In Vitro Activity of 30 Essential Oils against Bovine Clinical Isolates of Prototheca zopfii and Prototheca blaschkeae
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Roesler, U.; Möller, A.; Hensel, A.; Baumann, D.; Truyen, U. Diversity within the current algal species Prototheca zopfii: A proposal for two Prototheca zopfii genotypes and description of a novel species, Prototheca blaschkeae sp. nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Ooe, K.; Nagayama, H.; Makimura, K. Prototheca cutis sp. nov., a newly discovered pathogen of protothecosis isolated from inflamed human skin. Int. J. Syst. Evol. Microbiol. 2010, 60, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.; Huss, V.A.R.; Pfisterer, K.; Grosse, C.; Thompson, G. Internal transcribed spacer sequence-based rapid molecular identification of Prototheca zopfii and Prototheca blaschkeae directly from milk of infected cows. J. Dairy Sci. 2015, 98, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Hirose, N.; Ishikawa, T.; Ikawa, Y.; Nishimura, K. Prototheca miyajii sp. nov., isolated from a patient with systemic protothecosis. Int. J. Syst. Evol. Microbiol. 2016, 66, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Roesler, U.; Hensel, A. Longitudinal analysis of Prototheca zopfii-specific immune responses: Correlation with disease progression and carriage in dairy cows. J. Clin. Microbiol. 2003, 41, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.L.; Lee, S.H.I.; de Paula Arruda, E.; Galles, D.P.; Caetano, V.C.; de Oliveira, C.A.F.; Fernandes, A.M.; dos Santos, M.V. Biofilm-producing ability and efficiency of sanitizing agents against Prototheca zopfii isolates from bovine subclinical mastitis. J. Dairy Sci. 2015, 98, 3613–3621. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J. Biofilm formation by pathogenic Prototheca algae. Lett. Appl. Microbiol. 2015, 61, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.; Capra, E.; Morandi, S.; Cremonesi, P.; Pantoja, J.C.F.; Langoni, H.; Vargas, A.P.C.; Costa, M.M.; Jagielski, T.; Bolaños, C.A.D.; et al. In vitro algicidal effect of guanidine on Prototheca zopfii genotype 2 strains isolated from clinical and subclinical bovine mastitis. Lett. Appl. Microbiol. 2017, 64, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Jánosi, S.; Ratz, F.; Szigeti, G.; Kulcsar, M.; Kerenyi, J.; Lauko, T.; Katona, F.; Huszenicza, G. Pathophysiology: Review of the microbiological, pathological, and clinical aspects of bovine mastitis caused by the alga Prototheca zopfii. Vet. Q. 2001, 23, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.; Silva, E.; Kraft, C.; Carvalheira, J.; Videira, A.; Huss, V.A.; Thompson, G. Bovine mastitis associated with Prototheca blaschkeae. J. Clin. Microbiol. 2008, 46, 1941–1945. [Google Scholar] [CrossRef] [PubMed]
- Ahrholdt, J.; Murugaiyan, J.; Straubinger, R.K.; Jagielski, T.; Roesler, U. Epidemiological analysis of worldwide bovine, canine and human clinical Prototheca isolates by PCR genotyping and MALDI-TOF mass spectrometry proteomic phenotyping. Med. Mycol. 2012, 50, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Mayr, A. Human protothecosis. Clin. Microbiol. Rev. 2007, 20, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Hoshi, S.; Nagai, K.; Ishidaira, H.; Onozaki, M.; Satoh, K.; Makimura, K. The first case of human protothecosis caused by Prototheca zopfii in Japan. J. Inf. Chemother. 2014, 20, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Turchetti, B.; Facelli, R.; Baudino, R.; Cavarero, F.; Mattalia, L.; Mosso, P.; Martini, A. First large-scale isolation of Prototheca zopfii from milk produced by dairy herds in Italy. Mycopathologia 2004, 158, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Tortorano, A.M.; Prigitano, A.; Dho, G.; Piccinini, R.; Dapra, V.; Viviani, M.A. In vitro activity of conventional antifungal drugs and natural essences against the yeast-like alga Prototheca. J. Antimicrob. Chemother. 2008, 61, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Buzzini, P.; Lassa, H.; Malinowski, E.; Branda, E.; Turchetti, B.; Polleichtner, A.; Lagneau, P.E.; Silva, E.; Marques, S.; et al. Multicentre Etest evaluation of in vitro activity of conventional antifungal drugs against European bovine mastitis Prototheca spp. isolates. J. Antimicrob. Chemother. 2012, 67, 1945–1947. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Pieroni, A. Antimicrobial activity of extracts of Clematis vitalba towards pathogenic yeast and yeast-like microorganisms. Fitoterapia 2003, 74, 397–400. [Google Scholar] [CrossRef]
- Turchetti, B.; Pinelli, P.; Buzzini, P.; Romani, A.; Heimler, D.; Franconi, F.; Martini, A. In vitro antimycotic activity of some plant extracts towards yeast and yeast-like strains. Phytother. Res. 2005, 191, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Vergis, J.; Gokulakrishnan, P.; Agarwal, R.K.; Kumar, A. Essential oils as natural food antimicrobial agents: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Artemisia sieberi Besser essential oil and treatment of fungal infections. Biomed. Pharmacother. 2017, 89, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Timbermont, L.; Lanckriet, A.; Dewulf, J.; Nollet, N.; Schwarzer, K.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 2010, 39, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, F.R.; Schmidt, G.; Romero, A.L.; Sartoretto, J.L.; Caparroz-Assef, S.M.; Bersani-Amado, C.A.; Cuman, R.K.N. Immunomodulatory activity of Zingiber officinale Roscoe, Salvia officinalis L. and Syzygium aromaticum L. essential oils: Evidence for humor-and cell-mediated responses. J. Pharm. Pharmacol. 2009, 61, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Chaabouni, Y.; Mahdouani, K.; Bakhrouf, A.; Chaieb, K. Use of carvacrol, thymol, and eugenol for biofilm eradication and resistance modifying susceptibility of Salmonella enterica serovar Typhimurium strains to nalidixic acid. Microb. Pathog. 2017, 104, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, L.R.; Rosalen, P.L.; Ferreira, G.L.S.; Freires, I.A.; de Carvalho, F.G.; Castellano, L.R.; de Castro, R.D. Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candida spp. Arch. Oral Biol. 2017, 73, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, B.; Głowacka, A.; Krukowski, H.; Lisowski, A.; Lassa, H.; Sienkiewicz, M. The in vitro efficacy of essential oils and antifungal drugs against Prototheca zopfii. Mycopathologia 2016, 181, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Bouari, C.; Bolfa, P.; Borza, G.; Nadăş, G.; Cătoi, C.; Fiţ, N. Antimicrobial activity of Mentha piperita and Saturenja hortensis in a murine model of cutaneous protothecosis. J. Mycol. Méd. 2014, 24, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Najar, B.; Giovanelli, S.; Lorenzini, L.; Tavarini, S.; Angelini, L.G. Agronomic and phytochemical evaluation of lavandin and lavender cultivars ciultivated in the Tyrrhenian area of Tuscany (Italy). Ind. Crops Prod. 2017, 109, 37–44. [Google Scholar] [CrossRef]
- Ricchi, M.; Cammi, G.; Garbarino, C.A.; Buzzini, P.; Belletti, G.L.; Arrigoni, N. A rapid real-time PCR/DNA resolution melting method to identify Prototheca species. J. Appl. Microbiol. 2011, 110, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2008. [Google Scholar]
- Pisseri, F.; Scuola Cimi Koinè Roma, Italy. Personal communication, 2017.
- Sobukawa, H.; Kano, R.; Ito, T.; Onozaki, M.; Makimura, K.; Hasegawa, A.; Kamata, H. In vitro susceptibility of Prototheca zopfii genotypes 1 and 2. Med. Mycol. 2011, 49, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.; De Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [PubMed]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Marei, G.I.K.; Rasoul, M.A.A.; Abdelgaleil, S.A. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pest Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Brennan, T.C.; Turner, C.D.; Krömer, J.O.; Nielsen, L.K. Alleviating monoterpene toxicity using a two-phase extractive fermentation for the bioproduction of jet fuel mixtures in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2012, 109, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Lassa, H.; Jagielski, T.; Malinowski, E. Effect of different heat treatments and disinfectants on the survival of Prototheca zopfii. Mycopathologia 2011, 171, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Bakuła, Z.; Di Mauro, S.; Casciari, C.; Cambiotti, V.; Krukowski, H.; Turchetti, B.; Ricchi, M.; Manuali, E.; Buzzini, P. A comparative study of the in vitro activity of iodopropynyl butylcarbamate and amphotericin B against Prototheca spp. isolates from European dairy herds. J. Dairy Sci. 2017, 100, 7435–7445. [Google Scholar] [CrossRef] [PubMed]
- Ricchi, M.; De Cicco, C.; Buzzini, P.; Cammi, G.; Arrigoni, N.; Cammi, M.; Garbarino, C. First outbreak of bovine mastitis caused by Prototheca blaschkeae. Vet. Microbiol. 2013, 162, 997–999. [Google Scholar] [CrossRef] [PubMed]
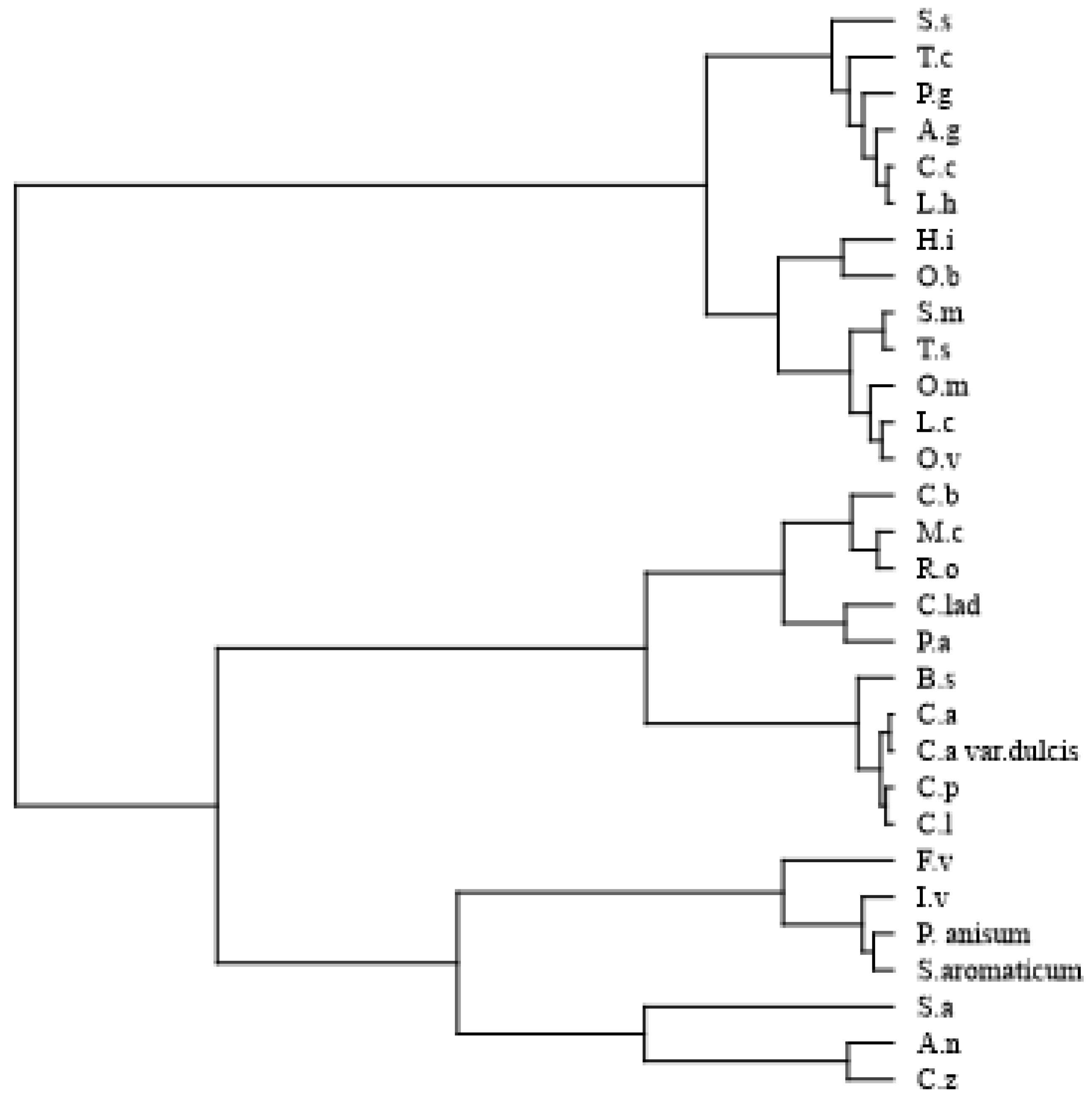
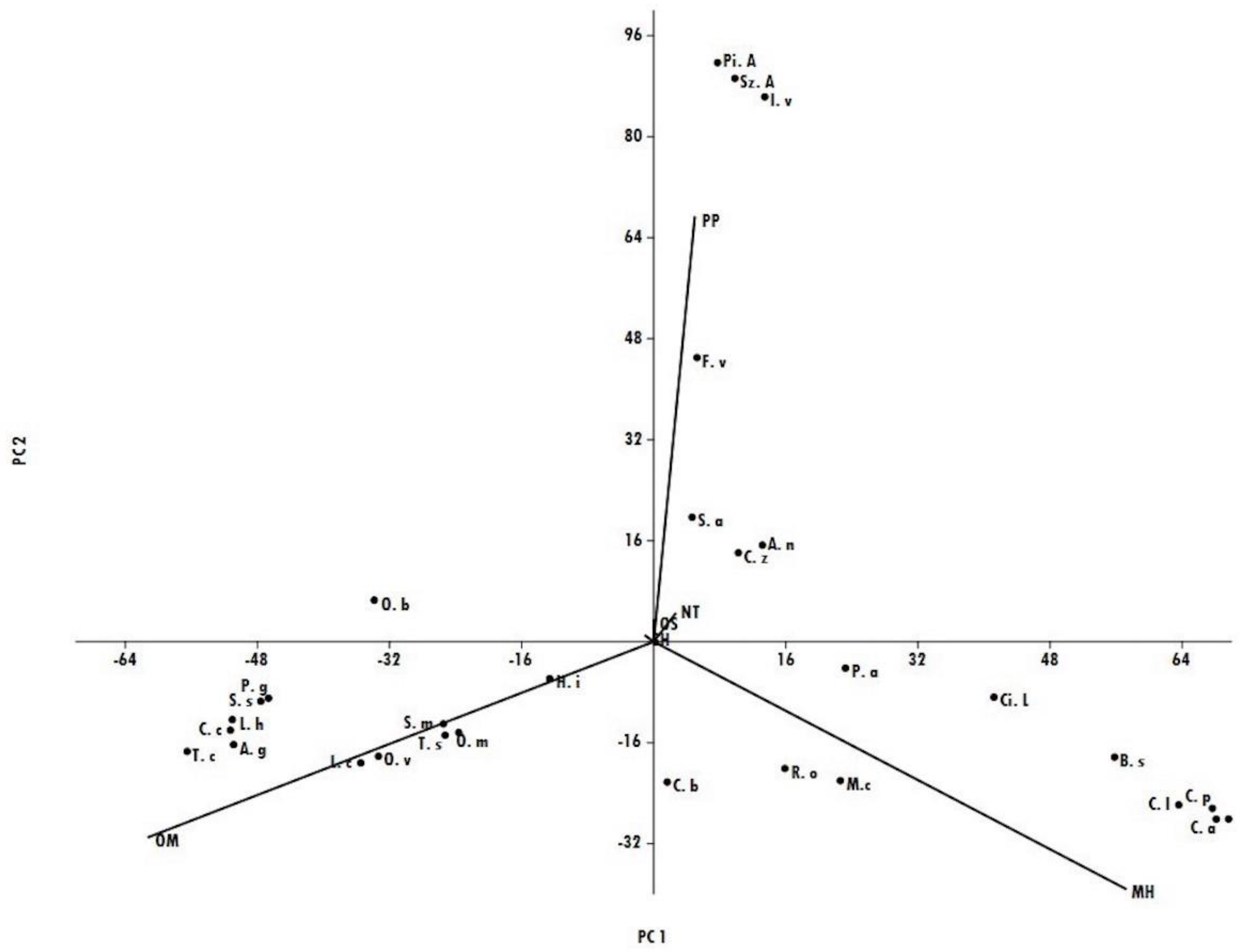
| Compounds * | Class | LRI § | A.n | A.g | B.s | C.z | C.a | C.b | C.l | C.p | C.a.dul | C.la | C.c | F.v | H.i | I.v |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Propyl butanoate | EST | 898 | 5.5 | |||||||||||||
| α-Thujene | MH | 932 | 54.2 | 0.3 | 0.3 | 0.4 | 0.1 | 7.2 | ||||||||
| Tricyclene | MH | 938 | 0.2 | 1.4 | 0.4 | 1.1 | 1.9 | 0.6 | 0.2 | 8.6 | 1.4 | |||||
| α-Pinene | MH | 940 | 1.2 | 0.3 | 6.2 | 0.5 | 45.0 | |||||||||
| α-Fenchene | MH | 951 | 1.2 | |||||||||||||
| Camphene | MH | 955 | 0.8 | 0.8 | 0.6 | 1.0 | 1.1 | 0.3 | ||||||||
| Thuja-2.4(10)-diene | MH | 959 | 7.3 | |||||||||||||
| β-Pinene | MH | 981 | 0.2 | 1.1 | 0.5 | 0.1 | 5.4 | 11.9 | 0.1 | 0.6 | 1.0 | 1.0 | ||||
| α-Terpinene | MH | 1019 | 5.1 | 1.0 | 0.2 | 0.1 | 0.1 | 0.2 | ||||||||
| p-Cymene | MH | 1028 | 3.2 | 3.0 | 0.1 | 0.2 | 6.1 | 1.9 | 1.1 | 0.1 | ||||||
| Limonene | MH | 1032 | 0.7 | 3.2 | 0.4 | 94.7 | 33.2 | 65.7 | 92.2 | 95.5 | 1.8 | 2.0 | 6.5 | 7.0 | 3.9 | |
| β-Phellandrene | MH | 1033 | 5.9 | |||||||||||||
| 1.8-Cineole | OM | 1036 | 0.3 | 2.3 | ||||||||||||
| Isobutyl angelate | EST | 1053 | 34.5 | |||||||||||||
| γ-Terpinene | MH | 1062 | 0.1 | 6.4 | 9.3 | 0.3 | 0.3 | 0.1 | ||||||||
| Artemisia ketone | MH | 1065 | 7.4 | |||||||||||||
| Fenchone | OM | 1090 | 20.1 | |||||||||||||
| trans-Sabinene hydrate | OM | 1101 | 0.2 | |||||||||||||
| Linalool | OM | 1102 | 0.2 | 6.3 | 0.4 | 14.2 | 0.2 | 0.5 | 0.5 | 1.5 | 0.4 | 0.8 | 0.2 | |||
| Camphor | OM | 1148 | 0.5 | |||||||||||||
| Isoamyl angelate | EST | 1162 | 18.7 | |||||||||||||
| Propyl tiglate | EST | 1166 | 5.3 | |||||||||||||
| 4-Terpineol | OM | 1180 | 0.3 | 0.2 | 0.3 | |||||||||||
| 3.9-Epoxy-p-menth-1-ene | OM | 1186 | 12.0 | |||||||||||||
| Menthyl chavicol | PP | 1198 | 8.6 | 0.3 | ||||||||||||
| Citronellol | OM | 1231 | ||||||||||||||
| Neral | OM | 1242 | 0.1 | 0.4 | 0.7 | 35.2 | ||||||||||
| Carvone | OM | 1248 | 70.4 | |||||||||||||
| Geraniol | OM | 1259 | 4.4 | |||||||||||||
| Linalyl acetate | OM | 1260 | 0.5 | 1.4 | 31.7 | |||||||||||
| (E)-Cinnamaldehyde | NT | 1274 | 56.4 | |||||||||||||
| Geranial | OM | 1276 | 0.4 | 1.2 | 0.1 | 38.4 | ||||||||||
| Citronellyl formate | OM | 1280 | ||||||||||||||
| iso-Bornyl acetate | OM | 1287 | ||||||||||||||
| (E)-Anethol | PP | 1290 | 0.3 | 46.9 | 89.8 | |||||||||||
| Carvacrol | OM | 1301 | ||||||||||||||
| Thymol | OM | 1307 | 0.3 | |||||||||||||
| α-Limonene diepoxide | OM | 1347 | ||||||||||||||
| Eugenol | PP | 1361 | 3.0 | |||||||||||||
| Neryl acetate | OM | 1368 | 0.8 | 0.7 | 31.8 | |||||||||||
| β-Caryophyllene | SH | 1418 | 10.3 | 0.2 | 0.7 | 0.4 | 0.7 | 0.2 | 2.3 | 3.1 | 0.3 | |||||
| Neryl propanoate | OM | 1454 | 5.1 | |||||||||||||
| ar-Curcumene | SH | 1484 | 5.6 | |||||||||||||
| Eugenol acetate | PP | 1529 | ||||||||||||||
| 5-epi-7-epi-α-Eudesmol | OS | 1606 | 5.5 | |||||||||||||
| γ-Eudesmol | OS | 1634 | 0.5 | |||||||||||||
| T-Cadinol | OS | 1642 | ||||||||||||||
| β-Eudesmol | OS | 1649 | 0.2 | |||||||||||||
| Valerianol | OS | 1655 | 0.3 | |||||||||||||
| 7-epi-α-Eudesmol | OS | 1664 | ||||||||||||||
| (Z)-α-Santalol | OS | 1675 | ||||||||||||||
| (Z)-β-trans-Santalol | OS | 1710 | ||||||||||||||
| Unknown | 7.9 | 2.8 | 5.1 | 0.3 | 0.8 | 6.2 | 2.4 | 0.3 | 5.8 | |||||||
| TOTAL | 92.1 | 97.2 | 94.9 | 99.7 | 100.0 | 100.0 | 100.0 | 99.2 | 100.0 | 93.9 | 97.6 | 99.7 | 94.3 | 100.0 |
| Compounds * | Class | LRI § | L.h | L.c | M.c | O.b | O.m | O.v | P.g | P.ab | P.a | R.o | S.s | S.al | S.m | S.a | T.c | T.v |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Propyl butanoate | EST | 898 | ||||||||||||||||
| α-Thujene | MH | 932 | 0.6 | 0.8 | 0.8 | 0.5 | 0.2 | 0.3 | 0.1 | |||||||||
| Tricyclene | MH | 938 | 0.5 | 1.5 | 49.0 | 0.2 | ||||||||||||
| α-Pinene | MH | 940 | 0.2 | 0.7 | 1.0 | 10.8 | 0.1 | 37.9 | 0.5 | 0.9 | ||||||||
| α-Fenchene | MH | 951 | 0.1 | |||||||||||||||
| Camphene | MH | 955 | 0.5 | 0.3 | 0.2 | 0.1 | 4.6 | 5.4 | 0.3 | 0.3 | ||||||||
| Thuja-2.4(10)-diene | MH | 959 | ||||||||||||||||
| β-Pinene | MH | 981 | 0.4 | 1.2 | 0.9 | 0.5 | 0.7 | 0.4 | 1.5 | 5.0 | 0.9 | |||||||
| α-Terpinene | MH | 1019 | 4.7 | 2.1 | 0.3 | 1.2 | 0.3 | 0.8 | ||||||||||
| p-Cymene | MH | 1028 | 0.2 | 2.7 | 4.2 | 9.3 | 9.0 | 2.4 | 15.3 | |||||||||
| Limonene | MH | 1032 | 16.3 | 5.9 | 0.3 | 2.1 | 0.7 | 20.4 | 3.3 | 0.4 | ||||||||
| β-Phellandrene | MH | 1033 | ||||||||||||||||
| 1.8-Cineole | OM | 1036 | 7.7 | 2.3 | 29.0 | 5.9 | 0.1 | 0.8 | 22.0 | 1.0 | 1.3 | 0.7 | ||||||
| Isobutyl angelate | EST | 1053 | ||||||||||||||||
| γ-Terpinene | MH | 1062 | 0.1 | 0.1 | 7.9 | 5.3 | 0.5 | 6.1 | 1.6 | 2.9 | ||||||||
| Artemisia ketone | MH | 1065 | ||||||||||||||||
| Fenchone | OM | 1090 | ||||||||||||||||
| trans-Sabinene hydrate | OM | 1101 | 12.8 | 1.8 | 3.8 | |||||||||||||
| Linalool | OM | 1102 | 31.5 | 1.5 | 1.5 | 46.0 | 3.9 | 3.5 | 0.6 | 8.1 | 3.1 | 2.3 | ||||||
| Camphor | OM | 1148 | 7.3 | 0.8 | 0.2 | 7.6 | 0.7 | 0.5 | ||||||||||
| Isoamyl angelate | EST | 1162 | ||||||||||||||||
| Propyl tiglate | EST | 1166 | ||||||||||||||||
| 4-Terpineol | OM | 1180 | 4.0 | 0.1 | 0.2 | 0.3 | 17.6 | 0.9 | 0.3 | 0.7 | 1.7 | 2.4 | ||||||
| 3.9-Epoxy-p-menth-1-ene | OM | 1186 | ||||||||||||||||
| Menthyl chavicol | PP | 1198 | 1.1 | 0.4 | ||||||||||||||
| Citronellol | OM | 1231 | 44.5 | |||||||||||||||
| Neral | OM | 1242 | 32.5 | 0.2 | ||||||||||||||
| Carvone | OM | 1248 | 0.6 | 0.8 | 1.9 | |||||||||||||
| Geraniol | OM | 1259 | 0.5 | 0.2 | 2.7 | 13.7 | ||||||||||||
| Linalyl acetate | OM | 1260 | 26.8 | 3.2 | 54.7 | 1.2 | ||||||||||||
| (E)-Cinnamaldehyde | NT | 1274 | ||||||||||||||||
| Geranial | OM | 1276 | 36.4 | 0.7 | ||||||||||||||
| Citronellyl formate | OM | 1280 | 7.3 | |||||||||||||||
| iso-Bornyl acetate | OM | 1287 | 1.6 | 0.2 | 0.1 | 8.9 | 3.3 | 0.1 | ||||||||||
| (E)-Anethol | PP | 1290 | 94.6 | |||||||||||||||
| Carvacrol | OM | 1301 | 20.8 | 65.9 | 47.1 | 82.5 | 0.2 | |||||||||||
| Thymol | OM | 1307 | 0.2 | 0.9 | 2.6 | 52.6 | ||||||||||||
| α-Limonene diepoxide | OM | 1347 | 8.6 | |||||||||||||||
| Eugenol | PP | 1361 | 11.5 | 77.9 | ||||||||||||||
| Neryl acetate | OM | 1368 | 0.4 | 0.1 | ||||||||||||||
| β-Caryophyllene | SH | 1418 | 2.2 | 0.8 | 0.5 | 0.3 | 1.7 | 3.7 | 0.7 | 4.4 | 4.1 | 3.6 | 8.9 | 3.0 | 6.8 | |||
| Neryl propanoate | OM | 1454 | ||||||||||||||||
| ar-Curcumene | SH | 1484 | 0.3 | |||||||||||||||
| Eugenol acetate | PP | 1529 | 12.2 | |||||||||||||||
| 5-epi-7-epi-α-Eudesmol | OS | 1606 | ||||||||||||||||
| γ-Eudesmol | OS | 1634 | 5.2 | |||||||||||||||
| T-Cadinol | OS | 1642 | 0.2 | 5.8 | 0.2 | |||||||||||||
| β-Eudesmol | OS | 1649 | 5.5 | |||||||||||||||
| Valerianol | OS | 1655 | 14.4 | |||||||||||||||
| 7-epi-α-Eudesmol | OS | 1664 | 5.9 | |||||||||||||||
| (Z)-α-Santalol | OS | 1675 | 27.1 | |||||||||||||||
| (Z)-β-trans-Santalol | OS | 1710 | 10.8 | |||||||||||||||
| Unknown | 6.2 | 0.8 | 2.0 | 1.8 | 0.0 | 1.7 | 1.4 | 5.1 | 13.9 | 5.6 | 5.4 | 1.3 | 1.7 | |||||
| TOTAL | 100.0 | 99.4 | 93.9 | 99.2 | 98.1 | 98.2 | 99.3 | 98.3 | 98.6 | 94.9 | 86.2 | 94.4 | 94.6 | 100.0 | 98.7 | 97.3 |
| Class of Compounds * | A.n | A.g | B.s | C.z | C.a | C.b | C.l | C.p | C.a.dul | C.la | C.c | F.v | H.i | I.v |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoterpene hydrocarbons (MH) | 10.3 | 10.1 | 84.3 | 15.5 | 97.4 | 49.0 | 94.3 | 96.2 | 98.7 | 57.0 | 3.9 | 22.1 | 19.2 | 7.3 |
| Oxygenated monoterpenes (OM) | 5.9 | 85.9 | 6.7 | 7.4 | 1.9 | 48.5 | 3.6 | 0.5 | 0.6 | 16.6 | 86.3 | 21.1 | 40.6 | 0.7 |
| Sesquiterpene hydrocarbons (SH) | 0.2 | 3.7 | 14.7 | 0.2 | 2.5 | 2.0 | 1.9 | 11.0 | 4.5 | 22.5 | 1.1 | |||
| Oxygenated sesquiterpenes (OS) | 0.2 | 0.8 | 6.6 | 0.9 | 8.9 | 0.1 | ||||||||
| Diterpene hydrocarbons (DH) | ||||||||||||||
| Oxygenated diterpenes (OD) | ||||||||||||||
| Phenylpropanoids (PP) | 0.3 | 0.2 | 3.4 | 56.5 | 90.8 | |||||||||
| Non-terpenes (NT) | 0.9 | 57.9 | 0.5 | 0.1 | 0.1 | 0.6 | 0.7 | 2.7 | 2.0 | |||||
| Esters (EST) | 75.6 | 3.1 | ||||||||||||
| TOTAL Identified | 92.1 | 97.2 | 94.9 | 99.7 | 100.0 | 100.1 | 100.0 | 99.2 | 100.0 | 93.9 | 97.6 | 99.7 | 94.3 | 100.0 |
| Class of Compounds * | L.h | L.c | M.c | O.b | O.m | O.v | P.g | P.ab | P.a | R.o | S.s | S.al | S.m | S.a | T.c | T.v |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoterpene hydrocarbons (MH) | 6.4 | 21.3 | 61.2 | 2.3 | 27.7 | 22.4 | 38.2 | 0.1 | 56.5 | 19.6 | 4.6 | 21.5 | ||||
| Oxygenated monoterpenes (OM) | 85.0 | 75.7 | 31.1 | 56.1 | 66.6 | 71.2 | 83.4 | 23.1 | 3.5 | 36.8 | 78.2 | 0.6 | 61.9 | 89.2 | 64.1 | |
| Sesquiterpene hydrocarbons (SH) | 5.4 | 0.9 | 1.2 | 20.0 | 3.2 | 4.2 | 7.8 | 25.0 | 4.4 | 0.9 | 5.1 | 11.9 | 9.5 | 3.8 | 9.2 | |
| Oxygenated sesquiterpenes (OS) | 1.3 | 7.9 | 0.4 | 0.4 | 6.9 | 12.0 | 0.3 | 4.9 | 88.8 | 1.1 | 0.4 | 0.7 | ||||
| Diterpene hydrocarbons (DH) | 0.2 | |||||||||||||||
| Oxygenated diterpenes (OD) | 1.3 | |||||||||||||||
| Phenylpropanoids (PP) | 12.7 | 1.2 | 95.0 | 90.1 | ||||||||||||
| Non-terpenes (NT) | 0.8 | 1.5 | 0.1 | 0.2 | 0.3 | 0.8 | ||||||||||
| Esters (EST) | 1.1 | 0.3 | 0.2 | 1.7 | ||||||||||||
| TOTAL Identified | 100.0 | 99.4 | 93.9 | 99.2 | 98.1 | 98.2 | 99.3 | 98.3 | 98.6 | 94.9 | 86.2 | 94.4 | 94.6 | 100.0 | 98.7 | 97.3 |
| EOs | Prototheca zopfii | Prototheca blaschkeae |
|---|---|---|
| MIC (%) | ||
| Pimpinella anisum | 4 | >4 |
| Illicium verum | >4 | >4 |
| Santalum album | >4 | >4 |
| Helichrysum italicum | >4 | >4 |
| Rosmarinus officinalis | >4 | >4 |
| Lavandula hybrida | >4 | >4 |
| Pelargonium graveolens | >4 | >4 |
| Salvia sclarea | >4 | >4 |
| Cynnamomum zeylanicum | >4 | >4 |
| Foeniculum vulgare | >4 | >4 |
| Syzygium aromaticum | >4 | >4 |
| Boswellia sacra | >4 | >4 |
| Anthemis nobilis | >4 | >4 |
| Citrus paradisi | 0.75 | 0.75 |
| Citrus bergamia | 2 | 0.75 |
| Citrus aurantium | >4 | >4 |
| Citrus aurantium var. dulcis | >4 | >4 |
| Citrus limon | >4 | >4 |
| Cymbopogon citratus | 1 | >4 |
| Ocimum basilicum | 1 | >4 |
| Origanum majorana | 1 | >4 |
| Thymus vulgaris | 0.75 | 1 |
| Litsea cubeba | 0.75 | 1 |
| Origanum vulgare | 0.75 | 1 |
| Satureja montana | 4 | 4 |
| Cistus ladanifer | 4 | 4 |
| Picea abies | 4 | 4 |
| Anethum graveolens | 4 | 4 |
| Thymus capitatus | 4 | 4 |
| Myrtus communis | 4 | 4 |
| Conventional drugs | MIC (μg/mL) | |
| Posaconazole | 0.38 | 0.5 |
| Voriconazole | 6 | 6 |
| Amphotericin B | 0.25 | 0.19 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardoni, S.; Pisseri, F.; Pistelli, L.; Najar, B.; Luini, M.; Mancianti, F. In Vitro Activity of 30 Essential Oils against Bovine Clinical Isolates of Prototheca zopfii and Prototheca blaschkeae. Vet. Sci. 2018, 5, 45. https://doi.org/10.3390/vetsci5020045
Nardoni S, Pisseri F, Pistelli L, Najar B, Luini M, Mancianti F. In Vitro Activity of 30 Essential Oils against Bovine Clinical Isolates of Prototheca zopfii and Prototheca blaschkeae. Veterinary Sciences. 2018; 5(2):45. https://doi.org/10.3390/vetsci5020045
Chicago/Turabian StyleNardoni, Simona, Francesca Pisseri, Luisa Pistelli, Basma Najar, Mario Luini, and Francesca Mancianti. 2018. "In Vitro Activity of 30 Essential Oils against Bovine Clinical Isolates of Prototheca zopfii and Prototheca blaschkeae" Veterinary Sciences 5, no. 2: 45. https://doi.org/10.3390/vetsci5020045







