Heart Failure in Patients with Preserved Ejection Fraction: Questions Concerning Clinical Progression
Abstract
:1. Introduction
2. Clinical Phenotypes of Heart Failure Based on the Ejection Fraction
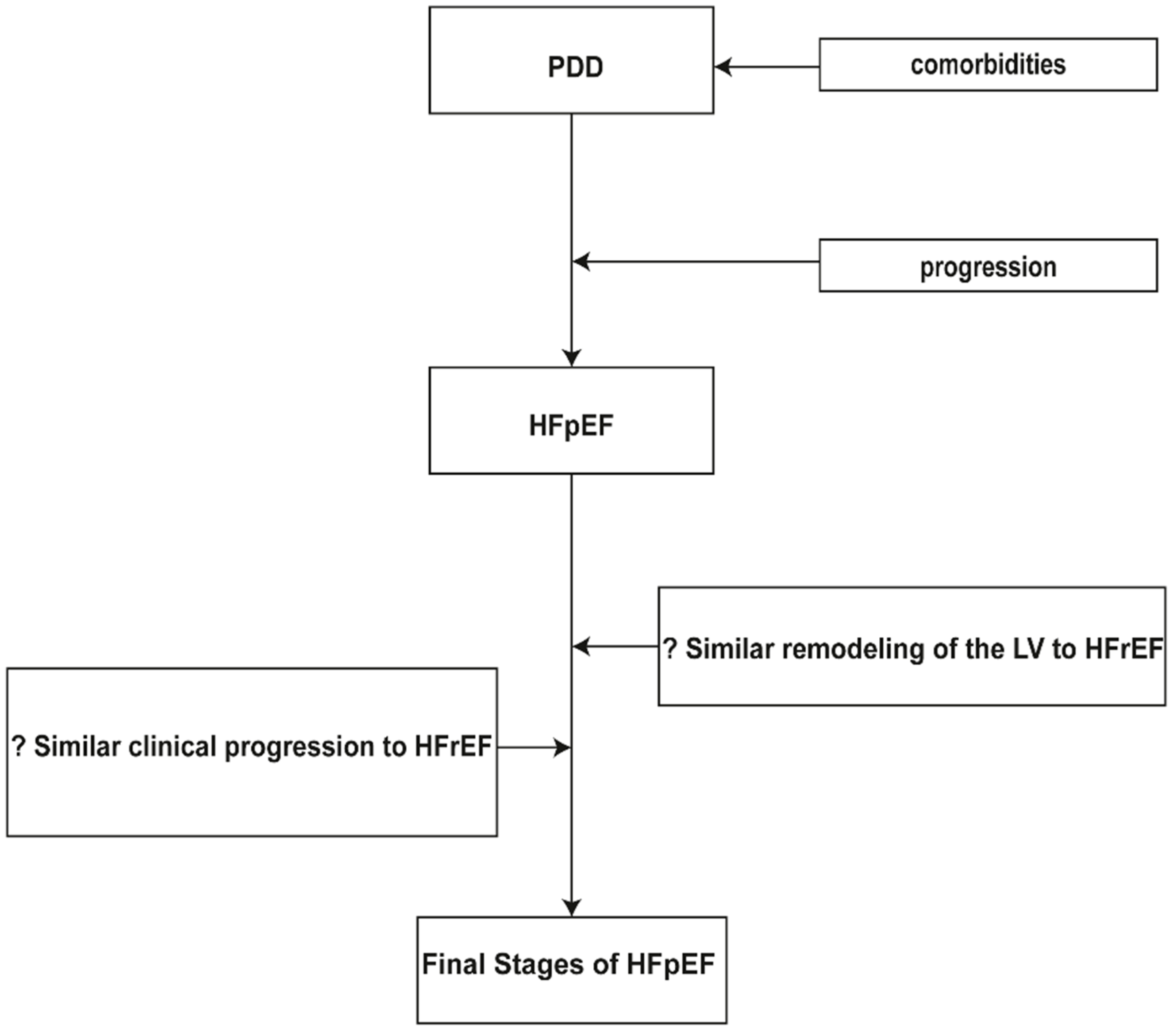
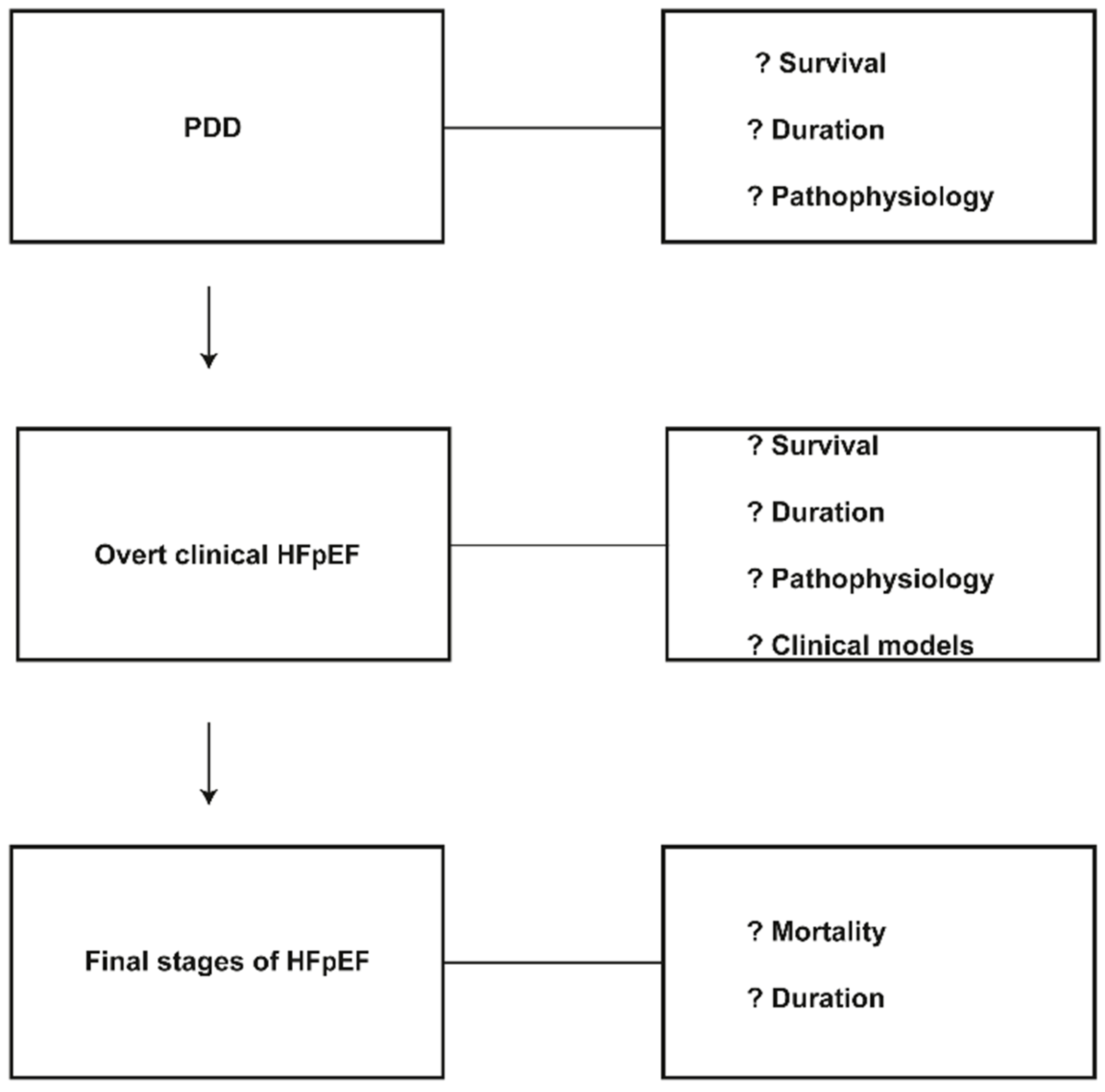
3. Clinical Progression in Patients with Preserved Ejection Fraction
4. Incomplete Knowledge of Clinical Progression
5. Conclusions
Conflicts of Interest
References
- Senni, M.; Paulus, W.J.; Gavazzi, A.; Fraser, A.G.; Díez, J.; Solomon, S.D.; Smiseth, O.A.; Guazzi, M.; Lam, C.S.P.; Maggioni, A.P.; et al. New strategies for heart failure with preserved ejection fraction: The importance of targeted therapies for heart failure phenotypes. Eur. Heart J. 2014, 35, 2797–2811. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B. Heart failure preserved ejection fraction with coronary artery disease: Time for a new classification? J. Am. Coll. Cardiol. 2014, 63, 2828–2830. [Google Scholar] [CrossRef] [PubMed]
- Bursi, F.; Weston, S.A.; Redfield, M.M.; Jacobsen, S.J.; Pakhomov, S.; Nkomo, V.T.; Meverden, R.A.; Roger, V.L. Systolic and diastolic heart failure in the community. JAMA 2006, 296, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Hatle, L.; Tajik, A.J.; Little, W.C. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J. Am. Coll. Cardiol. 2006, 47, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, A.P.; Fonarow, G.C.; Georgiopoulou, V.; Burkman, G.; Siwamogsatham, S.; Patel, A.; Li, S.; Papadimitriou, L.; Butler, J. Characteristics and Outcomes of Adult Outpatients with Heart Failure and Improved or Recovered Ejection Fraction. JAMA Cardiol. 2016, 1, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Redfield, M.M. Diastolic and Systolic Heart Failure are Distinct Phenotypes within the Heart Failure Spectrum. Circulation 2011, 123, 2006–2013. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Hay, I.; Fetics, B.; Kass, D.A. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: Implications for systolic and diastolic reserve limitations. Circulation 2003, 107, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Remme, E.W.; Opdahl, A.; Aakhus, S.; Skulsad, H. Heart failure with normal left ventricular ejection fraction: Basic principles and clinical diagnostics. In Translational Approach to Heart Failure; Bartunek, J., Vanderheyden, M., Eds.; Springer: New York, NY, USA, 2013; pp. 25–61. [Google Scholar]
- Redfield, M.M.; Jacobsen, S.J.; Burnett, J.C., Jr.; Mahoney, D.W.; Bailey, K.R.; Rodeheffer, R.J. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA 2003, 289, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.H.; Vogel, M.W.; Chen, H.H. Pre-clinical diastolic dysfunction. J. Am. Coll. Cardiol. 2014, 63, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Zaphiriou, A.; Robb, S.; Murray-Thomas, T.; Mendez, G.; Fox, K.; McDonagh, T.; Hardman, S.M.; Dargie, H.J.; Cowie, M.R. The diagnostic accuracy of plasma BNP and NT-proBNP in patients referred from primary care with suspected heart failure: Results of the UK natriuretic peptide study. Eur. J. Heart Fail. 2005, 7, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, E.T.; Jorge, A.J.; Souza Junior, C.V.; Cassino, J.P. Systems biology applied to heart failure with normal ejection fraction. Arq. Bras. Cardiol. 2014, 102, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Abhayaratna, W.P.; Marwick, T.H.; Smith, W.T.; Becker, N.G. Characteristics of left ventricular diastolic dysfunction in the community: An echocardiographic survey. Heart 2006, 92, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Lyass, A.; Kraigher-Krainer, E.; Massaro, J.M.; Lee, D.S.; Ho, J.E.; Levy, D.; Redfield, M.M.; Pieske, B.M.; Benjamin, E.J.; et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation 2011, 124, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Mureddu, G.F.; Agabiti, N.; Rizzello, V.; Forastiere, F.; Latini, R.; Cesaroni, G.; Masson, S.; Cacciatore, G.; Colivicchi, F.; Uguccioni, M.; et al. Prevalence of preclinical and clinical heart failure in the elderly. A population-based study in Central Italy. Eur. J. Heart Fail. 2012, 14, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Hebert, P.R. Prevention of disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trials. J. Am. Coll. Cardiol. 1996, 27, 1214–1218. [Google Scholar] [CrossRef]
- Kane, G.C.; Karon, B.L.; Mahoney, D.W.; Redfield, M.M.; Roger, V.L.; Burnett, J.C.; Jacobsen, S.J.; Rodeheffer, R.J. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011, 306, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Correa de Sa, D.D.; Hodge, D.O.; Slusser, J.P.; Redfield, M.M.; Simari, R.D.; Burnett, J.C.; Chen, H.H. Progression of preclinical diastolic dysfunction to the development of symptoms. Heart 2010, 96, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Maizel, J.; Mentaverri, R.; Chillon, J.M.; Six, I.; Giummelly, P.; Brazier, M.; Choukroun, G.; Tribouilloy, C.; Massy, Z.A.; et al. The onset of left ventricular diastolic dysfunction in SHR rats is not related to hypertrophy or hypertension. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1524–H1532. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.W.; Slusser, J.P.; Hodge, D.O.; Chen, H.H. The natural history of preclinical diastolic dysfunction: A population-based study. Circ. Heart Fail. 2012, 5, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Karter, A.J.; Go, A.S.; Ferrara, A.; Liu, J.Y.; Sidney, S.; Selby, J.V. Glycemic control and heart failure among adult patients with diabetes. Circulation 2001, 103, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- From, A.M.; Scott, C.G.; Chen, H.H. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction: A population-based study. J. Am. Coll. Cardiol. 2010, 55, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ristow, B.; Na, B.; Ali, S.; Schiller, N.B.; Whooley, M.A. Prevalence and prognosis of asymptomatic left ventricular diastolic dysfunction in ambulatory patients with coronary heart disease. Am. J. Cardiol. 2007, 99, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Gaasch, W.H.; Anand, I.S.; Haass, M.; Little, W.C.; Miller, A.B.; Lopez-Sendon, J.; Teerlink, J.R.; White, M.; McMurray, J.J.; et al. Mode of death in patients with heart failure and a preserved ejection fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-Preserve) trial. Circulation 2010, 121, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012, 14, 803–869. [Google Scholar]
- Massie, B.M.; Carson, P.E.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Zile, M.R.; Anderson, S.; Donovan, M.; Iverson, E.; Staiger, C.; et al. Irbesartan in patients with heart failure and preserved ejection fraction. N. Engl. J. Med. 2008, 359, 2456–2467. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Magrini, G.; Serio, A.; Klersy, C.; Fucili, A.; Ronaszèki, A.; Karpati, P.; Mordenti, G.; Capriati, A.; Poole-Wilson, P.A.; et al. Effects of nebivolol in elderly heart failure patients with or without systolic left ventricular dysfunction: Results of the SENIORS echocardiographic substudy. Eur. Heart J. 2006, 27, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; van Ballegoij, J.J.M. Treatment of heart failure with normal ejection fraction. An inconvenient truth! J. Am. Coll. Cardiol. 2010, 55, 526–537. [Google Scholar] [CrossRef] [PubMed]
- The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N. Engl. J. Med. 1997, 336, 525–533. [Google Scholar]
- Yusuf, S.; Pfeffer, M.A.; Swedberg, K.; Granger, C.; Held, P.; McMurray, J.; Michelson, E.; Olofsson, B.; Ostergren, J.; Comm, C. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-Preserved Trial. Lancet 2003, 362, 777–781. [Google Scholar] [CrossRef]
- Campbell, R.T.; Jhund, P.S.; Castagno, D.; Hawkins, N.M.; Petrie, M.C.; McMurray, J.J.V. What have we learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-Preserved, and I-PRESERVE? J. Am. Coll. Cardiol. 2012, 60, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Rich, M.W.; Fleg, J.L.; Zile, M.R.; Young, J.B.; Kitzman, D.W.; Love, T.E.; Aronow, W.S.; Adams, K.F.; Gheorghiade, M. Effects of digoxin on morbidity and mortality in diastolic heart failure: The ancillary digitalis investigation group trial. Circulation 2006, 114, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Origasa, H.; Hori, M. Effects of carvedilol on heart failure with preserved ejection fraction: The Japanese Diastolic Heart Failure Study (J-DHF). Eur. J. Heart Fail. 2013, 15, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.M.; Yusuf, S.; Young, J.; Mathew, J.; Johnstone, D.; Avezum, A.; Lonn, E.; Pogue, J.; Bosch, J. Prevention of heart failure in patients in the Heart Outcomes Prevention Evaluation (HOPE) study. Circulation 2003, 107, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.R.; Piller, L.B.; Cutler, J.A.; Furberg, C.; Dunn, K.; Franklin, S.; Goff, D.; Leenen, F.; Mohiuddin, S.; Papademetriou, V.; et al. Role of diuretics in the prevention of heart failure: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Circulation 2006, 113, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Verma, A.; Desai, A.; Hassanein, A.; Izzo, J.; Oparil, S.; Lacourciere, Y.; Lee, J.; Seifu, Y.; Hilkert, R.J.; et al. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension 2010, 55, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Russo, C.; Bella, J.N. Treatment of isolated left ventricular diastolic dysfunction in hypertension: Reaching blood pressure target matters. Hypertension 2010, 55, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Yip, G.W.; Wang, M.; Wang, T.; Chan, S.; Fung, J.W.; Yeung, L.; Yip, T.; Lau, S.T.; Lau, C.P.; Tang, M.O.; et al. The Hong Kong diastolic heart failure study: A randomized controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart 2008, 94, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; Lewis, E.F.; Li, R.; Solomon, S.D.; Assmann, S.F.; Boineau, R.; Clausell, N.; Diaz, R.; Fleg, J.L.; Gordeev, I.; et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: A randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am. Heart J. 2011, 162, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Claggett, B.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; Gordeev, I.; et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015, 131, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, F.; Wachter, R.; Schmidt, A.G.; Kraigher-Krainer, E.; Colantonio, C.; Kamke, W.; Duvinage, A.; Stahrenberg, R.; Durstewitz, K.; Löffler, M.; et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The Aldo-DHF randomized controlled trial. JAMA 2013, 309, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Van Heerebeek, L.; Hamdani, N.; Falcao-Pires, I.; Leite-Moreira, A.F.; Begieneman, M.P.; Bronzwaer, J.G.; van der Velden, J.; Stienen, G.J.; Laarman, G.J.; Somsen, A.; et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012, 126, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Giannetta, E.; Isidori, A.M.; Galea, N.; Carbone, I.; Mandosi, E.; Vizza, C.D.; Naro, F.; Morano, S.; Fedele, F.; Lenzi, A. Chronic inhibition of cGMP phosphodiesterase 5A improves diabetic cardiomyopath: Randomized, controlled clinical trial using magnetic resonance imaging with myocardial tagging. Circulation 2012, 125, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Kasner, M.; Westermann, D.; Lopez, B.; Gaub, R.; Escher, F.; Kühl, U.; Schultheiss, H.-P.; Tschöpe, C. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J. Am. Coll. Cardiol. 2011, 57, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, A.; Alogna, A.; Post, H.; Hamdani, N. Is enhancing cGMP-PKG signaling a promising therapeutic target for heart failure with preserved ejection fraction? Neth. Heart J. 2016, 24, 268–74. [Google Scholar] [CrossRef] [PubMed]
- Franssen, C.; Gonzalez Miqueo, A. The role of titin and extracellular matrix remodeling in heart failure with preserved ejection fraction. Neth. Heart J. 2016, 24, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Gottdiener, J.S.; Hetzel, S.J.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Baicu, C.F.; Massie, B.M.; Carson, P.E. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 2011, 124, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- Pellicori, P.; Zhang, J.; Lukaschuk, E.; Joseph, A.C.; Bourantas, C.V.; Loh, H.; Bragadeesh, T.; Clark, A.L.; Cleland, J.G. Left atrial function measured by cardiac magnetic resonance imaging in patients with heart failure: Clinical associations and prognostic value. Eur. Heart J. 2015, 36, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Lam, C.S.P. Function over form? Assessing the left atrium in heart failure. Eur. Heart J. 2015, 36, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Conceicao, G.; Heinonen, I.; Lourenco, A.P.; Duncker, D.J.; Falcao-Pires, I. Animal models of heart failure with preserved ejection fraction. Neth. Heart J. 2016, 24, 275–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louridas, G.E.; Lourida, K.G. A conceptual paradigm of heart failure and systems biology approach. Int. J. Cardiol. 2012, 159, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Kawakami, R.; Nishida, T.; Onoue, K.; Soeda, T.; Okayama, S.; Takeda, Y.; Watanabe, M.; Kawata, H.; Uemura, S.; et al. Left ventricular ejection fraction (EF) of 55% as cutoff for late transition from heart failure (HF) with preserved EF to HF with mildly reduced EF. Circ. J. 2015, 79, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Bohm, M.; Cleland, J.G.; Paulus, W.J.; Pieske, B.; Rapezzi, C.; Tavazzi, L. Heart failure with preserved ejection fraction: Uncertainties and dilemmas. Eur. J. Heart Fail. 2015, 17, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Lekavich, C.L.; Barksdale, D.J.; Neelon, V.; Wu, J.R. Heart failure preserved ejection fraction (HFpEF): An integrated and strategic review. Heart Fail. Rev. 2015, 20, 643–653. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louridas, G.E.; Lourida, K.G. Heart Failure in Patients with Preserved Ejection Fraction: Questions Concerning Clinical Progression. J. Cardiovasc. Dev. Dis. 2016, 3, 27. https://doi.org/10.3390/jcdd3030027
Louridas GE, Lourida KG. Heart Failure in Patients with Preserved Ejection Fraction: Questions Concerning Clinical Progression. Journal of Cardiovascular Development and Disease. 2016; 3(3):27. https://doi.org/10.3390/jcdd3030027
Chicago/Turabian StyleLouridas, George E., and Katerina G. Lourida. 2016. "Heart Failure in Patients with Preserved Ejection Fraction: Questions Concerning Clinical Progression" Journal of Cardiovascular Development and Disease 3, no. 3: 27. https://doi.org/10.3390/jcdd3030027




