Vertebrate Left-Right Asymmetry: What Can Nodal Cascade Gene Expression Patterns Tell Us?
Abstract
:1. Introduction
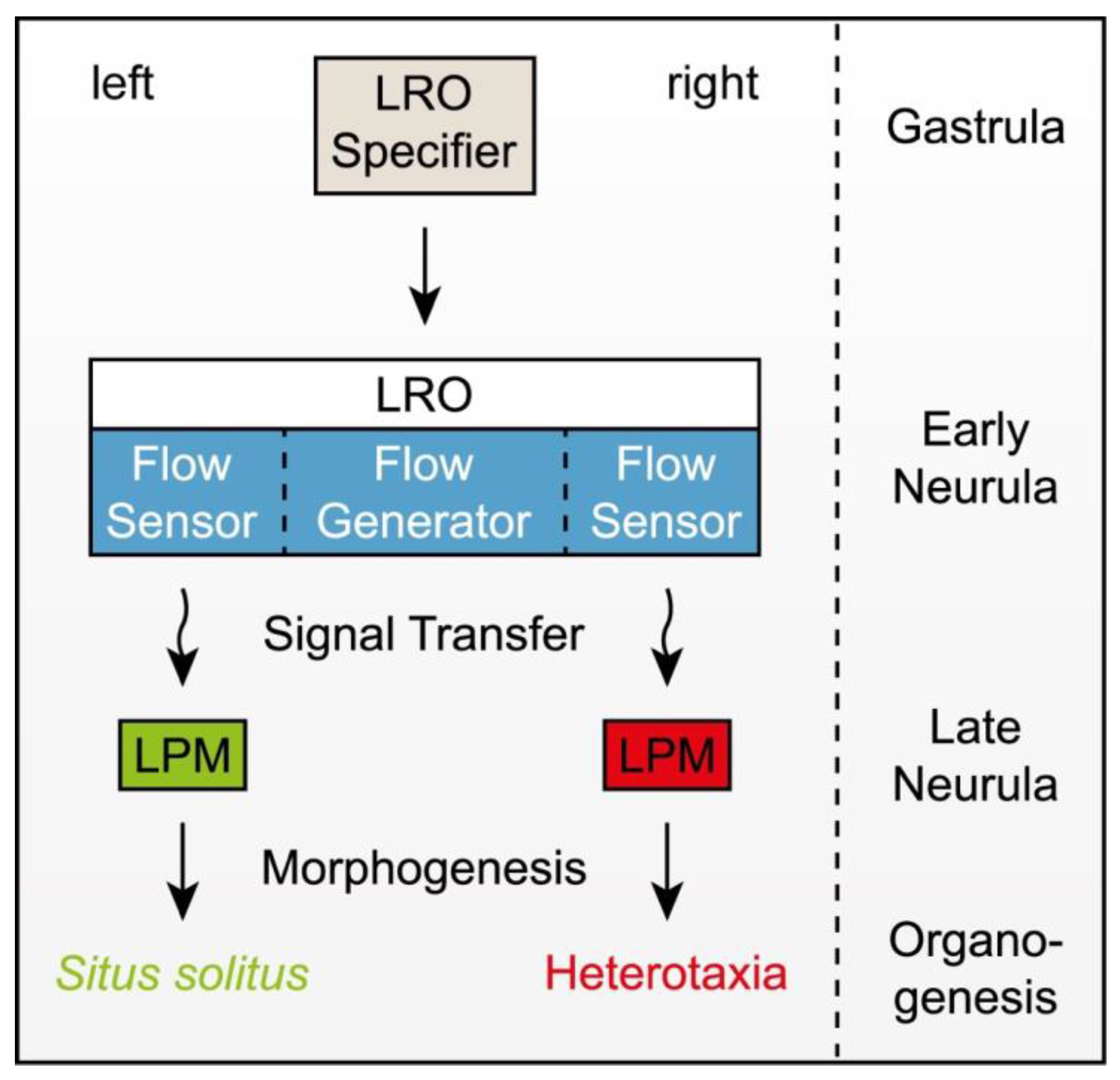
2. Cilia-Dependent Symmetry Breaking
2.1. LRO Specifier
2.2. Flow Generator
2.3. Flow Cargo
2.4. Flow Sensor
2.5. Signal Transfer
2.6. Precautions
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dareste, C. Recherches sur la Production Artificielle des Monstruosités, ou Essais de Tératogénie Expérimentale; C. Reinwald: Paris, France, 1891. [Google Scholar]
- Fol, H.; Warynsky, S. Sur la production artificielle de l’inversion viscérale ou hétérotaxie chez des embryons de poulet. CR Acad. Sci. Paris 1883, 96, 1674–1676. [Google Scholar]
- Tisler, M.; Schweickert, A.; Blum, M. Xenopus, an ideal model organism to study laterality in conjoined twins. Genesis 2017, 55. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Vogan, K.J.; Tabin, C.J.; Izpisúa Belmonte, J.C. Mechanisms of left-right determination in vertebrates. Cell 2000, 101, 9–21. [Google Scholar] [CrossRef]
- Campione, M.; Steinbeisser, H.; Schweickert, A.; Deissler, K.; van Bebber, F.; Lowe, L.A.; Nowotschin, S.; Viebahn, C.; Haffter, P.; Kuehn, M.R.; et al. The homeobox gene Pitx2: Mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development 1999, 126, 1225–1234. [Google Scholar] [PubMed]
- Levin, M.; Johnson, R.L.; Sterna, C.D.; Kuehn, M.; Tabin, C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 1995, 82, 803–814. [Google Scholar] [CrossRef]
- Nakamura, T.; Hamada, H. Left-right patterning: Conserved and divergent mechanisms. Development 2012, 139, 3257–3262. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mine, N.; Nakaguchi, E.; Mochizuki, A.; Yamamoto, M.; Yashiro, K.; Meno, C.; Hamada, H. Generation of robust left-right asymmetry in the mouse embryo requires a self-enhancement and lateral-inhibition system. Dev. Cell 2006, 11, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, H. Conserved regulation and role of Pitx2 in situs-specific morphogenesis of visceral organs. Development 2006, 133, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Inácio, J.M.; Marques, S.; Nakamura, T.; Shinohara, K.; Meno, C.; Hamada, H.; Belo, J.A. The dynamic right-to-left translocation of Cerl2 is involved in the regulation and termination of Nodal activity in the mouse node. PLoS ONE 2013, 8, e60406. [Google Scholar] [CrossRef] [PubMed]
- Oki, S.; Kitajima, K.; Marques, S.; Belo, J.A.; Yokoyama, T.; Hamada, H.; Meno, C. Reversal of left-right asymmetry induced by aberrant Nodal signaling in the node of mouse embryos. Development 2009, 136, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.L.; Danos, M.C.; Groth, T.W.; Yost, H.J. Maintenance of asymmetric nodal expression in Xenopus laevis. Dev. Genet. 1998, 23, 194–202. [Google Scholar] [CrossRef]
- Tisler, M.; Wetzel, F.; Mantino, S.; Kremnyov, S.; Thumberger, T.; Schweickert, A.; Blum, M.; Vick, P. Cilia are required for asymmetric nodal induction in the sea urchin embryo. BMC Dev. Biol. 2016, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Feistel, K.; Thumberger, T.; Schweickert, A. The evolution and conservation of left-right patterning mechanisms. Development 2014, 141, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Schweickert, A.; Vick, P.; Wright, C.V.E.; Danilchik, M.V. Symmetry breakage in the vertebrate embryo: When does it happen and how does it work? Dev. Biol. 2014, 393, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.T.; Burdine, R.D. Left–Right Patterning: Breaking Symmetry to Asymmetric Morphogenesis. Trends Genet. 2017, 33, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Supp, D.M.; Brueckner, M.; Kuehn, M.R.; Witte, D.P.; Lowe, L.A.; McGrath, J.; Corrales, J.; Potter, S.S. Targeted deletion of the ATP binding domain of left-right dynein confirms its role in specifying development of left-right asymmetries. Development 1999, 126, 5495–5504. [Google Scholar] [PubMed]
- Supp, D.M.; Witte, D.P.; Potter, S.S.; Brueckner, M. Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature 1997, 389, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Lowe, L.A.; Supp, D.M.; Sampath, K.; Yokoyama, T.; Wright, C.V.; Potter, S.S.; Overbeek, P.; Kuehn, M.R. Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature 1996, 381, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Brown, N.A.; Ho, S.Y.; Anderson, R.H. Abnormal laterality and congenital cardiac anomalies. Relations of visceral and cardiac morphologies in the iv/iv mouse. Circulation 1992, 86, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Kontarakis, Z.; Gerri, C.; Nolte, H.; Hölper, S.; Krüger, M.; Stainier, D.Y. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 2015, 524, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; De Robertis, E.M.; Wallingford, J.B.; Niehrs, C. Morpholinos: Antisense and Sensibility. Dev. Cell 2015, 35, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Nonaka, S.; Tanaka, Y.; Saijoh, Y.; Hamada, H.; Hirokawa, N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol. Cell 1999, 4, 459–468. [Google Scholar] [CrossRef]
- Grimes, D.T.; Keynton, J.L.; Buenavista, M.T.; Jin, X.; Patel, S.H.; Kyosuke, S.; Vibert, J.; Williams, D.J.; Hamada, H.; Hussain, R.; et al. Genetic Analysis Reveals a Hierarchy of Interactions between Polycystin-Encoding Genes and Genes Controlling Cilia Function during Left-Right Determination. PLoS Genet. 2016, 12, e1006070. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Levin, M. Consistent left-right asymmetry cannot be established by late organizers in Xenopus unless the late organizer is a conjoined twin. Development 2010, 137, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Levin, M. Far from solved: A perspective on what we know about early mechanisms of left-right asymmetry. Dev. Dyn. 2010, 239, 3131–3146. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Levin, M. Perspectives and open problems in the early phases of left-right patterning. Semin. Cell Dev. Biol. 2009, 20, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Melby, A.E.; Warga, R.M.; Kimmel, C.B. Specification of cell fates at the dorsal margin of the zebrafish gastrula. Development 1996, 122, 2225–2237. [Google Scholar] [PubMed]
- Shook, D.R.; Majer, C.; Keller, R. Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis and Xenopus tropicalis. Dev. Biol. 2004, 270, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Andre, P.; Muders, K.; Schweickert, A.; Fischer, A.; Bitzer, E.; Bogusch, S.; Beyer, T.; van Straaten, H.W.; Viebahn, C. Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation 2007, 75, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Bajoghli, B.; Aghaallaei, N.; Soroldoni, D.; Czerny, T. The roles of Groucho/Tle in left-right asymmetry and Kupffer’s vesicle organogenesis. Dev. Biol. 2007, 303, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Essner, J.J.; Amack, J.D.; Nyholm, M.K.; Harris, E.B.; Yost, H.J. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 2005, 132, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Beyer, T.; Weber, T.; Vick, P.; Andre, P.; Bitzer, E.; Schweickert, A. Xenopus, an ideal model system to study vertebrate left-right asymmetry. Dev. Dyn. 2009, 238, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- King, T.; Beddington, R.S.; Brown, N.A. The role of the brachyury gene in heart development and left-right specification in the mouse. Mech. Dev. 1998, 79, 29–37. [Google Scholar] [CrossRef]
- Amack, J.D.; Yost, H.J. The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Curr. Biol. 2004, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, D.; Papaioannou, V.E. Nature and extent of left/right axis defects in TWis/TWis mutant mouse embryos. Dev. Dyn. 2014, 243, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Beckers, A.; Alten, L.; Viebahn, C.; Andre, P.; Gossler, A. The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left right patterning. Proc. Natl. Acad. Sci. USA 2007, 104, 15765–15770. [Google Scholar] [CrossRef] [PubMed]
- Walentek, P.; Beyer, T.; Thumberger, T.; Schweickert, A.; Blum, M. ATP4a Is Required for Wnt-Dependent Foxj1 Expression and Leftward Flow in Xenopus Left-Right Development. Cell Rep. 2012, 1, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Walentek, P.; Schneider, I.; Schweickert, A.; Blum, M. Wnt11b is involved in cilia-mediated symmetry breakage during Xenopus left-right development. PLoS ONE 2013, 8, e73646. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Hu, J.; Chen, W.; Elliott, G.; Andre, P.; Gao, B.; Yang, Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature 2010, 466, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Antic, D.; Stubbs, J.L.; Suyama, K.; Kintner, C.; Scott, M.P.; Axelrod, J.D. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS ONE 2010, 5, e8999. [Google Scholar] [CrossRef] [PubMed]
- Pintado, P.; Sampaio, P.; Tavares, B.; Montenegro-Johnson, T.D.; Smith, D.J.; Lopes, S.S. Dynamics of cilia length in left-right development. R. Soc. Open Sci. 2017, 4, 161102. [Google Scholar] [CrossRef] [PubMed]
- Vick, P.; Schweickert, A.; Weber, T.; Eberhardt, M.; Mencl, S.; Shcherbakov, D.; Beyer, T.; Blum, M. Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev. Biol. 2009, 331, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Kawasumi, A.; Takamatsu, A.; Yoshiba, S.; Botilde, Y.; Motoyama, N.; Reith, W.; Durand, B.; Shiratori, H.; Hamada, H. Two rotating cilia in the node cavity are sufficient to break left–right symmetry in the mouse embryo. Nat. Commun. 2011, 3, 622–628. [Google Scholar] [CrossRef]
- Takeda, S.; Yonekawa, Y.; Tanaka, Y.; Okada, Y.; Nonaka, S.; Hirokawa, N. Left-right asymmetry and kinesin superfamily protein KIF3A: New insights in determination of laterality and mesoderm induction by kif3A-/- mice analysis. J. Cell Biol. 1999, 145, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Bisgrove, B.W.; Snarr, B.S.; Emrazian, A.; Yost, H.J. Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer’s vesicle are required for specification of the zebrafish left-right axis. Dev. Biol. 2005, 287, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Taulman, P.D.; Haycraft, C.J.; Balkovetz, D.F.; Yoder, B.K. Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol. Biol. Cell 2001, 12, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Yoder, B.K.; Tousson, A.; Millican, L.; Wu, J.H.; Bugg, C.E.; Schafer, J.A.; Balkovetz, D.F. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am. J. Physiol. Renal Physiol. 2002, 282, F541–F552. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Okada, Y.; Hirokawa, N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature 2005, 435, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Tanaka, Y.; Okada, Y. Left-Right Determination: Involvement of Molecular Motor KIF3, Cilia, and Nodal Flow. Cold Spring Harb. Perspect. Biol. 2009, 1, a000802. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Shiratori, H.; Saijoh, Y.; Hamada, H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 2002, 418, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhao, L.; Brueckner, M.; Sun, Z. Intraciliary calcium oscillations initiate vertebrate left-right asymmetry. Curr. Biol. 2015, 25, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.P.; Jackson, P.K. Cell biology: Calcium contradictions in cilia. Nature 2016, 531, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Delling, M.; Indzhykulian, A.A.; Liu, X.; Li, Y.; Xie, T.; Corey, D.P.; Clapham, D.E. Primary cilia are not calcium-responsive mechanosensors. Nature 2016, 531, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.; Norris, D.P.; Robertson, E.J. Nodal activity in the node governs left-right asymmetry. Genes Dev. 2002, 16, 2339–2344. [Google Scholar] [CrossRef] [PubMed]
- Sampath, K.; Robertson, E.J. Keeping a lid on nodal: Transcriptional and translational repression of nodal signalling. Open Biol. 2016, 6, 150200–150208. [Google Scholar] [CrossRef] [PubMed]
- Toyoizumi, R.; Ogasawara, T.; Takeuchi, S.; Mogi, K. Xenopus nodal related-1 is indispensable only for left-right axis determination. Int. J. Dev. Biol. 2005, 49, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Schweickert, A.; Vick, P.; Getwan, M.; Weber, T.; Schneider, I.; Eberhardt, M.; Beyer, T.; Pachur, A.; Blum, M. The nodal inhibitor Coco is a critical target of leftward flow in Xenopus. Curr. Biol. 2010, 20, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.; Borges, A.C.; Silva, A.C.; Freitas, S.; Cordenonsi, M.; Belo, J.A. The activity of the Nodal antagonist Cerl-2 in the mouse node is required for correct L/R body axis. Genes Dev. 2004, 18, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Vonica, A.; Brivanlou, A.H. The left-right axis is regulated by the interplay of Coco, Xnr1 and derrière in Xenopus embryos. Dev. Biol. 2007, 303, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Yoshiba, S.; Shiratori, H.; Kuo, I.Y.; Kawasumi, A.; Shinohara, K.; Nonaka, S.; Asai, Y.; Sasaki, G.; Belo, J.A.; Sasaki, H.; et al. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science 2012, 338, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Pennekamp, P.; Karcher, C.; Fischer, A.; Schweickert, A.; Skryabin, B.; Horst, J.; Blum, M.; Dworniczak, B. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr. Biol. 2002, 12, 938–943. [Google Scholar] [CrossRef]
- Field, S.; Riley, K.-L.; Grimes, D.T.; Hilton, H.; Simon, M.; Powles-Glover, N.; Siggers, P.; Bogani, D.; Greenfield, A.; Norris, D.P. Pkd1l1 establishes left-right asymmetry and physically interacts with Pkd2. Development 2011, 138, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Hanafusa, H.; Masuyama, N.; Kusakabe, M.; Shibuya, H.; Nishida, E. The TGF-β family member derrière is involved in regulation of the establishment of left-right asymmetry. EMBO Rep. 2000, 1, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Viotti, M.; Niu, L.; Shi, S.-H.; Hadjantonakis, A.-K. Role of the gut endoderm in relaying left-right patterning in mice. PLoS Biol. 2012, 10, e1001276. [Google Scholar] [CrossRef] [PubMed]
- Bessodes, N.; Haillot, E.; Duboc, V.; Röttinger, E.; Lahaye, F.; Lepage, T. Reciprocal signaling between the ectoderm and a mesendodermal left-right organizer directs left-right determination in the sea urchin embryo. PLoS Genet. 2012, 8, e1003121. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.; Thumberger, T.; Schweickert, A.; Blum, M. Connexin26-mediated transfer of laterality cues in Xenopus. Biol. Open 2012, 1, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Schweickert, A.; Weber, T.; Beyer, T.; Vick, P.; Bogusch, S.; Feistel, K.; Blum, M. Cilia-driven leftward flow determines laterality in Xenopus. Curr. Biol. 2007, 17, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bolfing, M.F.; Knowles, H.J.; Karnes, H.; Hackett, B.P. Foxj1 regulates asymmetric gene expression during left-right axis patterning in mice. Biochem. Biophys. Res. Commun. 2004, 324, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ng, C.P.; Habacher, H.; Roy, S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat. Genet. 2008, 40, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.I.; Peyrot, S.M.; LeBoeuf, S.; Park, T.J.; McGary, K.L.; Marcotte, E.M.; Wallingford, J.B. RFX2 is broadly required for ciliogenesis during vertebrate development. Dev. Biol. 2012, 363, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Bisgrove, B.W.; Makova, S.; Yost, H.J.; Brueckner, M. RFX2 is essential in the ciliated organ of asymmetry and an RFX2 transgene identifies a population of ciliated cells sufficient for fluid flow. Dev. Biol. 2012, 363, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Tözser, J.; Earwood, R.; Kato, A.; Brown, J.; Tanaka, K.; Didier, R.; Megraw, T.L.; Blum, M.; Kato, Y. TGF-β Signaling Regulates the Differentiation of Motile Cilia. Cell Rep. 2015, 11, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.P.; Robertson, E.J. Asymmetric and node-specific nodal expression patterns are controlled by two distinct cis-acting regulatory elements. Genes Dev. 1999, 13, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Ohi, Y.; Wright, C.V.E. Anteriorward shifting of asymmetric Xnr1 expression and contralateral communication in left-right specification in Xenopus. Dev. Biol. 2007, 301, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Marjoram, L.; Wright, C. Rapid differential transport of Nodal and Lefty on sulfated proteoglycan-rich extracellular matrix regulates left-right asymmetry in Xenopus. Development 2011, 138, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Guimier, A.; Gabriel, G.C.; Bajolle, F.; Tsang, M.; Liu, H.; Noll, A.; Schwartz, M.; El Malti, R.; Smith, L.D.; Klena, N.T.; et al. MMP21 is mutated in human heterotaxy and is required for normal left-right asymmetry in vertebrates. Nat. Genet. 2015, 47, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Gros, J.; Feistel, K.; Viebahn, C.; Blum, M.; Tabin, C.J. Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science 2009, 324, 941–944. [Google Scholar] [CrossRef] [PubMed]
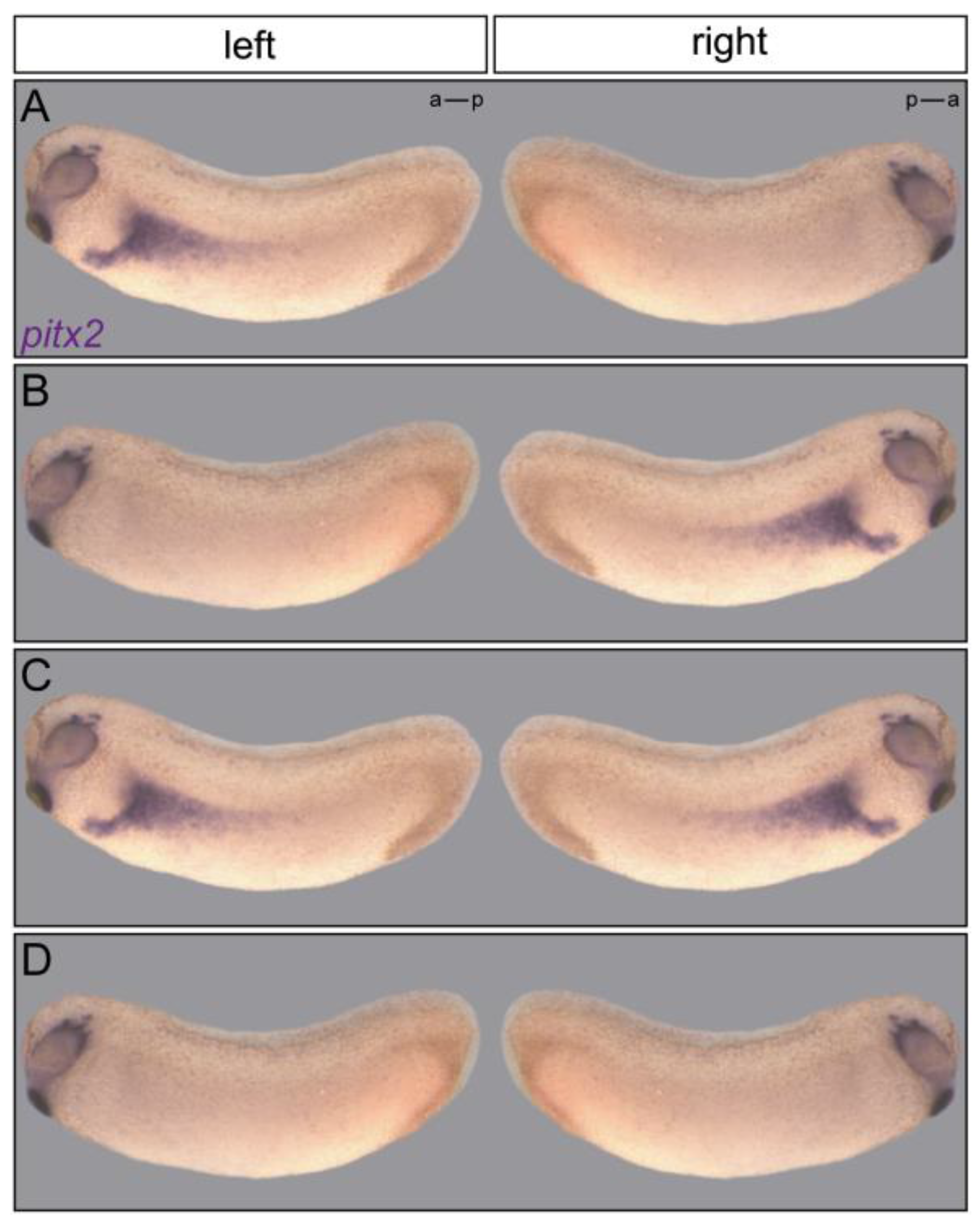
| Process | Gene/Treatment | Species | Mutant | Morphant | Nodal Cascade * | References |
|---|---|---|---|---|---|---|
| LRO specifier | brachyury | mouse | T/T | absent | [34,36] | |
| TWis/TWis | absent | |||||
| fish | ntl | absent | [35] | |||
| SK & MB | ||||||
| frog | Xbra | TBMO | absent | (unpublished) | ||
| LRO flow | methyl cellulose | Xenopus | absent | [44,68] | ||
| mouse | bilateral | |||||
| flow generator | FOXJ1 | Zebrafish | TBMO | random | [69,70] | |
| mouse | Foxj1neo/neo | random | ||||
| absent, bilateral | ||||||
| KIF3A | mouse | -/- | bilateral | [45,61] | ||
| RFX2 | Xenopus | SBMO | absent, bilateral | [71,72] | ||
| zebrafish | TBMO | absent | ||||
| cilia motility | DNAH11 | mouse | Dnah11iv/iv | random | [5,19,43] | |
| DNAH9 | Xenopus | TBMO | absent | |||
| DNAH5 | Xenopus | TBMO | absent | |||
| DYX1C1 | zebrafish | TBMO | absent | |||
| ciliapolarity | VANGL1 | mouse | Vangl1gt/gt | bilateral | [40,41] | |
| VANGL2 | Vangl2−/− | bilateral | ||||
| Xenopus | TBMO | absent | ||||
| LRO sensor | NODAL | mouse | absent | [55] | ||
| zebrafish | TBMO | random | [56,57,58] | |||
| Xenopus | TBMO | absent | ||||
| DAND5 | mouse | -/- | random | [58,59,60] | ||
| Xenopus | TBMO | bilateral | ||||
| zebrafish | TBMO | bilateral | ||||
| GDF1/GDF3 | mouse | -/- | absent | [60] | ||
| zebrafish | TBMO | absent | ||||
| zebrafish | mz-/- | absent | ||||
| PKD2 | mouse | Pkd2lacZ/lacZ | absent | [62] | ||
| zebrafish | TBMO | bilateral | ||||
| zebrafish | SBMO | absent | ||||
| PKD1L1 | mouse | Pkd1l1rks/rks | absent | [63] | ||
| medaka | abcaA12 | absent |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweickert, A.; Ott, T.; Kurz, S.; Tingler, M.; Maerker, M.; Fuhl, F.; Blum, M. Vertebrate Left-Right Asymmetry: What Can Nodal Cascade Gene Expression Patterns Tell Us? J. Cardiovasc. Dev. Dis. 2018, 5, 1. https://doi.org/10.3390/jcdd5010001
Schweickert A, Ott T, Kurz S, Tingler M, Maerker M, Fuhl F, Blum M. Vertebrate Left-Right Asymmetry: What Can Nodal Cascade Gene Expression Patterns Tell Us? Journal of Cardiovascular Development and Disease. 2018; 5(1):1. https://doi.org/10.3390/jcdd5010001
Chicago/Turabian StyleSchweickert, Axel, Tim Ott, Sabrina Kurz, Melanie Tingler, Markus Maerker, Franziska Fuhl, and Martin Blum. 2018. "Vertebrate Left-Right Asymmetry: What Can Nodal Cascade Gene Expression Patterns Tell Us?" Journal of Cardiovascular Development and Disease 5, no. 1: 1. https://doi.org/10.3390/jcdd5010001




