Tongue Squamous Cell Carcinoma in a European Lynx (Lynx Lynx): Papillomavirus Infection and Histologic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Information and Histologic Analysis
2.2. Transmission Electron Microscope (TEM) Analysis
2.3. Polymerase Chain Reaction (PCR) for Detection of PV DNA
3. Results
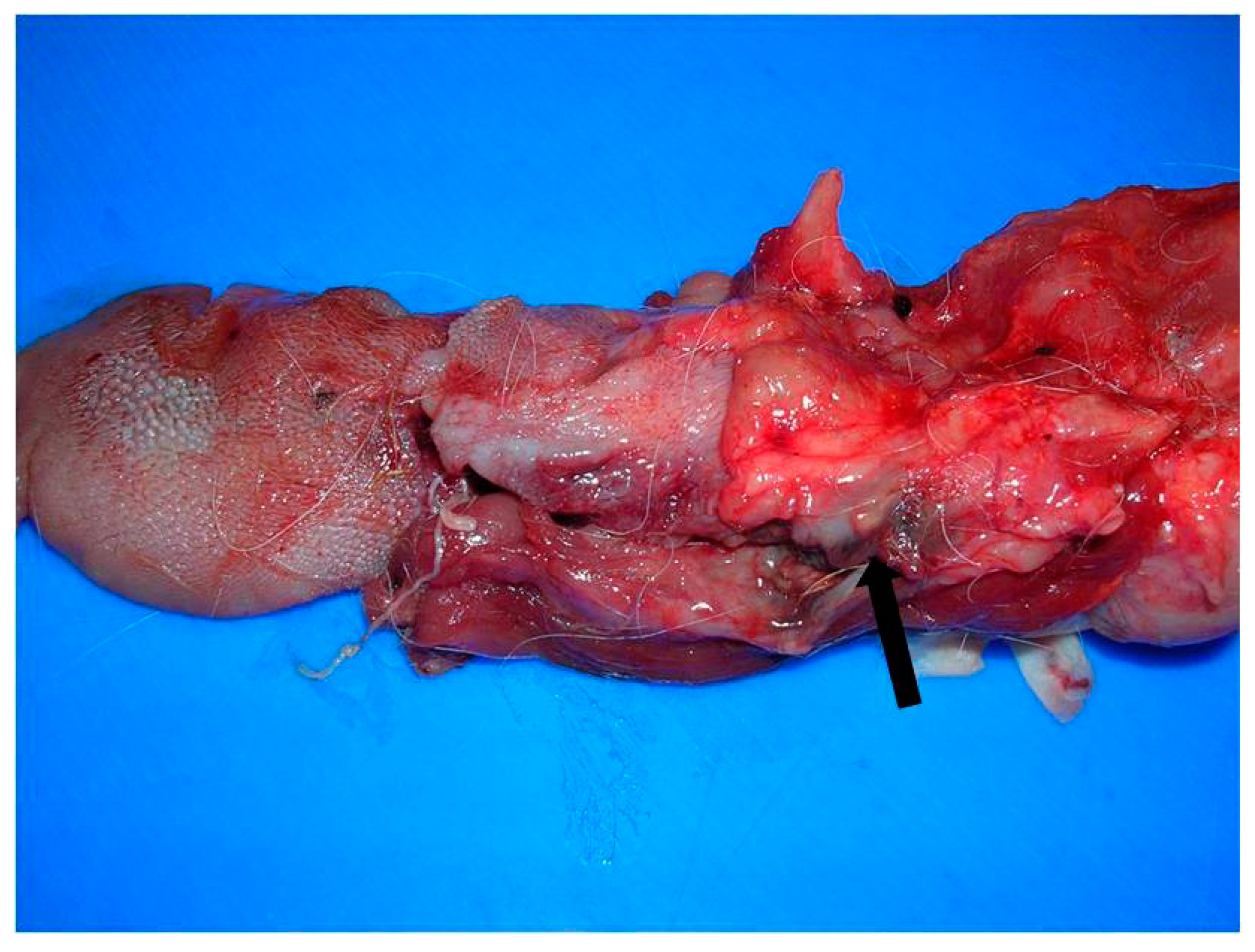
3.1. Gross Pathological Findings
3.2. Histological Findings
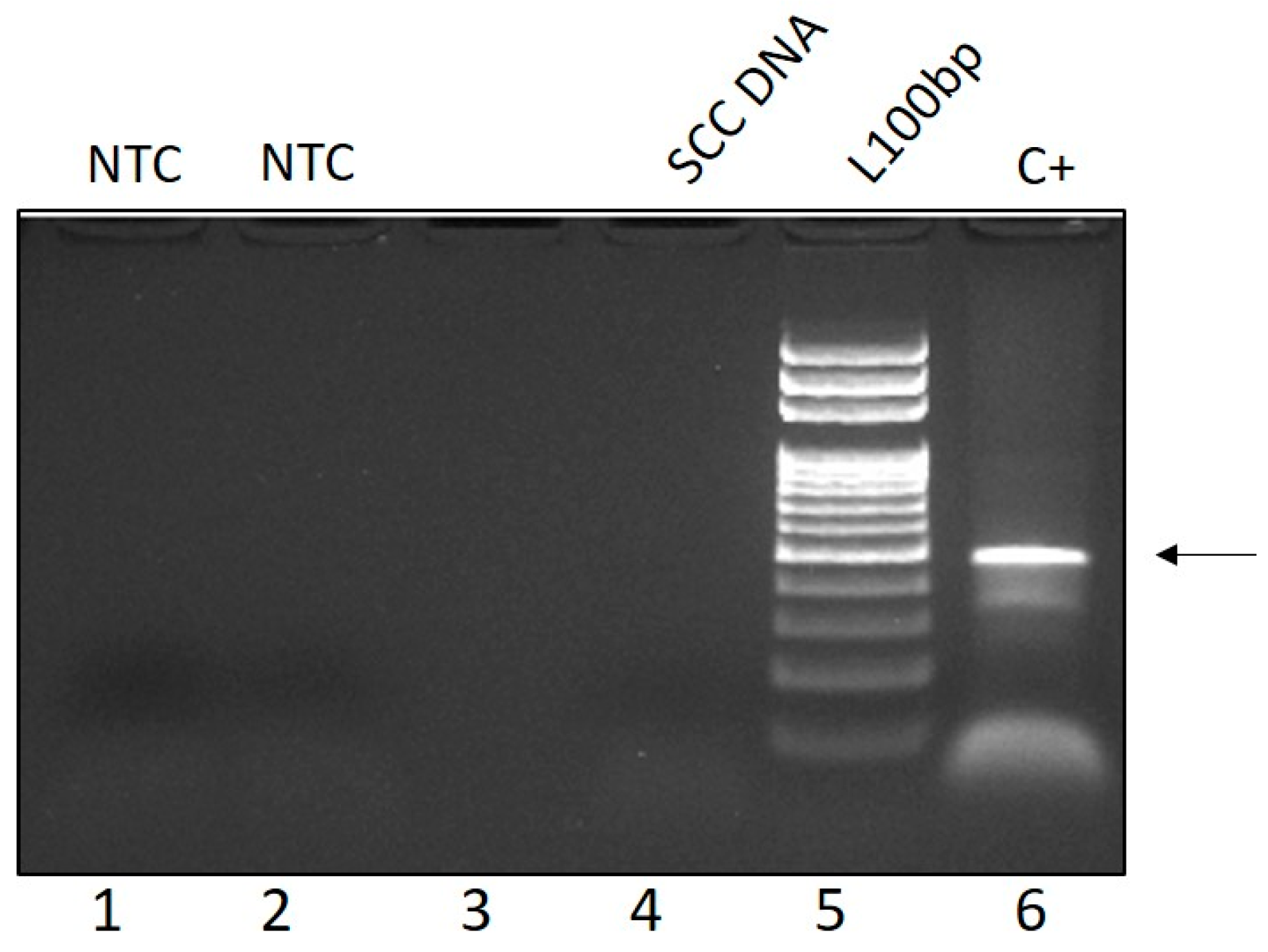
3.3. Molecular and Ultrastructural Viral Investigations
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gunson, D.E.; Klein, L.V.; Reid, C.F. Gingival squamous cell carcinoma in a Canadian lynx. J. Am. Vet. Med. Assoc. 1978, 173, 1228–1230. [Google Scholar] [PubMed]
- Sladakovic, I.; Burnum, A.; Blas-Machado, U.; Kelly, L.S.; Garner, B.C.; Holmes, S.P.; Divers, S.J. Mandibular Squamous Cell Carcinoma in a Bobcat (Lynx Rufus). J. Zoo Wildl. Med. 2016, 47, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Supsavhad, W.; Dirksen, W.P.; Martin, C.K.; Rosol, T.J. Animal models of head and neck squamous cell carcinoma. Vet. J. 2016, 210, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Altamura, G.; Corteggio, A.; Pacini, L.; Conte, A.; Pierantoni, G.M.; Tommasino, M.; Accardi, R.; Borzacchiello, G. Transforming properties of Feliscatus papillomavirus type 2 E6 and E7 putative oncogenes in vitro and their transcriptional activity in feline squamous cell carcinoma in vivo. Virology 2016, 496, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Thomson, N.A.; Luff, J.A. Papillomaviruses in dogs and cats. Vet. J. 2017, 225, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; French, A.F. Felis catus papillomavirus types 1 and 4 are rarely present in neoplastic and inflammatory oral lesions of cats. Res. Vet. Sci. 2015, 100, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Rector, A.; Lemey, P.; Tachezy, R.; Mostmans, S.; Ghim, S.J.; Van Doorslaer, K.; Roelke, M.; Bush, M.; Montali, R.J.; Joslin, J.; et al. Ancient papillomavirus-host co-speciation in Felidae. Genome Biol. 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, L.L.; Spraker, T.R. Oral papillomatosis in Canada lynx (Lynx canadensis). J. Wildl. Dis. 2007, 43, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Biel, S.S.; Gelderblom, H.R. Electron microscopy of viruses. In Virus Culture—A practical Approach; Cann, A.J., Ed.; Oxford University Press: New York, NY, USA, 1999; pp. 111–147. [Google Scholar]
- Forslund, O.; Antonsson, A.; Nordin, P.; Stenquist, B.; Hansson, B.G. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 1999, 80, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Resnick, R.M.; Cornelissen, M.T.E.; Wright, D.K.; Eichinger, G.H.; Fox, H.S.; ter Schegget, J.; Manos, M.M. Detection and Typing of Human Papillomavirus in Archival Cervical-Cancer Specimens by DNA Amplification with Consensus Primers. J. Natl. Cancer Inst. 1990, 82, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Bocaneti, F.; Altamura, G.; Corteggio, A.; Martano, M.; Roperto, F.; Velescu, E.; Borzacchiello, G. Expression of platelet derived growth factor beta receptor, its activation and downstream signals in bovine cutaneous fibropapillomas. Res. Vet. Sci. 2013, 94, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Eleni, C.; Corteggio, A.; Altamura, G.; Meoli, R.; Cocumelli, C.; Rossi, G.; Friedrich, K.G.; Di Cerbo, P.; Borzacchiello, G. Detection of papillomavirus DNA in cutaneous squamous cell carcinoma and multiple papillomas in captive reptiles. J. Comp. Pathol. 2017, 157, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, M.H.; Goldschmidt, K.H. Epithelial and Melanocytic Tumors of the Skin. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons: Ames, IA, USA, 2017; pp. 97–99. ISBN 978-0-8138-2179-5. [Google Scholar]
- Altamura, G.; Corteggio, A.; Borzacchiello, G. Feliscatus papillomavirus type 2 E6 oncogene enhances mitogen-activated protein kinases and Akt activation but not EGFR expression in an in vitro feline model of viral pathogenesis. Vet. Microbiol. 2016, 195, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Mallen-St Clair, J.; Alani, M.; Wang, M.B.; Srivatsan, E.S. Human papillomavirus in oropharyngeal cancer: The changing face of a disease. Biochim. Biophys. Acta 2016, 1866, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Dunowska, M.; Laurie, R.E.; Hills, S. Genomic characterisation of canine papillomavirus type 17, a possible rare cause of canine oral squamous cell carcinoma. Vet. Microbiol. 2016, 182, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Howley, P.M.; Pfister, H.J. Beta genus papillomaviruses and skin cancer. Virology 2015, 479–480, 290–296. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamura, G.; Eleni, C.; Meoli, R.; Cardeti, G.; Friedrich, K.G.; Borzacchiello, G. Tongue Squamous Cell Carcinoma in a European Lynx (Lynx Lynx): Papillomavirus Infection and Histologic Analysis. Vet. Sci. 2018, 5, 1. https://doi.org/10.3390/vetsci5010001
Altamura G, Eleni C, Meoli R, Cardeti G, Friedrich KG, Borzacchiello G. Tongue Squamous Cell Carcinoma in a European Lynx (Lynx Lynx): Papillomavirus Infection and Histologic Analysis. Veterinary Sciences. 2018; 5(1):1. https://doi.org/10.3390/vetsci5010001
Chicago/Turabian StyleAltamura, Gennaro, Claudia Eleni, Roberta Meoli, Giusy Cardeti, Klaus Günther Friedrich, and Giuseppe Borzacchiello. 2018. "Tongue Squamous Cell Carcinoma in a European Lynx (Lynx Lynx): Papillomavirus Infection and Histologic Analysis" Veterinary Sciences 5, no. 1: 1. https://doi.org/10.3390/vetsci5010001




