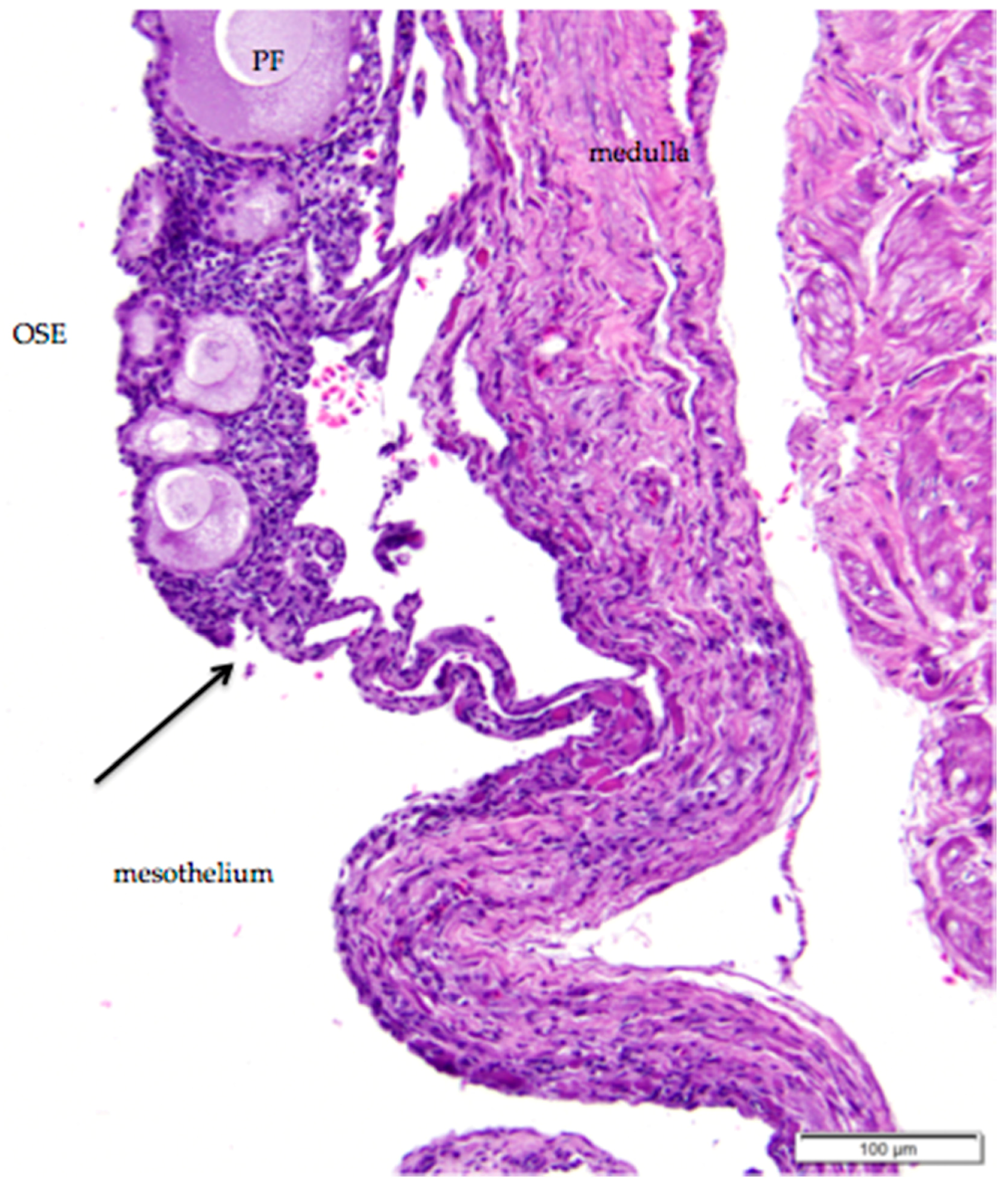
3.2. Ovarian Surface Epithelium
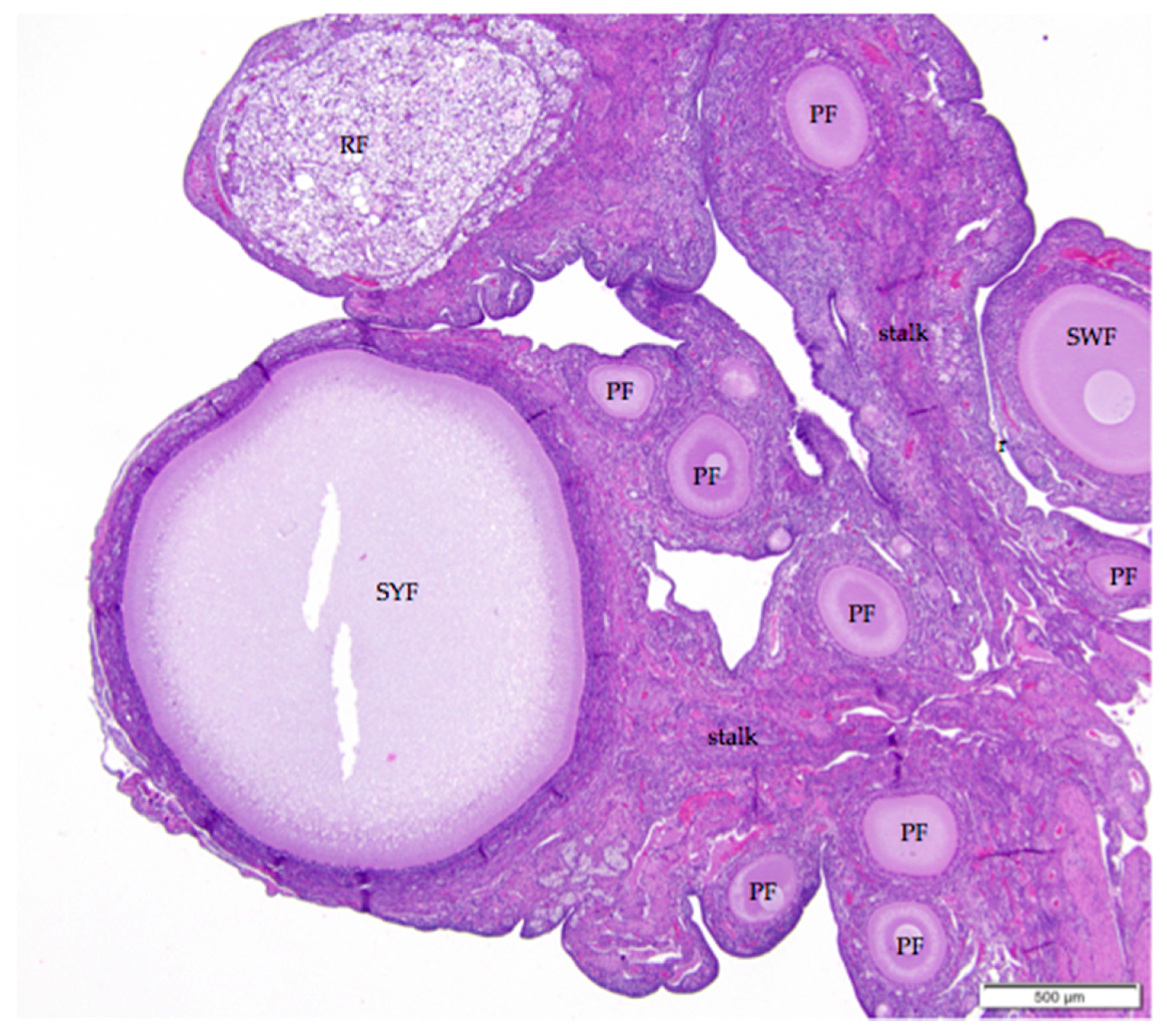
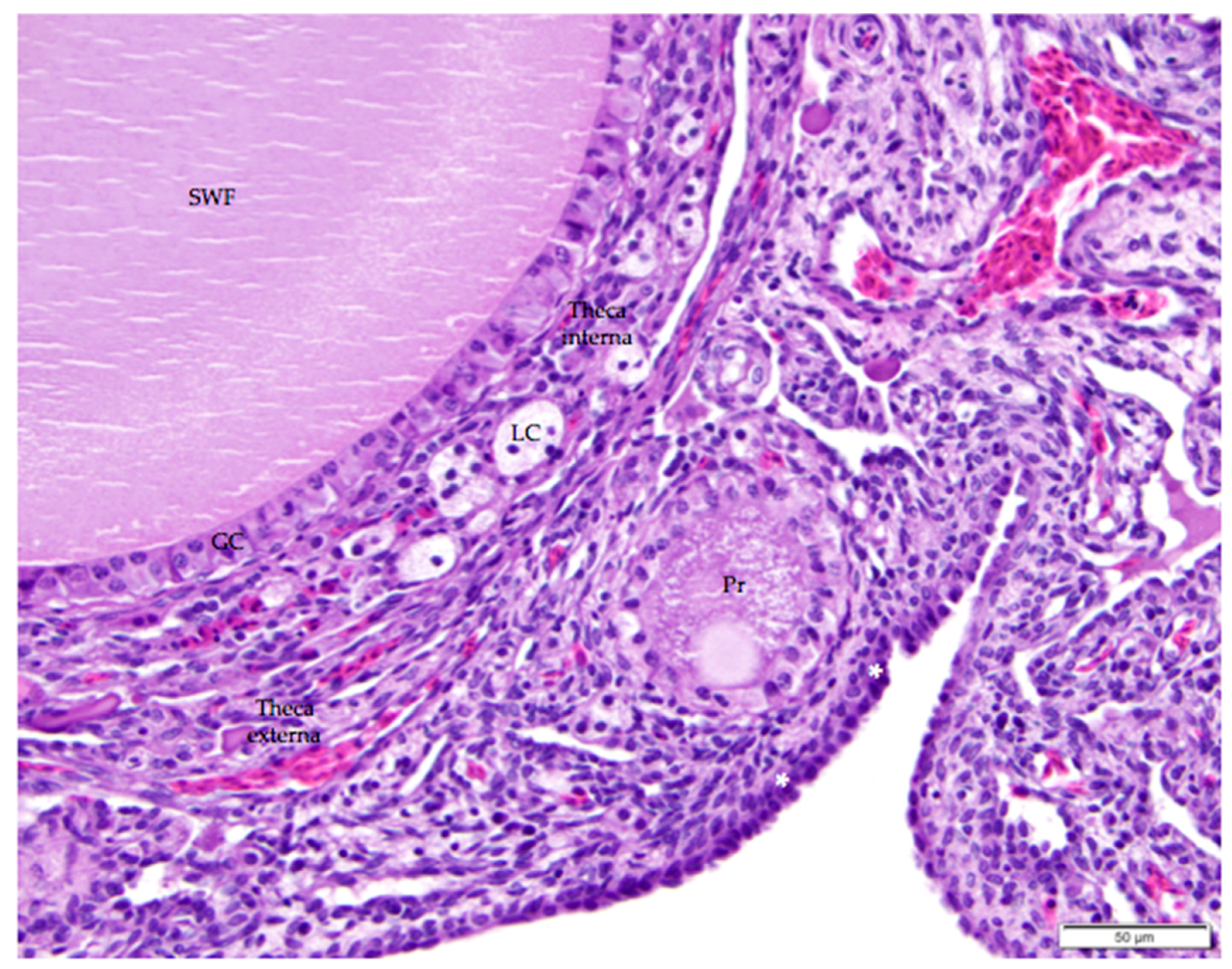
3.3. Follicles and Endocrine Cells in the Ovarian Cortex
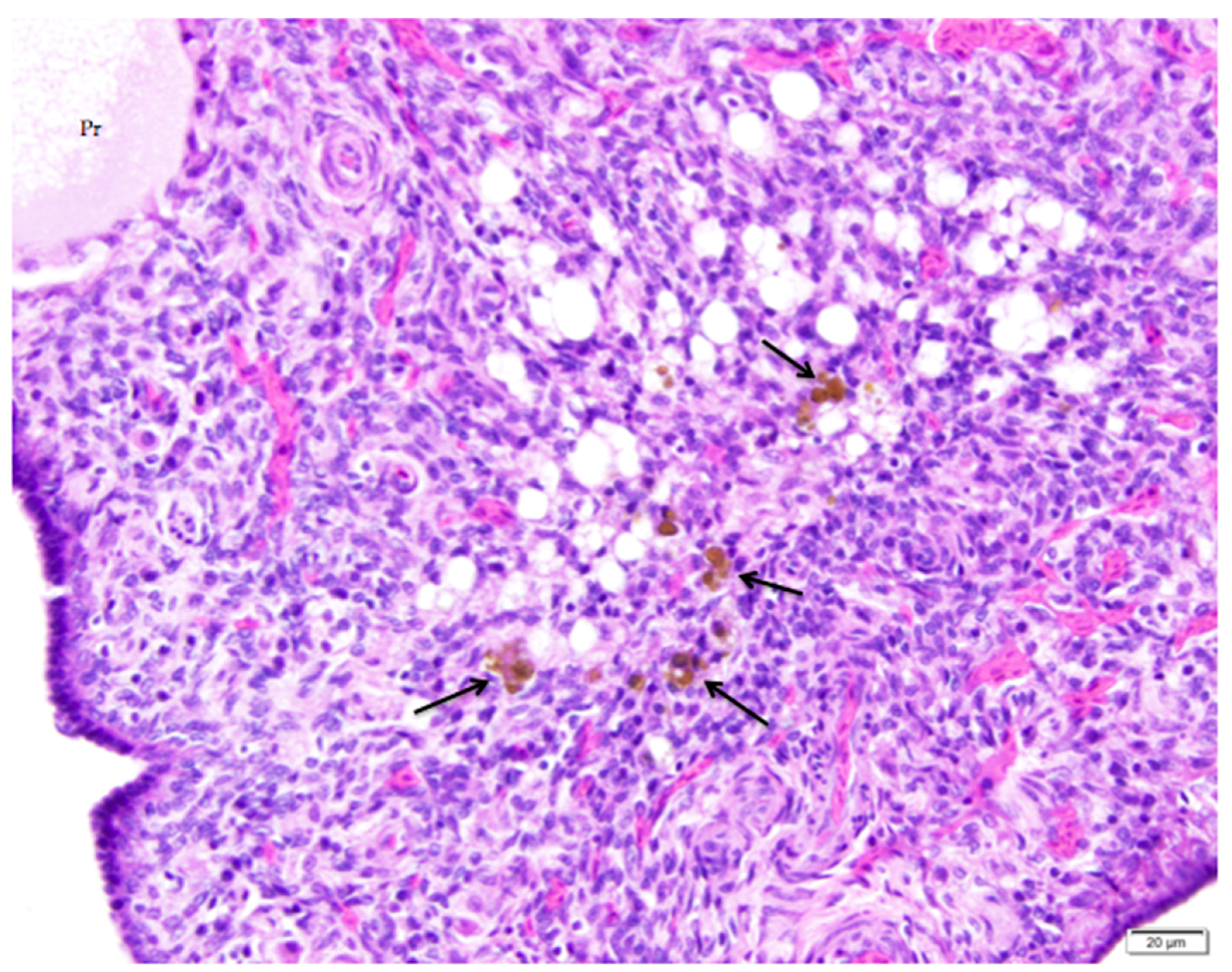
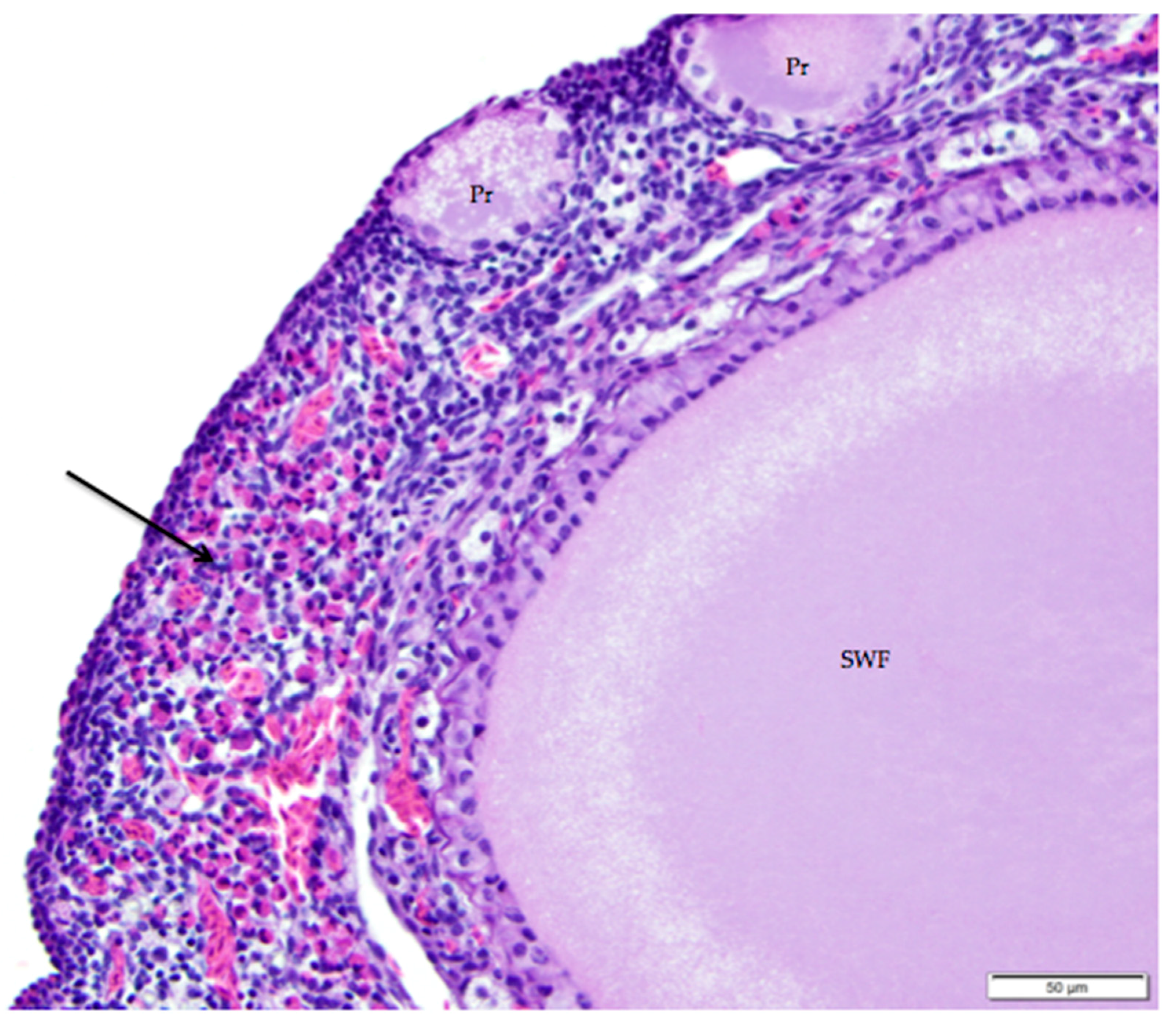
3.4. Follicular Regression and Atresia
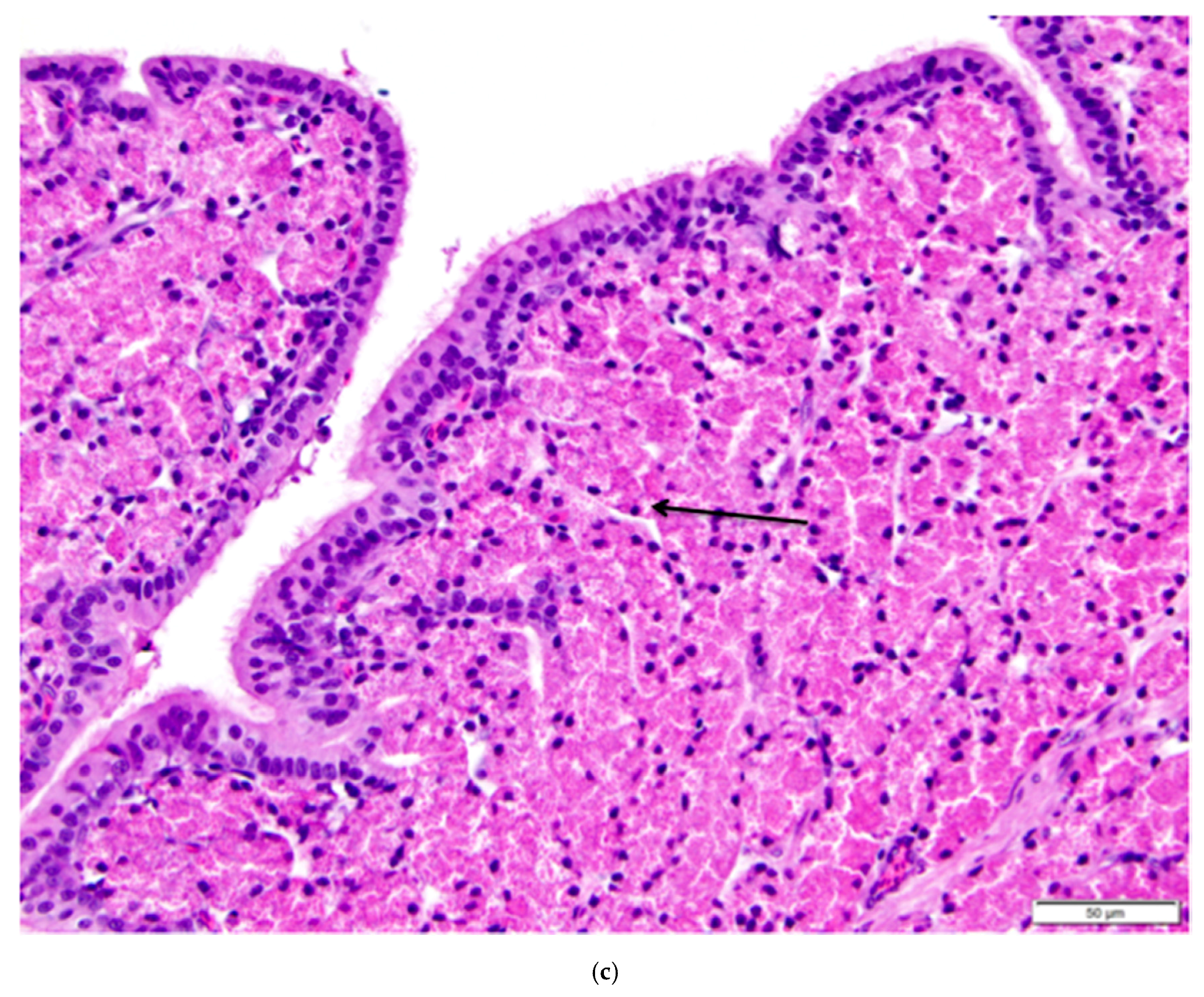
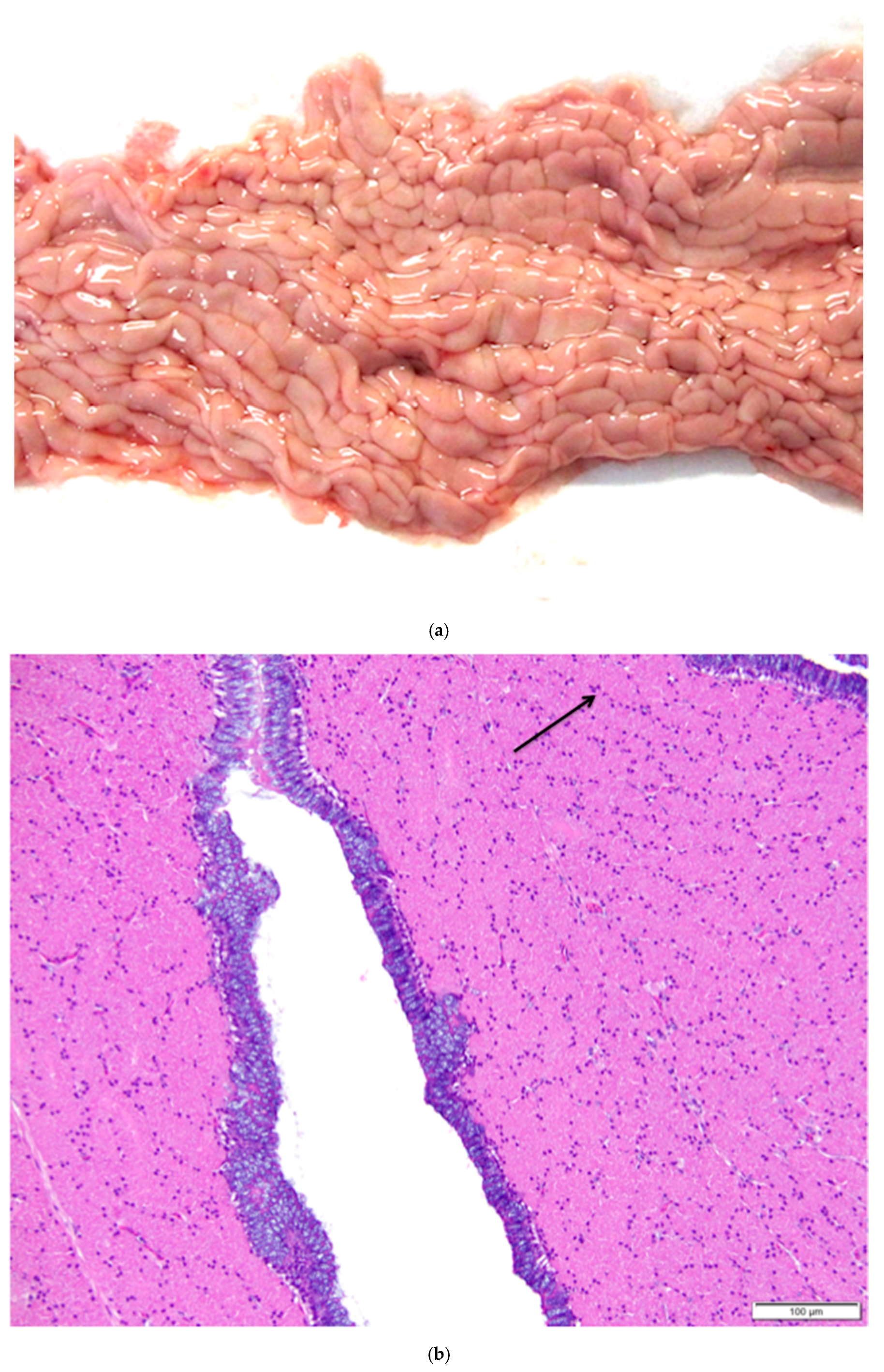
3.5. Infundibulum and Magnum of the Proximal Oviduct
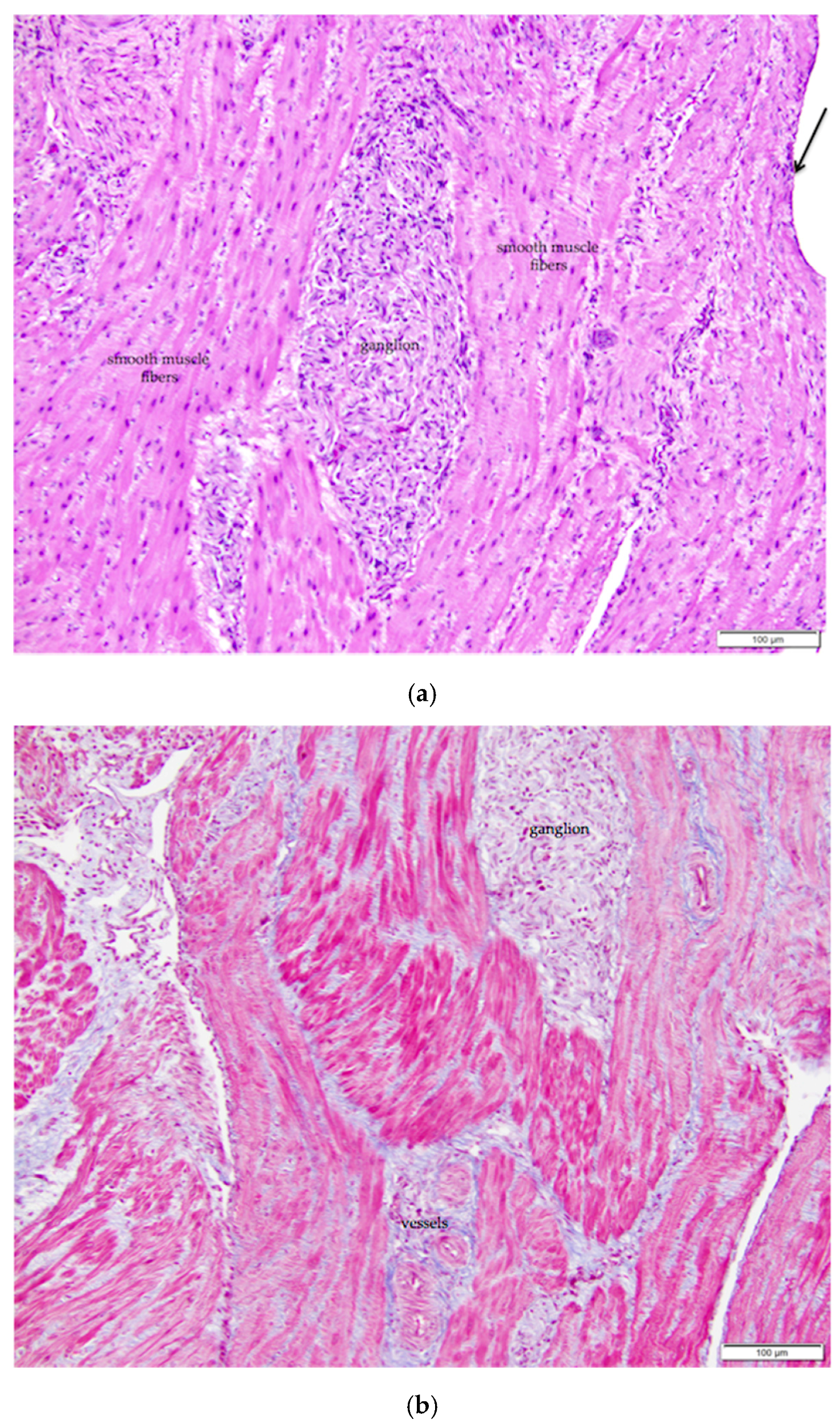
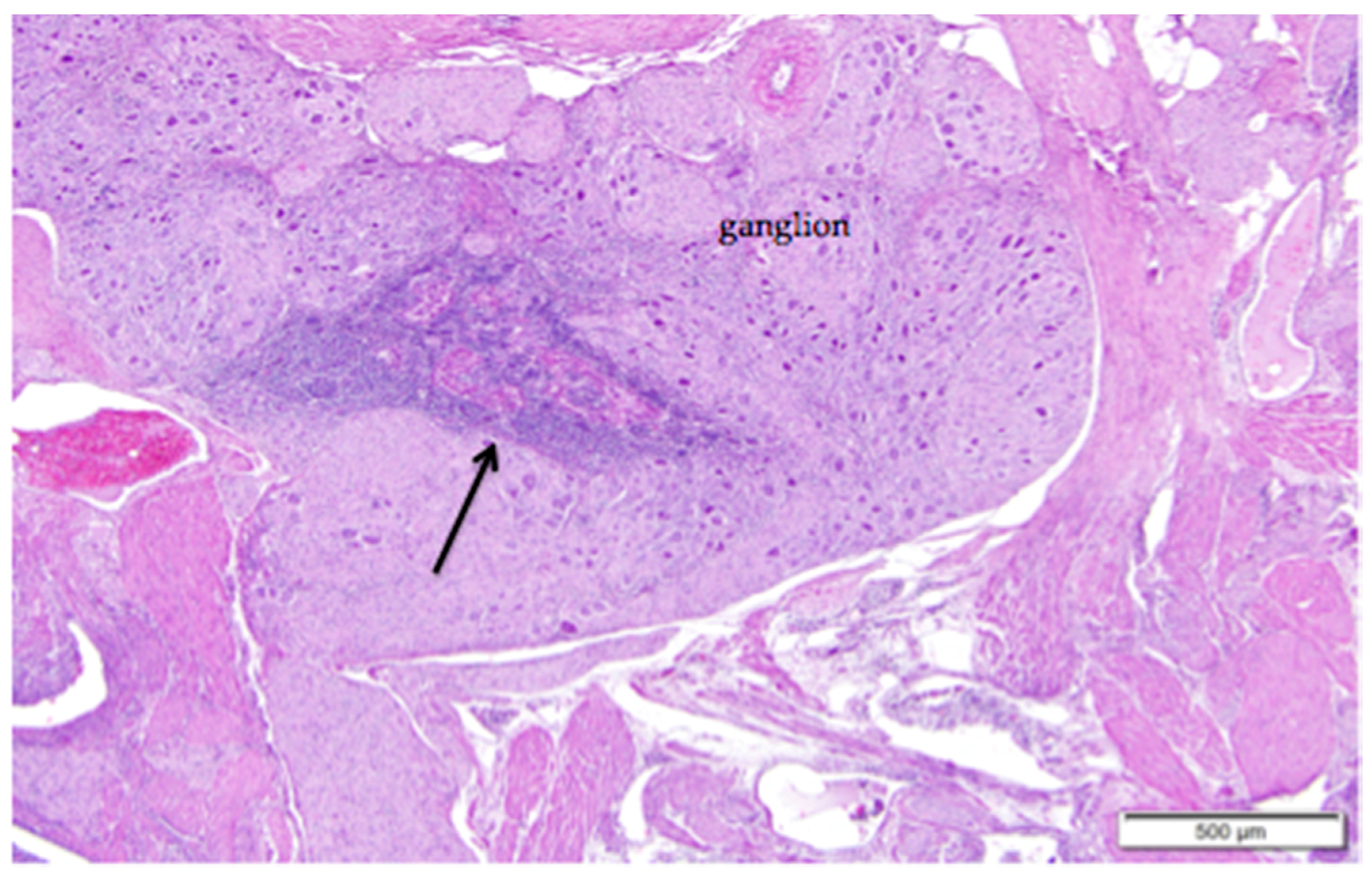
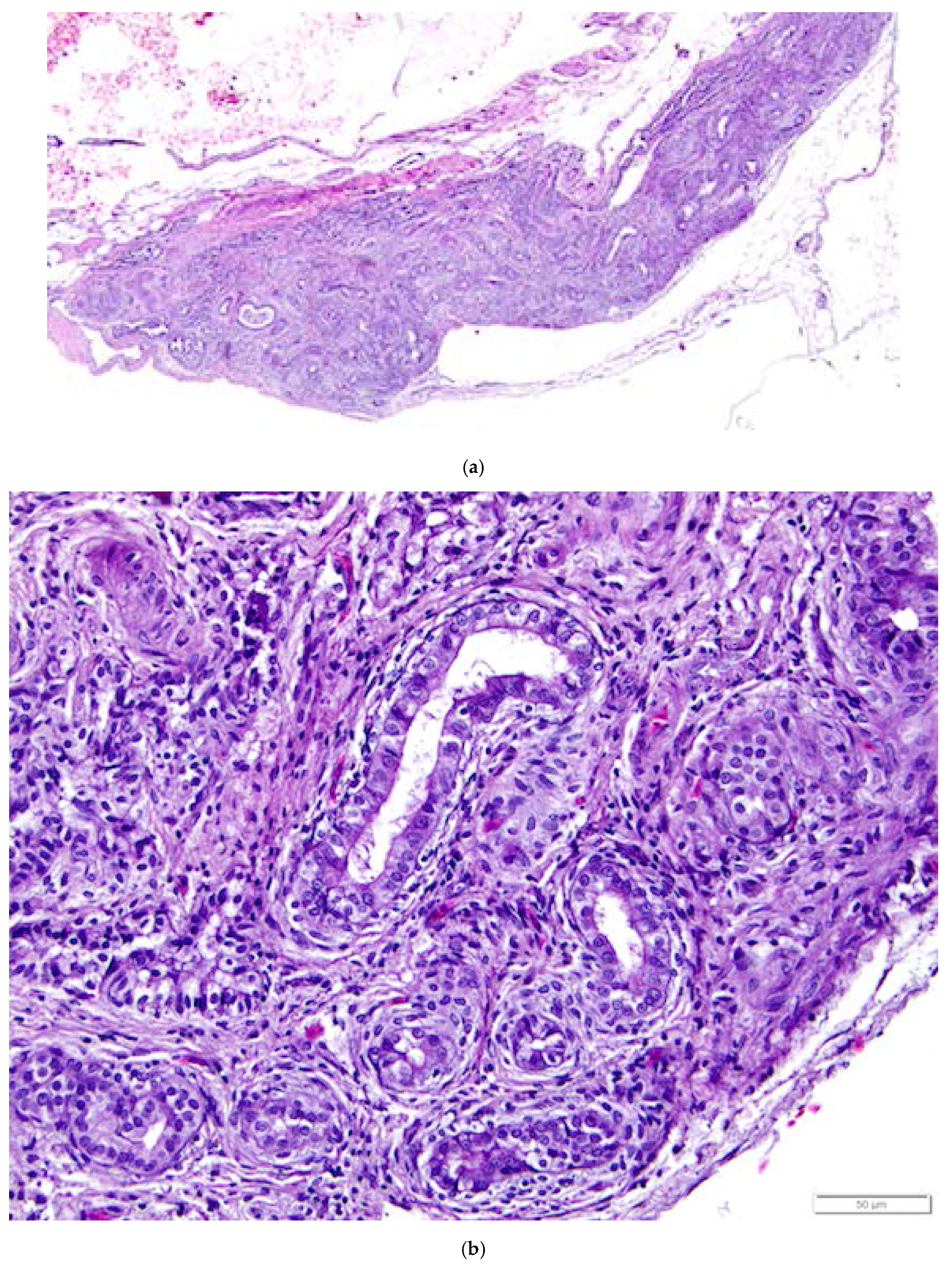
3.6. Rete Ovarii in the Laying Hen Ovary
3.7. Immunohistochemistry of the Laying Hen Ovary
4. Discussion
4.1. Ovarian Surface Epithelium
4.2. Oviductal Origin for Some EOC Tumors
4.3. Ovulation and the Follicular Hierarchy in Birds
4.4. Mesonephric Tissues in Laying Hen Ovaries
4.5. Immunohistochemical Response of Ovarian Tissues to Pancytokeratin and Vimentin
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- NIH National Cancer Institute. Cancer Stat Facts: Ovarian Cancer. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 19 July 2017).
- Tavassoli, F.A.; Devilee, P. Pathology and Genetics of Tumours of the Breast and Female Genital Organs; World Health Organization classification of tumours; IARC Press: Lyon, France, 2003; ISBN 92-832-2412-4. [Google Scholar]
- Vanderhyden, B.C.; Shaw, T.J.; Ethier, J.-F. Animal models of ovarian cancer. Reprod. Biol. Endocrinol. 2003, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, J.R.; Elkin, R.G.; Trevino, L.S.; Urick, M.E.; Ramachandran, R.; Johnson, P.A. The restricted ovulator chicken: A unique animal model for investigating the etiology of ovarian cancer. Int. J. Gynecol. Cancer 2010, 20, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.A.; Giles, J.R. The hen as a model of ovarian cancer. Nat. Rev. Cancer 2013, 13, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.A.; Stephens, C.S.; Giles, J.R. The domestic chicken: Causes and consequences of an egg a day. Poult. Sci. 2015, 94, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E.; Burdette, J.E.; Kenny, H.A.; Matei, D.; Pilrose, J.; Haluska, P.; Nephew, K.P.; Hales, D.B.; Stack, M.S. Epithelial ovarian cancer experimental models. Oncogene 2014, 33, 3619–3633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.H.; Yates, M.S.; Mok, S.C. The monkey, the hen, and the mouse: Models to advance ovarian cancer chemoprevention. Cancer Prev. Res. 2009, 2, 773–775. [Google Scholar] [CrossRef] [PubMed]
- De Melo Bernado, A.; Thorsteinsdottir, S.; Mummery, C. Advantages of the avian model for human ovarian cancer (review). Mol. Clin. Oncol. 2015, 3, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.S.; Beaugié, C.R.; Haviv, I.; Chenevix-Trench, G.; Tan, O.L. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: Revisting old hypotheses. Mol. Cell. Endocrinol. 2006, 247, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.A.; Barry, C.P.; Barnes, H.J.; Anderson, K.E.; Petitte, J.N.; Whitaker, R.; Lancaster, J.M.; Wenham, R.M.; Carver, D.K.; Turbov, J.; et al. Ovarian adenocarcinomas in the laying hen and women share similar alterations in p53, ras, and her-2/neu. Cancer Prev. Res. 2009, 2, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.A.; Fletcher, O.J.; Anderson, K.E.; Petitte, J.N.; Kopelovich, L.; Mozdziak, P.E. Epithelial cell tumors of the hen reproductive tract. Avian Dis. 2014, 58, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, T.N. Ovarian tumors of the hen. Environ. Health Perspect. 1987, 73, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Bitterman, P.; Abramowicz, J.S.; Dirks, A.L.; Bahr, J.M.; Hales, D.B.; Bradaric, M.J.; Edassery, S.L.; Rotmensch, J.; Luborsky, J.L. Histopathology of ovarian tumors in laying hens, a preclinical model of human ovarian cancer. Int. J. Gynecol. Cancer 2009, 19, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Hadley, J.A.; Hendricks, G.L.; Elkin, R.G.; Cooper, T.; Ramachandran, R. Characterization of ascites-derived ovarian tumor cells from spontaneously occuring ovarian tumors of the chicken: Evidence for e-cadherin upregulation. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Bosquet, J.; Peedicayil, A.; Maquire, J.; Chien, J.; Rodriguez, G.C.; Whitaker, R.; Petitte, J.N.; Anderson, K.E.; Barnes, H.J.; Shridhar, V.; et al. Comparison of gene expression patterns between avian and human ovarian cancers. Gynecol. Oncol. 2011, 120, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Auersperg, N.; Wong, A.S.T.; Choi, K.-C.; Kang, S.K.; Leung, P.C.K. Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr. Rev. 2001, 22, 255–288. [Google Scholar] [CrossRef] [PubMed]
- Fathalla, M.F. Incessant ovulation—A factor in ovarian neoplasia? Lancet 1971, 2. [Google Scholar] [CrossRef]
- Fathalla, M.F. Incessant ovulation and ovarian cancer—A hypothesis re-visted. Facts Views Vis. ObGyn 2013, 5, 292–297. [Google Scholar] [PubMed]
- Dubeau, L. The cell of origin of ovarian epithelial tumours. Lancet 2008, 9, 1191–1197. [Google Scholar] [CrossRef]
- Reade, C.J.; Ruaidhri, M.M.; Tone, A.A.; Finlayson, S.J.; McAlpine, J.N.; Fung-Kee-Fung, M.; Ferguson, S.E. The fallopian tube as the origin of high grade serous ovarian cancer: Review of a paradigm shift. J. Obstet. Gynaecol. Can. 2014, 36, 133–140. [Google Scholar] [CrossRef]
- Coles, B.H. Essentials of Avian Medicine and Surgery, 3rd ed.; Blackwell Publishing: Oxford, UK, 1997; ISBN 978-1-4051-5755-1. [Google Scholar]
- Barnes, H.J.; Fletcher, O.J.; Abdul-Aziz, T. Reproductive system. In Avian Histopathology, 3rd ed.; Fletcher, O.J., Abdul-Aziz, T., Eds.; American Association of Avian Pathologists: Jacksonville, FL, USA, 2008; pp. 348–391. ISBN 978-0-0789163-3-6. [Google Scholar]
- Gilbert, A.B. Female genital organs. In Form and Function in Birds; King, A.S., McLelland, J., Eds.; Academic Press: London, UK, 1979; Volume 1, pp. 237–360. ISBN 978-0124075016. [Google Scholar]
- Johnson, A.L.; Woods, D.C. Ovarian dynamics and follicle development. In Reproductive Biology and Phylogeny of Birds; Jamieson, B.G.M., Ed.; Science Publishers: Enfield, NH, USA, 2007; Volume 6A, pp. 243–277. [Google Scholar]
- Baumel, J.J.; King, A.S.; Breazile, J.E.; Evans, H.E.; Vanden Berge, J.C. Handbook of Avian Anatomy: Nomina Anatomica Avium (Publications of the Nuttall Ornithological Club), 2nd ed.; Nuttall Ornithological Club: Cambridge, MA, USA, 1993; ISBN 978-1877973345. [Google Scholar]
- Hodges, R.D. The Histology of the Fowl; Academic Press: London, UK, 1974; ISBN 978-0123513502. [Google Scholar]
- Giles, J.R.; Olson, L.M.; Johnson, P.A. Characterization of ovarian surface epithelial cells from the hen: A unique model for ovarian cancer. Exp. Biol. Med. 2006, 231, 1718–1725. [Google Scholar] [CrossRef]
- Woolnough, E.; Russo, L.; Khan, M.S.; Heatley, M.K. An immunohistochemical study of the rete ovarii and epoophoron. Pathology 2000, 32, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Czernobilsky, B.; Moll, R.; Levy, R.; Franke, W.W. Co-expression of cytokeratin and vimentin filaments in mesothelial, granulosa, and rete ovarii cells of the human ovary. Eur. J. Cell Biol. 1985, 37, 175–190. [Google Scholar] [PubMed]
- Van den Hurk, R.; Dijkstra, G.; Van Mil, F.N.; Hulshof, S.C.J.; Van den Ingh, T.S.G.A.M. Distribution of the intermediate filament proteins vimentin, keratin, and desmin in the bovine ovary. Mol. Reprod. Dev. 1995, 41, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Katabuchi, H.; Nitta, M.; Ohtake, H. Structural changes and cell properties of human ovarian surface epithelium in ovarian pathophysiology. Microsc. Res. Tech. 2006, 69, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Auersperg, N.; Edelson, M.I.; Mok, S.C.; Johnson, S.W.; Hamilton, T.C. The biology of ovarian cancer. Semin. Oncol. 1998, 25, 281–304. [Google Scholar] [PubMed]
- Whitaker, D.; Papadimitriou, J.M.; Walters, M.N.-I. The mesothelium and its reactions: A review. CRC Crit. Rev. Toxicol. 1982, 10, 81–144. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-M. Estrogen, progesterone and epithelial ovarian cancer. Reprod. Biol. Endocrinol. 2003, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, S.M.; Hilliard, T.S.; Wu, L.Y.; Jaffe, R.C.; Fazleabas, A.T.; Burdette, J.E. The impact of ovulation on fallopian tube epithelial cells: Evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr. Relat. Cancer 2011, 18, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.W.; Rengaraj, D.; Choi, J.W.; Park, K.J.; Song, G.; Han, J.Y. The expression profile of apoptosis-related genes in the chicken as a human epithelial ovarian cancer model. Oncol. Rep. 2011, 25, 49–56. [Google Scholar] [PubMed]
- Murdoch, W.J.; Van Kirk, E.A.; Alexander, B.M. DNA damages in ovarian surface epithelial cells of ovulatory hens. Exp. Biol. Med. 2005, 230, 429–433. [Google Scholar] [CrossRef]
- Rodler, D.; Sinowatz, F. Immunohistochemical and ultrastructural characterization of the ovarian surface epithelium of japanese quail (coturnix japonica). Anim. Sci. J. 2011, 82, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.; Anderson, K.; Ashwell, C.; Petitte, J.N.; Mozdziak, P.E. Ca125 expression in spontaneous ovarian adenocarcinomas from laying hens. Gynecol. Oncol. 2007, 104, 192–198. [Google Scholar] [CrossRef] [PubMed]
- O’Shannessy, D.J.; Jackson, S.M.; Twine, N.C.; Hoffmann, B.E.; Dezso, Z.; Agoulnik, S.I.; Somers, E.B. Gene expression analysis support fallopian tube epithelium as the cell of origin of epithelial ovarian cancer. Int. J. Mol. Sci. 2013, 14, 13687–13703. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.R.; Shivaprasad, H.L.; Johnson, P.A. Ovarian tumor expression of an oviductal protein in the hen: A model for human serous ovarian adenocarcinoma. Gynecol. Oncol. 2004, 95, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Trevino, L.S.; Giles, J.R.; Wang, W.; Urick, M.E.; Johnson, P.A. Gene expression profiling reveals differentially expressed genes in ovarian cancer of the hen: Support for oviductal origin? Horm. Cancer 2010, 1, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L. The avian ovarian hierarchy: A balance between follicle differentiation and atresia. Poult. Avian Biol. Rev. 1996, 7, 99–110. [Google Scholar]
- Johnson, A.L. Reproduction in the female. In Sturkie's Avian Physiology, 5th ed.; Whittow, G.C., Ed.; Academic Press: Cambridge, MA, USA, 2000; pp. 569–596. ISBN 978-0127476056. [Google Scholar]
- Johnson, A.L. Ovarian follicle selection and granulosa differentiation. Poult. Sci. 2015, 94, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.A. Follicle selection in the avian ovary. Reprod. Domest. Anim. 2012, 47, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Tilly, J.L.; Kowalski, K.I.; Johnson, A.L. Stage of ovarian follicular development associated with the initiation of steroidogenic competence in avian granulosa cells. Biol. Reprod. 1991, 44, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.R.; Saxena, V.K.; Sastry, K.V.H.; Nagarajan, K.; Jain, P.; Singh, R.; Anish, D.; Ravindra, P.V.; Saxena, M.; Ahmed, K.A. Cytokines and chemokines in postovulatory follicle regression of domestic chicken (gallus gallus domesticus). Dev. Comp. Immunol. 2008, 32, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L. Intracellular mechanisms regulating cell survival in ovarian follicles. Anim. Reprod. Sci. 2003, 78, 185–201. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Saxena, V.K.; Sastry, K.V.H.; Anish, D.; Marcus Leo, M.D.; Kantaraja, C.; Saxena, M.; Ahmed, K.A. Caspase-mediated apoptosis in chicken postovulatory follicle regression. Vet. Res. Commun. 2008, 32, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Yoshimura, Y.; Tamura, T. Localization of macrophages in the ovarian follicles during the follicular growth and postovulatory regression in chicken, gallus domesticus. Poult. Sci. 1998, 77, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Genovese, K.J.; He, H.; Swaggerty, C.L.; Kogut, J.H. The avian heterophil. Dev. Comp. Immunol. 2013, 41, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B.G. Avian heterophils in inflammation and disease resistance. Poult. Sci. 1998, 77, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Lowry, V.K.; Moyes, R.B.; Bowden, L.L.; Bowden, R.; Genovese, K.; Deloach, J.R. Lymphokine-augmented activation of avian heterophils. Poult. Sci. 1998, 77, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.A.; Ferrandina, G.; Gordinier, M.; Loercher, A.; Freedman, R.S. The role of cytokines in both the normal and malignant ovary. Endocr. Relat. Cancer 1999, 6, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.G.; Odend’hal, S. The mammalian rete ovarii: A literature review. Cornell Vet. 1985, 75, 411–425. [Google Scholar] [PubMed]
- Chambers, J.K.; Uchida, K.; Ise, K.; Nakayama, H. Cystic rete ovarii and uterine tube adenoma in a rabbit. J. Vet. Med. Sci. 2014, 76, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Gelberg, H.B.; McEntee, K.; Heath, E.H. Feline cystic rete ovarii. Vet. Pathol. 1984, 21, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Heatley, M.K. Adenomatous hyperplasia of the rete ovarii. Histopathology 2000, 36, 383–384. [Google Scholar] [CrossRef] [PubMed]
- McEntee, K. Reproductive Pathology of Domestic Mammals; Academic Press: Cambridge, MA, USA, 1990; ISBN 978-0124833753. [Google Scholar]
- Moerman, P.; Amant, F.; Vergote, I. Mesonephric (wolffian) pseudoendometrioid carcinoma of the broad ligament, arising from a papillary cystadenoma. Int. J. Surg. Pathol. 2016, 24, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.; Abdulla, A.; Razvi, K.; Pandeva, I.; Al-Nafussi, A. Cystadenofibroma of the rete ovarii: A case report with review of the literature. Rare Tumors 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Rutgers, J.L.; Scully, R.E. Cysts (cystadenomas) and tumors of the rete ovarii. Int. J. Gynecol. Pathol. 1988, 7, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Byskov, A.G. The role of the rete ovarii in meiosis and follicle formation in the cat, mink and ferret. J. Reprod. Fertil. 1975, 45, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Byskov, A.G.; Skakkebaek, N.E.; Stafanger, G.; Peters, H. Influence of ovarian surface epithelium and rete ovarii on follicle formation. J. Anat. 1977, 123, 77–86. [Google Scholar] [PubMed]


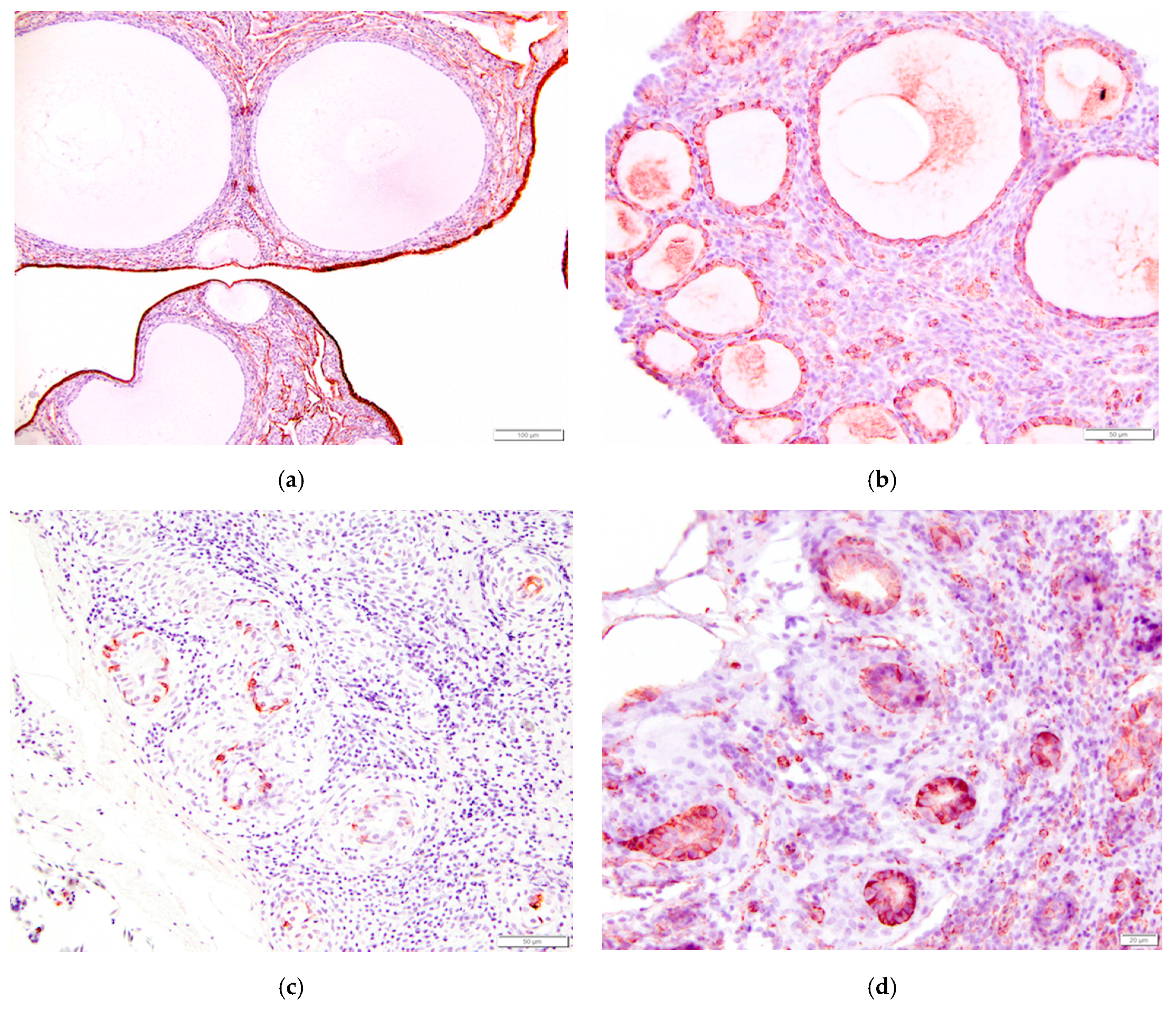
| Tissue | Pancytokeratin | Vimentin |
|---|---|---|
| ovarian surface epithelium | + laying hen (this study), [28] + human [29,30] + pig, rat, mouse [30] + mouse, fetal cow, adult cow [31] | − laying hen (this study), [28] − human [29] + human (periphery of the cells) [30] − pig [30] − rat, pig, fetal cow, adult cow [31] |
| granulosa cells | − laying hen (this study) + human [30] − pig, rat [30] + mouse, fetal cow [31] − adult cow [31] | + laying hen (this study), [28] + human, particularly mature cells [30] + pig, rat, mouse [30] + fetal cow, adult cow [31] |
| rete ovarii epithelium | − laying hen (this study) + human [29,30] + fetal cow [31] − adult cow [31] | + laying hen (this study) + human [29,30] − human rete testis [30] + rat, pig, fetal cow, adult cow [31] |
| epithelium lining tubules of epoöphoron | + laying hen (this study) + human [29] | + laying hen (this study) + human [29] |
| oviduct epithelium | + laying hen (this study) + human [29] | + human [29] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apperson, K.D.; Bird, K.E.; Cherian, G.; Löhr, C.V. Histology of the Ovary of the Laying Hen (Gallus domesticus). Vet. Sci. 2017, 4, 66. https://doi.org/10.3390/vetsci4040066
Apperson KD, Bird KE, Cherian G, Löhr CV. Histology of the Ovary of the Laying Hen (Gallus domesticus). Veterinary Sciences. 2017; 4(4):66. https://doi.org/10.3390/vetsci4040066
Chicago/Turabian StyleApperson, K. Denise, Karyn E. Bird, Gita Cherian, and Christiane V. Löhr. 2017. "Histology of the Ovary of the Laying Hen (Gallus domesticus)" Veterinary Sciences 4, no. 4: 66. https://doi.org/10.3390/vetsci4040066






