A Multiparametric Approach Based on NT-proBNP, ST2, and Galectin3 for Stratifying One Year Prognosis of Chronic Heart Failure Outpatients
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Correlates of Biomarkers
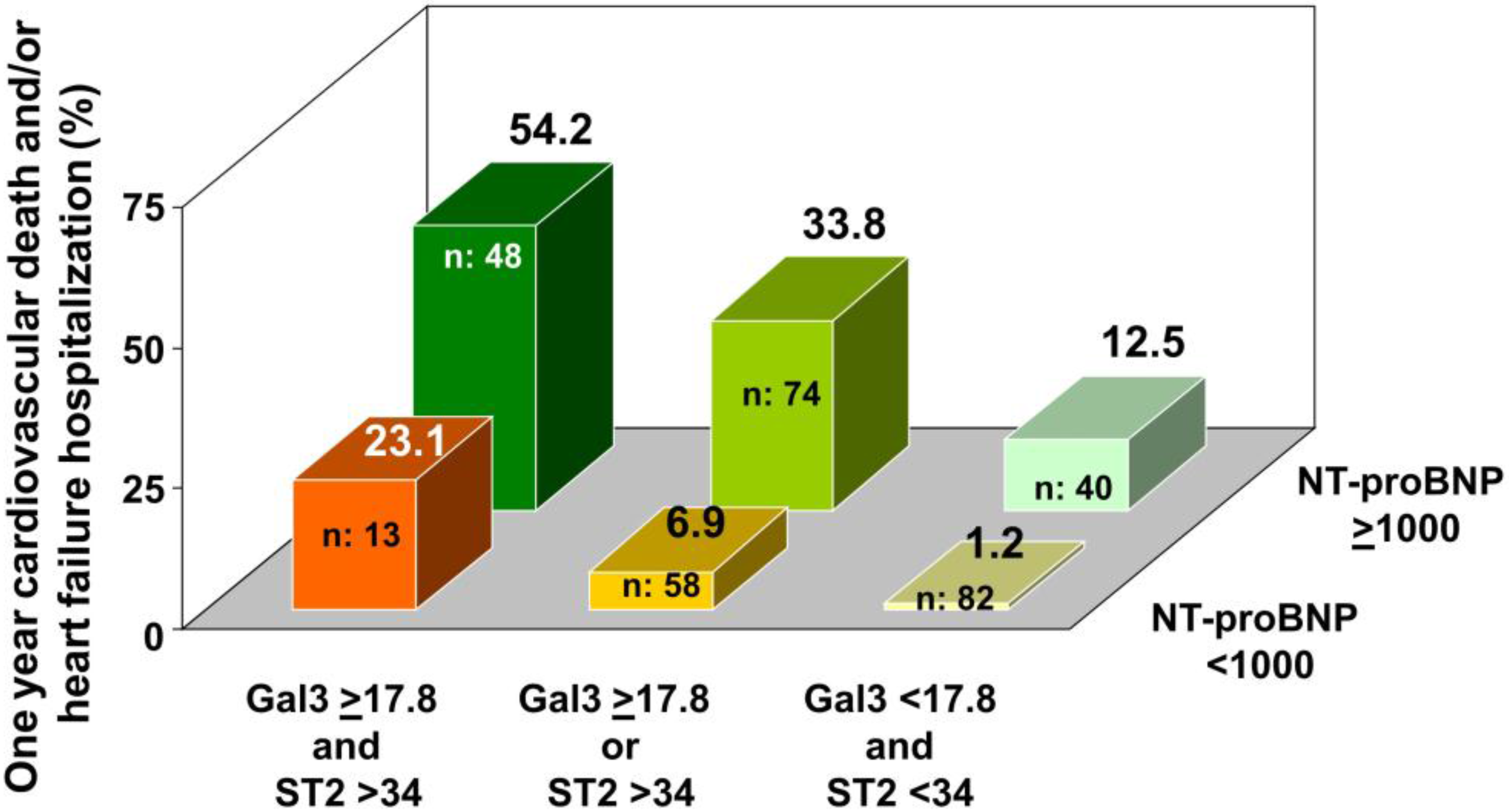
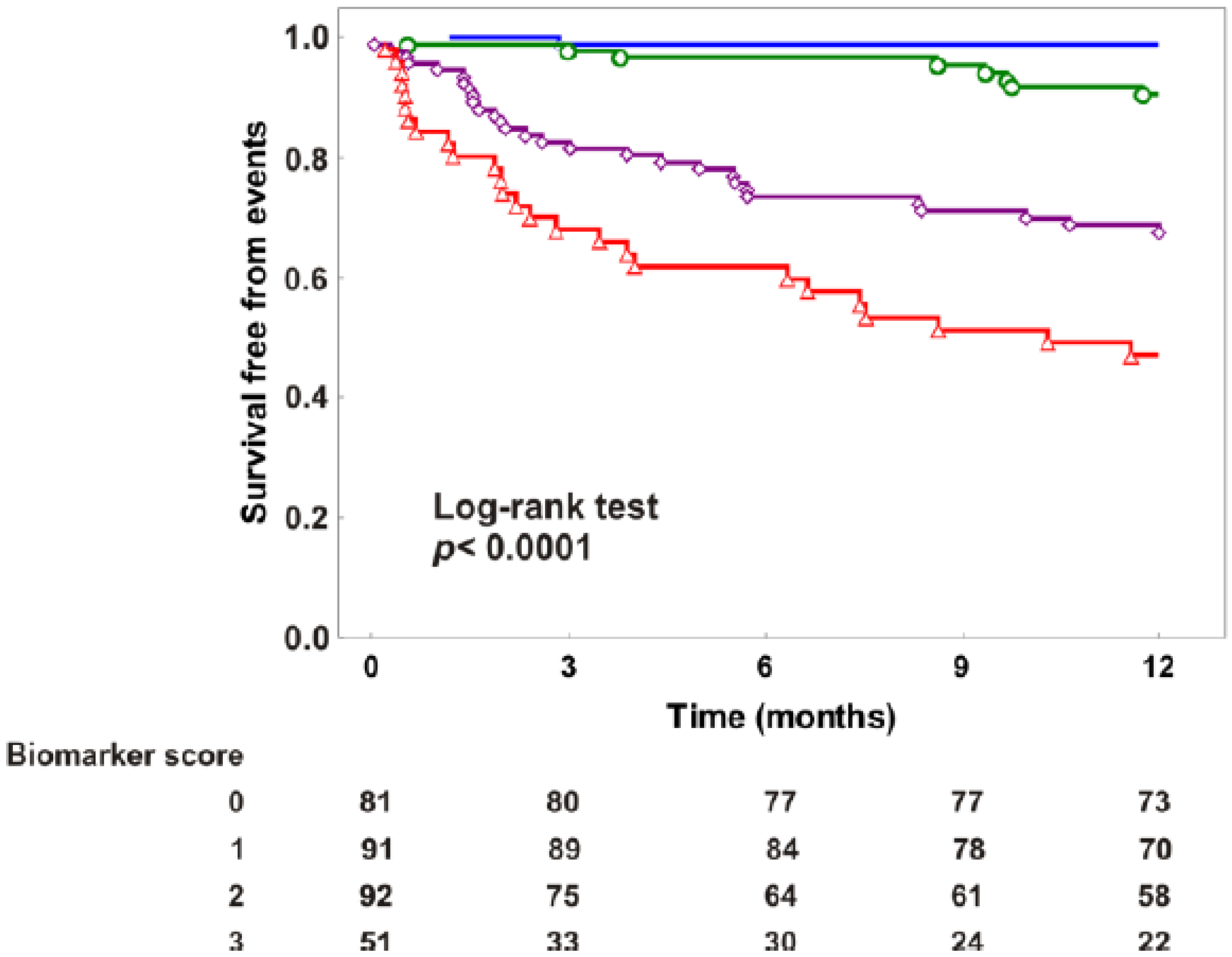
3.2. Independent and Significant Association of Biomarkers with Events
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moriates, C.; Maisel, A. The Utility of Biomarkers in Sorting Out the Complex Patient. Am. J. Med. 2010, 123, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Gopal, D.; Kommineni, M.; Ayalon, N.; Koelbl, C.; Ayalon, R.; Biolo, A.; Dember, L.; Downing, J.; Siwik, D.; Liang, C.; et al. Relationship of Plasma Galectin-3 to Renal Function in Patients With Heart Failure: Effects of Clinical Status, Pathophysiology of Heart Failure, and Presence or Absence of Heart Failure. J. Am. Heart Assoc. 2012, 1, e000760. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, M.; Aspromonte, N.; Leone, M.; Paradies, V.; Antoncecchi, V.; Valle, R.; Caldarola, P.; Ciccone, M.; Gesualdo, L.; Serio, F. Galectin-3 Serum Levels Are Independently Associated With Microalbuminuria in Chronic Heart Failure Outpatients. Res. Cardiovasc. Med. 2015, 5, e28952. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Hou, W.; Zhang, Y.; Chen, Y.; He, B. Prognostic value of serum galectin-3 in patients with heart failure: A meta-analysis. Int. J. Cardiol. 2015, 182, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Lok, D.; Van Der Meer, P.; de la Porte, P.; Lipsic, E.; Van Wijngaarden, J.; Hillege, H.; van Veldhuisen, D. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the DEAL-HF study. Clin. Res. Cardiol. 2010, 99, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Shrestha, K.; Shao, Z.; Borowski, A.; Troughton, R.; Thomas, J.; Klein, A. Usefulness of Plasma Galectin-3 Levels in Systolic Heart Failure to Predict Renal Insufficiency and Survival. Am. J. Cardiol. 2011, 108, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Lok, D.; Lok, S.; Bruggink-André de la Porte, P.; Badings, E.; Lipsic, E.; van Wijngaarden, J.; de Boer, R.; van Veldhuisen, D.; van der Meer, P. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin. Res. Cardiol. 2012, 102, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Stolen, C.; Adourian, A.; Meyer, T.; Stein, K.; Solomon, S. Plasma Galectin-3 and Heart Failure Outcomes in MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy). J. Card. Fail. 2014, 20, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.; Fiuzat, M.; Shaw, L.; Clare, R.; Whellan, D.; Bettari, L.; Shirolkar, S.; Donahue, M.; Kitzman, D.; Zannad, F.; et al. Galectin-3 in Ambulatory Patients With Heart Failure: Results From the HF-ACTION Study. Circ.: Heart Fail. 2011, 5, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gullestad, L.; Ueland, T.; Kjekshus, J.; Nymo, S.; Hulthe, J.; Muntendam, P.; McMurray, J.; Wikstrand, J.; Aukrust, P. The predictive value of galectin-3 for mortality and cardiovascular events in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Am. Heart J. 2012, 164, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Andrès, N.; Rossignol, P.; Iraqi, W.; Fay, R.; Nuée, J.; Ghio, S.; Cleland, J.; Zannad, F.; Lacolley, P. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: Insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur. J. Heart Fail. 2012, 14, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ky, B.; French, B.; McCloskey, K.; Rame, J.; McIntosh, E.; Shahi, P.; Dries, D.; Tang, W.; Wu, A.; Fang, J.; et al. High-Sensitivity ST2 for Prediction of Adverse Outcomes in Chronic Heart Failure. Circ.: Heart Fail. 2010, 4, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; de Antonio, M.; Galán, A.; Sanz, H.; Urrutia, A.; Cabanes, R.; Cano, L.; González, B.; Díez, C.; Pascual, T.; et al. Combined use of high-sensitivity ST2 and NT-proBNP to improve the prediction of death in heart failure. Eur. J. Heart Fail. 2012, 14, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.; Fiuzat, M.; Thompson, V.; Shaw, L.; Neely, M.; Adams, K.; Whellan, D.; Donahue, M.; Ahmad, T.; Kitzman, D.; et al. Soluble ST2 in Ambulatory Patients With Heart Failure: Association With Functional Capacity and Long-Term Outcomes. Circ.: Heart Fail. 2013, 6, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.; Rector, T.; Kuskowski, M.; Snider, J.; Cohn, J. Prognostic Value of Soluble ST2 in the Valsartan Heart Failure Trial. Circ.: Heart Fail. 2014, 7, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.; Lai, W.; Afilalo, J.; Hua, L.; Handschumacher, M.; Chandrasekaran, K.; Solomon, S.; Louie, E.; Schiller, N. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef] [PubMed]
- Masson, S. Direct Comparison of B-Type Natriuretic Peptide (BNP) and Amino-Terminal proBNP in a Large Population of Patients with Chronic and Symptomatic Heart Failure: The Valsartan Heart Failure (Val-HeFT) Data. Clin. Chem. 2006, 52, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Dieplinger, B. The Presage®ST2 Assay: Analytical considerations and clinical applications for a high-sensitivity assay for measurement of soluble ST2. Expert Rev. Mol. Diagn. 2013, 13, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.; Januzzi, J.; de Filippi, C.; Adourian, A.; Shah, S.; van Veldhuisen, D.; de Boer, R. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: A pooled analysis of 3 clinical trials. Am. Heart J. 2014, 167, 853.e4–860.e4. [Google Scholar] [CrossRef] [PubMed]
- Slinker, B.; Glantz, S. Multiple Linear Regression: Accounting for Multiple Simultaneous Determinants of a Continuous Dependent Variable. Circulation 2008, 117, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Pencina, M.; D’Agostino, R.; Steyerberg, E. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat. Med. 2010, 30, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.; Ouwerkerk, W.; Zannad, F.; van Veldhuisen, D.; Samani, N.; Ponikowski, P.; Ng, L.; Metra, M.; Ter Maaten, J.; Lang, C.; et al. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur. J. Heart Fail. 2017, 19, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.; Maggioni, A.; Køber, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.; Drazner, M.; Fonarow, G.; Geraci, S.; Horwich, T.; Januzzi, J.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed]
- Raymond, I. The influence of age, sex and other variables on the plasma level of N-terminal pro brain natriuretic peptide in a large sample of the general population. Heart 2003, 89, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Wettersten, N.; Maisel, A. Biomarkers for Heart Failure: An Update for Practitioners of Internal Medicine. Am. J. Med. 2016, 129, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.; Rodeheffer, R.; Jacobsen, S.; Mahoney, D.; Bailey, K.; Burnett, J. Plasma brain natriuretic peptide concentration: Impact of age and gender. J. Am. Coll. Cardiol. 2002, 40, 976–982. [Google Scholar] [CrossRef]
- Mehra, M.; Uber, P.; Park, M.; Scott, R.; Ventura, H.; Harris, B.; Frohlich, E. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J. Am. Coll. Cardiol. 2004, 43, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Biomarkers in Heart Failure. New Engl. J. Med. 2008, 358, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Richards, A. What We May Expect from Biomarkers in Heart Failure. Heart Fail. Clin. 2009, 5, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Suarez, G.; Meyerrose, G. Heart failure and galectin 3. Ann. Transl. Med. 2014, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Boulogne, M.; Sadoune, M.; Launay, J.; Baudet, M.; Cohen-Solal, A.; Logeart, D. Inflammation versus mechanical stretch biomarkers over time in acutely decompensated heart failure with reduced ejection fraction. Int. J. Cardiol. 2017, 226, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.; Richards, A.; Pascual-Figal, D.; Mueller, C. Serial ST2 Testing in Hospitalized Patients with Acute Heart Failure. Am. J. Cardiol. 2015, 115, 32B–37B. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Mueller, T.; Januzzi, J. Characteristics of the Novel Interleukin Family Biomarker ST2 in Patients with Acute Heart Failure. J. Am. Coll. Cardiol. 2008, 52, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Zamora, E.; de Antonio, M.; Galán, A.; Vila, J.; Urrutia, A.; Díez, C.; Coll, R.; Altimir, S.; Lupón, J. Soluble ST2 Serum Concentration and Renal Function in Heart Failure. J. Card. Fail. 2013, 19, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Dieplinger, B.; Mueller, T. Soluble ST2 in heart failure. Clin. Chim. Acta 2015, 30, 57–70. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.; Lok, D.; Jaarsma, T.; van der Meer, P.; Voors, A.; Hillege, H.; van Veldhuisen, D. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann. Med. 2010, 43, 60–68. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Number of Increased Biomarkers | p | ||||
|---|---|---|---|---|---|---|
| All Patients | Zero | One | Two | Three | ||
| n: 315 | n: 81 | n: 91 | n: 92 | n: 51 | ||
| Age (years) | 64 ± 13 | 59 ± 13 | 64 ± 13 * | 65 ± 13 * | 71 ± 10 *,†,‡ | <0.001 |
| Males (%) | 81 | 88 | 79 | 76 | 80 | 0.247 |
| NYHA class | 2.4 ± 0.6 | 2.0 ± 0.5 | 2.2 ± 0.5 * | 2.6 ± 0.5 *,† | 2.8 ± 0.5 *,†,‡ | <0.001 |
| MBP (mm Hg) | 90 ± 11 | 94 ± 10 | 94 ± 11 | 88 ± 10 *,† | 85 ± 9 *,† | <0.001 |
| BMI (kg/m2) | 27 ± 5 | 29 ± 5 | 29 ± 5 | 27 ± 5 | 27 ± 5 | 0.026 |
| Atrial fibrillation (%) | 20 | 4 | 15 * | 30 *,† | 35 *,† | <0.001 |
| Ace-inhibitors/ARBs (%) | 79 | 89 | 85 | 73 *,† | 57 *†,‡ | <0.001 |
| Beta-blockers (%) | 96 | 100 | 97 | 95 | 92 | <0.001 |
| Diuretics (%) | 92 | 84 | 91 * | 97 * | 100 *,† | <0.001 |
| Digoxin (%) | 11 | 4 | 13 | 13 | 18 | 0.476 |
| MRAs (%) | 70 | 70 | 64 | 78 † | 65 ‡ | 0.030 |
| ICD (%) | 87 | 91 | 82 | 87 | 86 | 0.549 |
| CRT (%) | 34 | 37 | 37 | 32 | 26 | 0.149 |
| LVEF (%) | 33 ± 9 | 37 ± 7 | 34 ± 8 * | 31 ± 10 * | 31 ± 11 * | <0.001 |
| E/e’ | 14 ± 7 | 10 ± 4 | 13 ± 6 | 16 ± 8 *,† | 17 ± 10 *,† | <0.001 |
| TAPSE (mm) | 19 ± 4 | 20 ± 4 | 20 ± 4 | 18 ± 4 *,† | 17 ± 10 *,† | <0.001 |
| MR (a.u.) | 1.8 ± 0.9 | 1.4 ± 0.8 | 1.6 ± 0.8 | 2.0 ± 1.0 *,† | 2.1 ± 0.9 *,† | <0.001 |
| TR (a.u.) | 1.8 ± 1.0 | 1.4 ± 0.8 | 1.5 ± 0.7 | 2.0 ± 1.1 *,† | 2.4 ± 1.1 *,†,‡ | <0.001 |
| CVP (mmHg) | 5 ± 4 | 3 ± 2 | 4 ± 3 | 6 ± 5 *,† | 8 ± 5 *,†,‡ | <0.001 |
| PASP (mmHg) | 37 ± 14 | 31 ± 11 | 33 ± 9 | 40 ± 12 *,† | 48 ± 19 *,†,‡ | <0.001 |
| GFR-EPI (mL/min/1.73 m2) | 71 ± 26 | 87 ± 20 | 75 ± 24 * | 66 ± 25 *,† | 48 ± 17 *,†,‡ | <0.001 |
| Sodium (mmol/L) | 139 ± 8 | 139 ± 15 | 140 ± 3 | 139 ± 3 | 138 ± 5 | 0.726 |
| Hb (g/dL) | 13.5 ± 1.6 | 14.1 ± 1.2 | 13.8 ± 1.6 | 13.4 ± 1.7 * | 12.4 ± 1.3 *,†,‡ | <0.001 |
| CRP (mg/L) | 5.4 ± 7.7 | 4.0 ± 2.6 | 4.9 ± 6.1 | 6.4 ± 10.3 | 6.9 ± 9.6 | 0.084 |
| NT-proBNP (pg/mL) | 2294 ± 3642 | 376 ± 256 | 1127 ± 1007 | 3145 ± 2125 *,† | 5888 ± 6625 *,†,‡ | <0.001 |
| ST2 (ng/mL) | 40.70 ± 27.52 | 24.98 ± 5.73 | 35.94 ± 13.88 * | 47.20 ± 27.16 *,† | 62.47 ± 44.84 *,†,‡ | <0.001 |
| Galectin-3 (ng/mL) | 16.0 ± 7.1 | 11.2 ± 2.9 | 13.6 ± 4.2 * | 17.3 ± 6.7 *,† | 25.7 ± 6.7 *,†,‡ | <0.001 |
| Variables | Age | NYHA | BMI | LVESV | LVEF | E/e’ | MR | TR | PAPS | CVP | TAPSE | HB | GFR-EPI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NT-proBNP | r: 0.160 | r: 0.399 | r: −0.102 | r: 0.297 | r: −0.350 | r: 0.326 | r: 0.335 | r: 0.244 | r: 0.506 | r: 0.347 | r: −0.244 | r: −0.315 | r: −0.376 |
| p: 0.005 | p < 0.001 | p: 0.071 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| ST2 | r: 0.057 | r: 0.223 | r: −0.121 | r: 0.016 | r: −0.145 | r: 0.114 | r: 0.146 | r: 0.146 | r: 0.336 | r: 0.333 | r: −0.146 | r: −0.189 | r:−0.125 |
| p: 0.314 | p < 0.001 | p: 0.032 | p: 0.787 | p < 0.001 | p: 0.058 | p: 0.010 | p: 0.011 | p < 0.001 | p < 0.001 | p: 0.011 | p: 0.001 | p: 0.027 | |
| Galectin-3 | r: 0.344 | r: 0.333 | r: −0.060 | r: 0.071 | r: −0.114 | r: 0.315 | r: 0.191 | r: 0.251 | r: 0.2910 | r: 0.252 | r: −0.161 | r: −0.313 | r: −0.646 |
| p < 0.001 | p < 0.001 | p: 0.287 | p: 0.223 | p: 0.044 | p < 0.001 | p: 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p: 0.005 | p < 0.001 | p < 0.001 |
| Variables | Univariate Cox Regression Analysis | Multivariate Cox Regression Analysis | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | C-index | HR (95% CI) | p | C-index | |
| NT-proBNP > 1000 | 7.94 (3.78–16.67) | <0.001 | 0.71 | 5.15 (2.39–11.08) | <0.001 | |
| ST2 > 34 | 4.06 (2.28–7.24) | <0.001 | 0.66 | 2.95 (1.64–5.25) | <0.001 | 0.79 |
| Galectin-3 > 17.9 | 3.18 (2.01–5.48) | <0.001 | 0.64 | 2.04 (1.22–3.41) | 0.007 | |
| Variables | Univariate Cox Regression Analysis | ||||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | C-index | |||||
| Biomarker score | 2.96 (2.21–3.95) | <0.001 | 0.78 | ||||
| Multivariate Cox Regression Analysis | |||||||
| HR (95% CI) | p | C-index | IDI | (p) | Free NRI | ||
| Biomarker score added to reference model * | 1.52 (1.06–2.17) | 0.023 | 0.87 | 0.026 | 0.031 | 0.398 | <0.001 |
| Biomarker score added to traditional biomarkers † | 2.64 (1.90–3.67) | <0.001 | 0.80 | 0.109 | <0.001 | 0.849 | <0.001 |
| Biomarker score added to BIOSTAT-CHF | 2.49 (1.84–3.36) | <0.001 | 0.80 | 0.118 | <0.001 | 0.43 | <0.001 |
| Biomarker score added to magic | 1.89 (1.35–2.65) | <0.001 | 0.83 | 0.777 | <0.001 | 0.589 | <0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande, D.; Leone, M.; Rizzo, C.; Terlizzese, P.; Parisi, G.; Gioia, M.I.; Leopizzi, T.; Segreto, A.; Guida, P.; Romito, R.; et al. A Multiparametric Approach Based on NT-proBNP, ST2, and Galectin3 for Stratifying One Year Prognosis of Chronic Heart Failure Outpatients. J. Cardiovasc. Dev. Dis. 2017, 4, 9. https://doi.org/10.3390/jcdd4030009
Grande D, Leone M, Rizzo C, Terlizzese P, Parisi G, Gioia MI, Leopizzi T, Segreto A, Guida P, Romito R, et al. A Multiparametric Approach Based on NT-proBNP, ST2, and Galectin3 for Stratifying One Year Prognosis of Chronic Heart Failure Outpatients. Journal of Cardiovascular Development and Disease. 2017; 4(3):9. https://doi.org/10.3390/jcdd4030009
Chicago/Turabian StyleGrande, Dario, Marta Leone, Caterina Rizzo, Paola Terlizzese, Giuseppe Parisi, Margherita Ilaria Gioia, Tiziana Leopizzi, Antonio Segreto, Piero Guida, Roberta Romito, and et al. 2017. "A Multiparametric Approach Based on NT-proBNP, ST2, and Galectin3 for Stratifying One Year Prognosis of Chronic Heart Failure Outpatients" Journal of Cardiovascular Development and Disease 4, no. 3: 9. https://doi.org/10.3390/jcdd4030009





