Is an Appreciation of Isomerism the Key to Unlocking the Mysteries of the Cardiac Findings in Heterotaxy?
Abstract
:1. Introduction
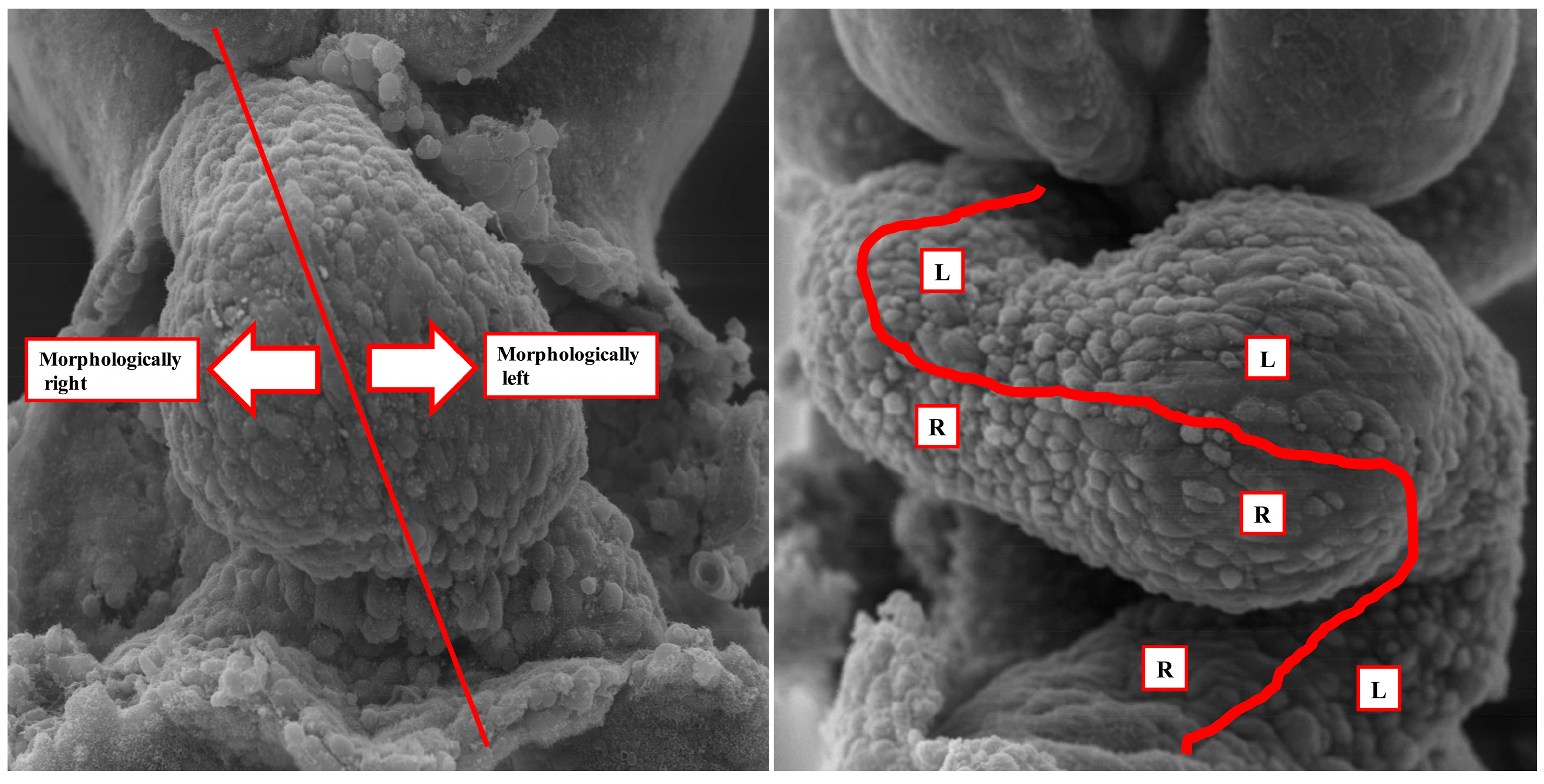
2. What Is “lateralization” and “Breaking of Symmetry”?
3. What Is Heterotaxy?
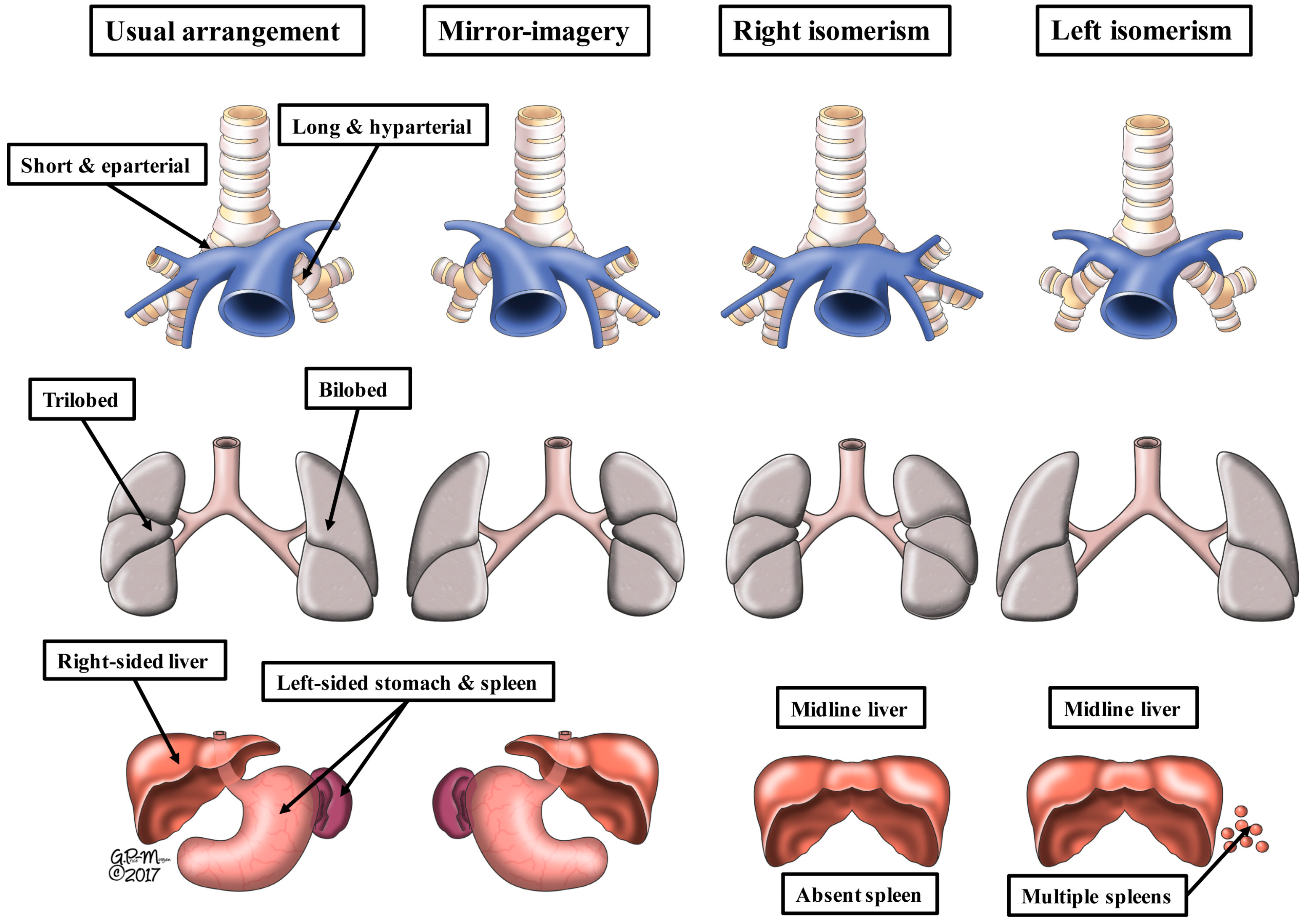
4. What Is Isomerism?
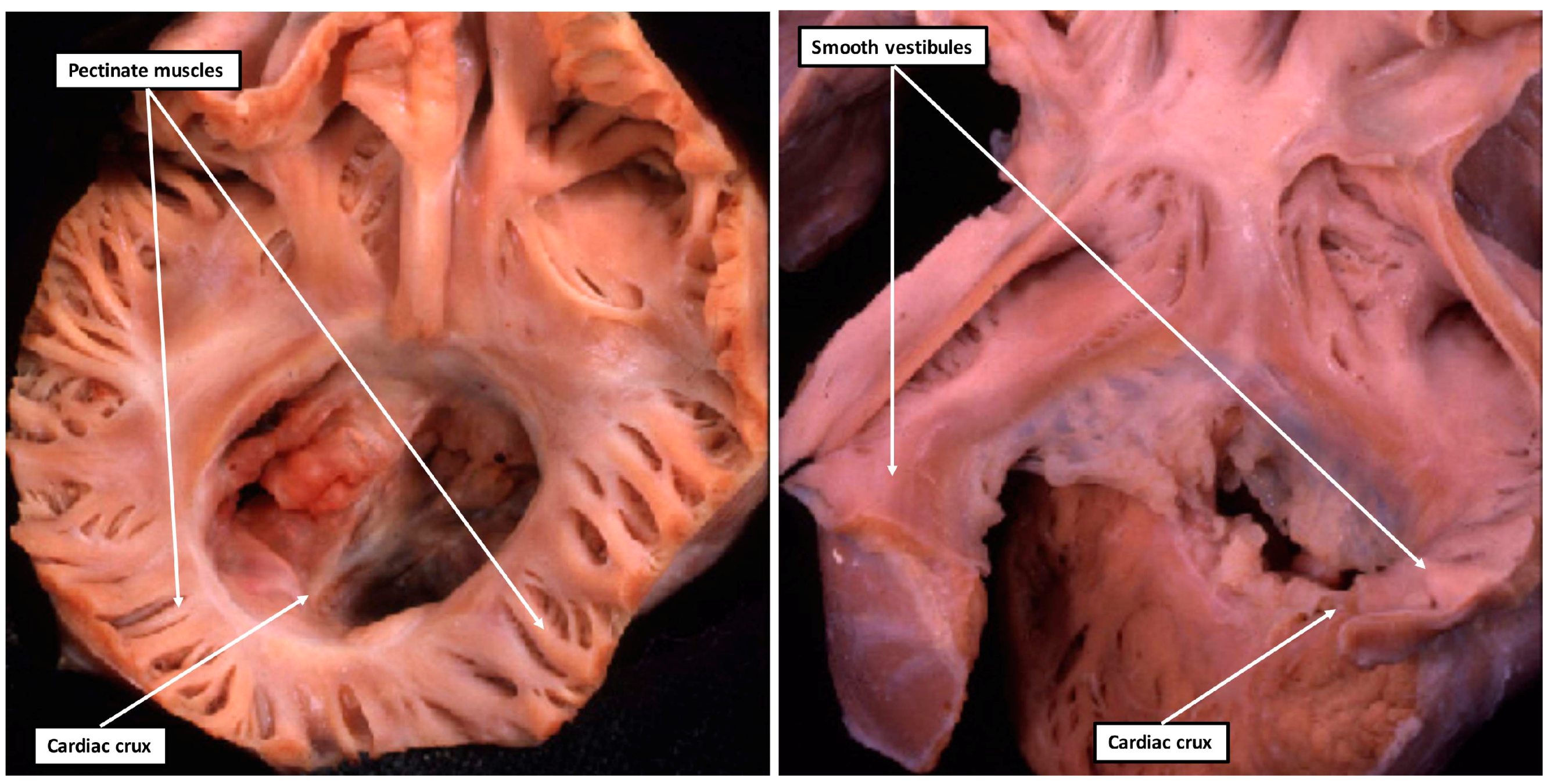
5. What Is Isomeric within the Heart?
6. How Do the Morphological Findings Relate to Cardiac Development?
7. Does “Dextrocardia” Have Any Meaning When the Heart is Congenitally Malformed?
8. Is “Transposition” a Disturbance of Laterality?
9. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Freedom, R.M.; Jaeggi, E.T.; Lim, J.S.; Anderson, R.H. Hearts with isomerism of the right atrial appendages—One of the worst forms of disease in 2005. Cardiol. Young 2005, 15, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Van Mierop, L.H.S.; Gessner, I.H.; Schiebler, G.L. Asplenia and polysplenia syndromes. Birth Defects Orig. Artic. Ser. 1972, 8, 36–52. [Google Scholar]
- Van Praagh, R.; Van Praagh, S. Atrial isomerism in the heterotaxy syndromes with asplenia, or polysplenia, or normally formed spleen: An erroneous concept. Am. J. Cardiol. 1990, 66, 1504–1506. [Google Scholar] [CrossRef]
- Loomba, R.S.; Ahmed, M.M.; Spicer, D.E.; Backer, C.L.; Anderson, R.H. Manifestations of bodily isomerism. Cardiovasc. Pathol. 2016, 30, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, C.; Loomba, R.S.; Frommelt, P.C.; Perrin, D.; Spicer, D.E.; Backer, C.; Anderson, R.H. Segregating bodily isomerism or heterotaxy: Potential echocardiographic correlations of morphological findings. Cardiol. Young 2017, 27, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Uemura, H.; Ho, S.Y.; Devine, W.A.; Anderson, R.H. Analysis of visceral heterotaxy according to splenic status, appendage morphology, or both. Am. J. Cardiol. 1995, 76, 846–849. [Google Scholar] [CrossRef]
- Loomba, R.S.; Pelech, A.N.; Shah, P.H.; Anderson, R.H. Determining bronchial morphology for the purposes of segregating so-called heterotaxy. Cardiol. Young 2016, 26, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Campione, M.; Franco, D.J. Current Perspectives in Cardiac Laterality. J. Cardiovasc. Dev. Dis. 2016, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.T.; Burdine, R.D. Left-Right Patterning: Breaking Symmetry to Asymmetric Morphogenesis. Trends Genet. 2017, 33, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.P.; Anderson, R.H.; Weinberg, P.M.; Walters, H.L., 3rd; Tchervenkov, C.I.; Del Duca, D.; Franklin, R.C.; Aiello, V.D.; Béland, M.J.; Colan, S.D.; et al. The nomenclature, definition and classification of cardiac structures in the setting of heterotaxy. Cardiol. Young 2007, 17 (Suppl. S2), 1–28. [Google Scholar] [PubMed]
- Loomba, R.S.; Hlavacek, A.M.; Spicer, D.E.; Anderson, R.H. Isomerism or heterotaxy: Which term leads to better understanding? Cardiol. Young 2015, 25, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.D.; Merriner, D.J.; Berger, S.; Rhodes, D.; Jamsai, D.; O’Bryan, M.K. Mutations in the Katnb1 Gene Cause Left–Right Asymmetry and Heart Defects. Dev. Dyn. 2017, 246, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Männer, J. The anatomy of cardiac looping: A step towards the understanding of the morphogenesis of several forms of congenital cardiac malformations. Clin. Anat. 2009, 22, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Campione, M.; Ros, M.A.; Icardo, J.M.; Piedra, E.; Christoffels, V.M.; Schweickert, A.; Blum, M.; Franco, D.; Moorman, A.F.M. Pitx2 Expression Defines a Left Cardiac Lineage of Cells: Evidence for Atrial and Ventricular Molecular Isomerism in the iv/iv Mice. Dev. Biol. 2001, 231, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Bamforth, S.D.; Bragança, J.; Farthing, C.R.; Schneider, J.E.; Broadbent, C.; Michell, A.C.; Clarke, K.; Neubauer, S.; Norris, D.; Brown, N.A.; et al. Cited2 controls left-right patterning and heart development through a Nodal-Pitx2c pathway. Nat. Genet. 2004, 36, 1189–1991. [Google Scholar] [CrossRef] [PubMed]
- Meno, C.; Shimono, A.; Saijoh, Y.; Yashiro, K.; Mochida, K.; Ohishi, S.; Noji, S.; Kondoh, H.; Hamada, H. Lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal. Cell 1998, 94, 287–297. [Google Scholar] [CrossRef]
- Macartney, F.J.; Zuberbuhler, J.R.; Anderson, R.H. Morphological considerations pertaining to recognition of atrial isomerism. Consequences for sequential chamber localisation. Br. Heart J. 1980, 44, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Shirali, G. Sequential segmental analysis. Ann. Pediatr. Cardiol. 2009, 2, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Van Praagh, R.; Van Praagh, S.; Vlad, P.; Keith, J.D. Anatomic types of congenital dextrocardia. Diagnostic and embryologic implications. Am. J. Cardiol. 1964, 13, 510–531. [Google Scholar] [CrossRef]
- Béland, M.J.; Franklin, R.C.; Aiello, V.D.; Houyel, L.; Weinberg, P.M.; Anderson, R.H. Nomenclature and classification of cardiac defects. In Pediatric and Congenital Cardiology, Cardiac Surgery and Intensive Care; da Cruz, E.M., Ivy, D., Hraska, V., Jaggers, J., Eds.; Springer: London, UK, 2016. [Google Scholar] [CrossRef]
- Liu, X.; Yagi, H.; Saeed, S.; Bais, A.S.; Gabriel, G.C.; Chen, Z.; Peterson, K.A.; Li, Y.; Schwartz, M.C.; Reynolds, W.T.; et al. The complex genetics of hypoplastic left heart syndrome. Nat. Genet. 2017, 49, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Crucean, A.; Alqahtani, A.; Barron, D.J.; Brawn, W.J.; Richardson, R.V.; O’Sullivan, J.; Anderson, R.H.; Henderson, D.J.; Chaudhry, B. Re-evaluation of hypoplastic left heart syndrome from a developmental and morphological perspective. Orphanet J. Rare Dis. 2017, 12, 138. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, R.H.; Spicer, D.E.; Loomba, R. Is an Appreciation of Isomerism the Key to Unlocking the Mysteries of the Cardiac Findings in Heterotaxy? J. Cardiovasc. Dev. Dis. 2018, 5, 11. https://doi.org/10.3390/jcdd5010011
Anderson RH, Spicer DE, Loomba R. Is an Appreciation of Isomerism the Key to Unlocking the Mysteries of the Cardiac Findings in Heterotaxy? Journal of Cardiovascular Development and Disease. 2018; 5(1):11. https://doi.org/10.3390/jcdd5010011
Chicago/Turabian StyleAnderson, Robert H., Diane E. Spicer, and Rohit Loomba. 2018. "Is an Appreciation of Isomerism the Key to Unlocking the Mysteries of the Cardiac Findings in Heterotaxy?" Journal of Cardiovascular Development and Disease 5, no. 1: 11. https://doi.org/10.3390/jcdd5010011




