Effect of a Chitosan-Based Biodegradable Middle Meatal Dressing after Endoscopic Sinus Surgery: A Prospective Randomized Comparative Study
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Results
| Demographics | |
|---|---|
| Number of Patients | 35 |
| Number of Implants (n) | 70 |
| Average Age | 39 |
| std dev | 21 |
| min age | 9 |
| max age | 77 |
| % Female | 34 |
| % Male | 66 |
| % CRSwNP | 11 |
| % CRSsNP | 89 |
| % Primary ESS | 77 |
| % Revision ESS | 23 |
| % AR | 46 |
| % DM | 3 |
| % Coagulopathic | 3 |
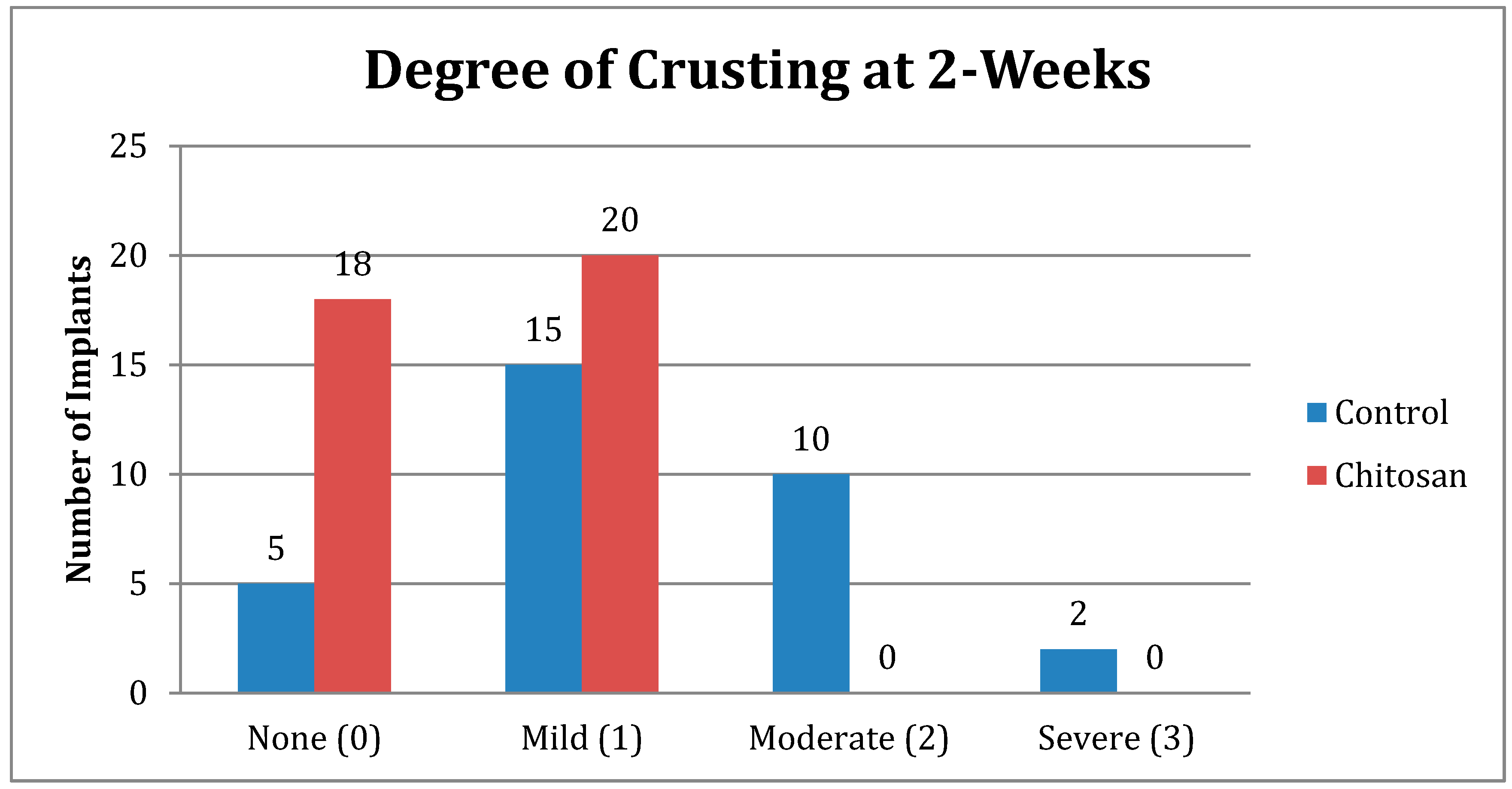
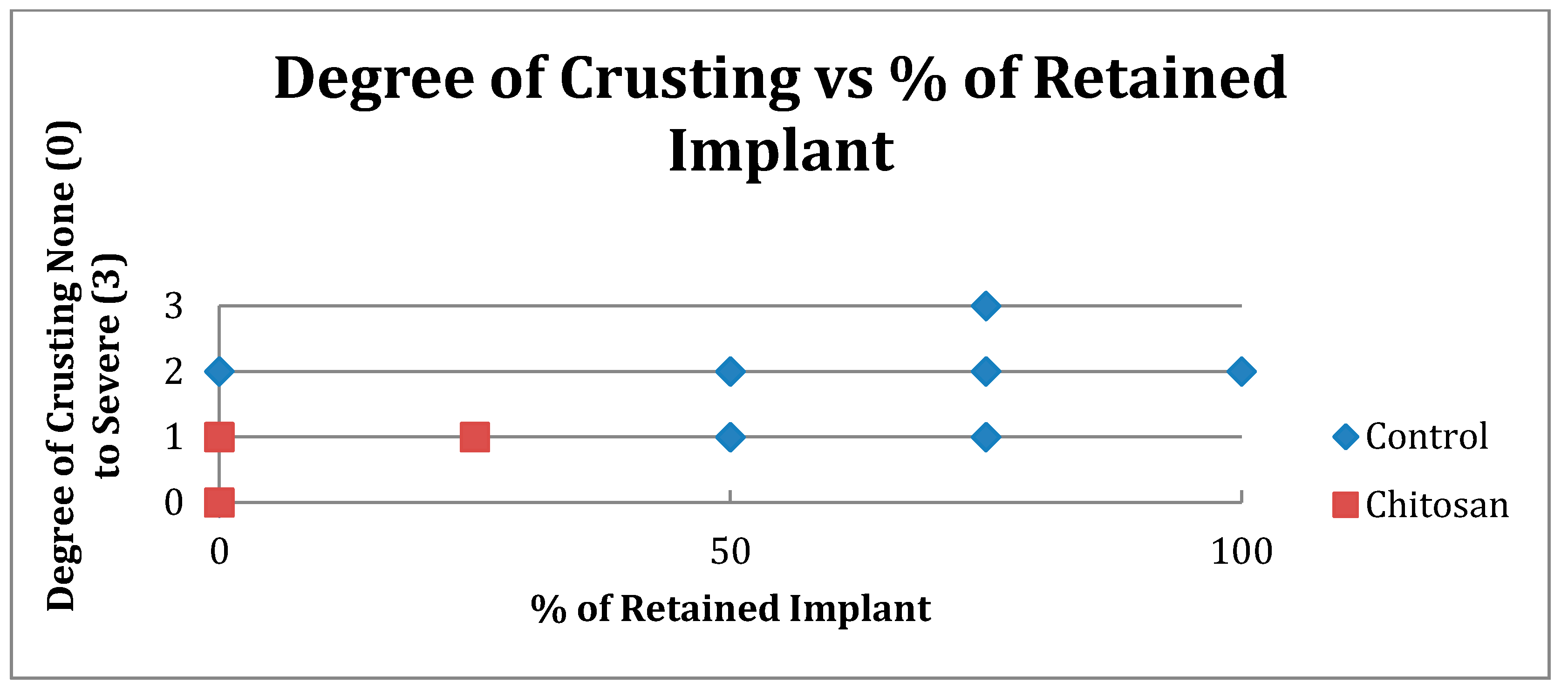
| Crusting | Control | Chitosan |
|---|---|---|
| None (0) | 5 | 18 |
| Mild (1) | 15 | 20 |
| Moderate (2) | 10 | 0 |
| Severe (3) | 2 | 0 |
| Total Number | 32 | 38 |

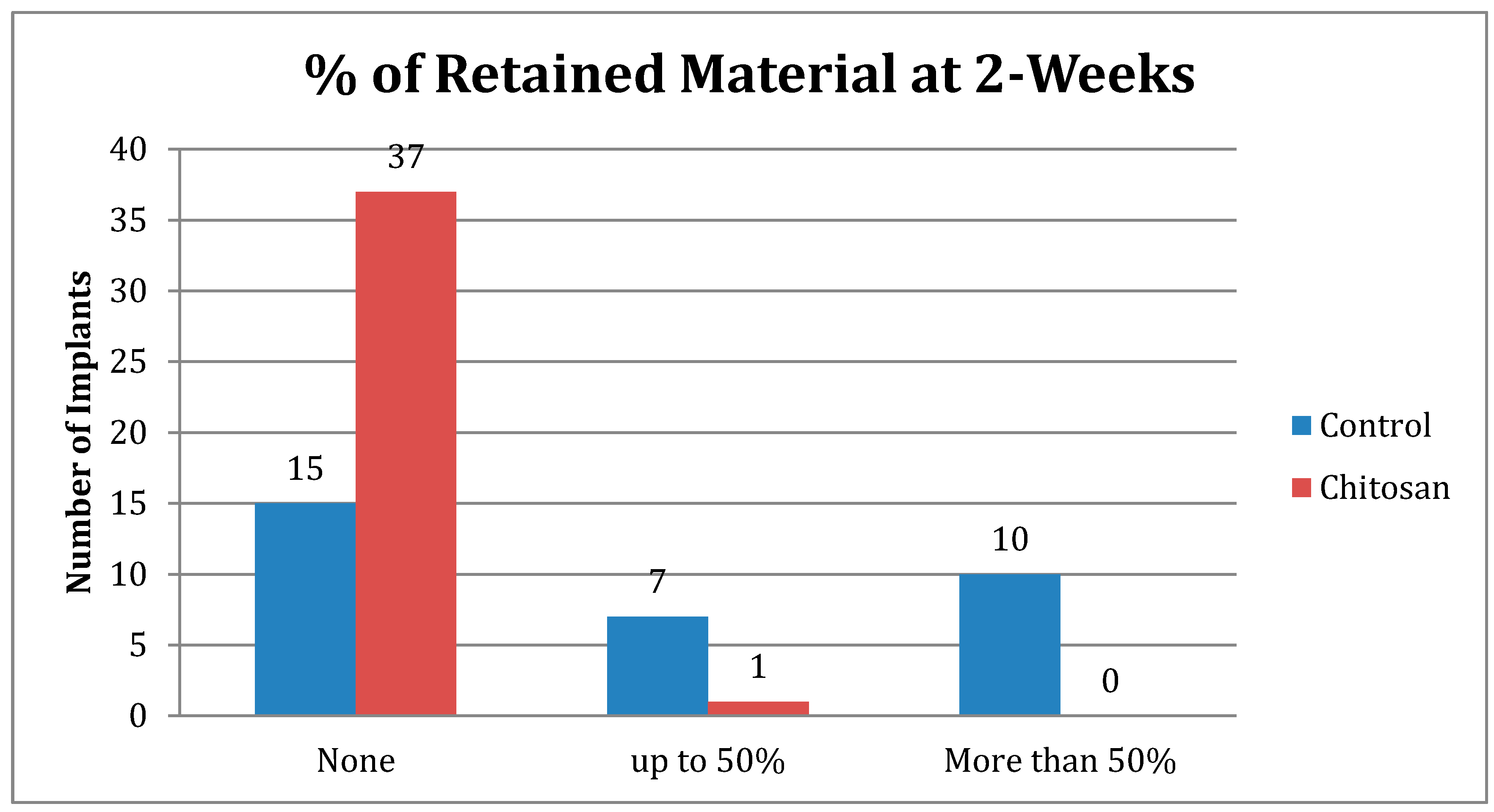
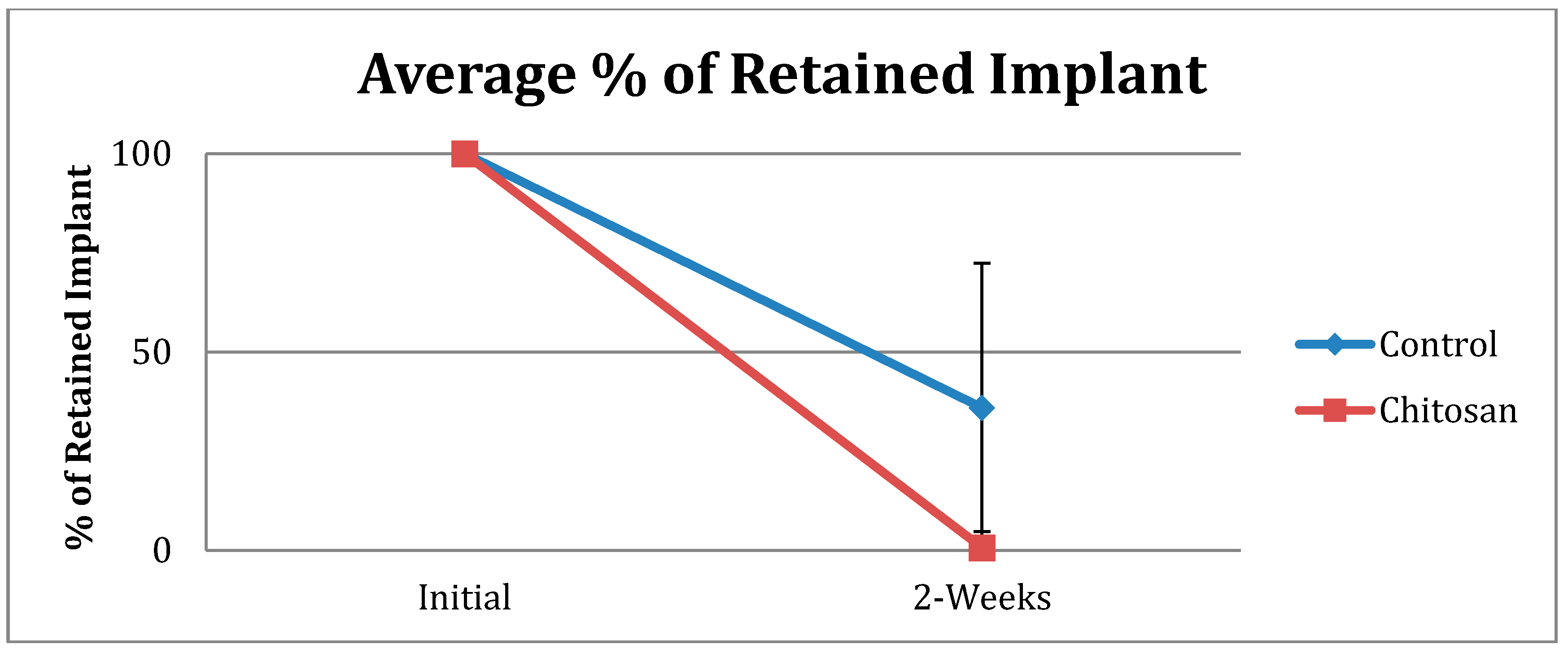
| Retained Dressing | Control | Chitosan |
|---|---|---|
| None | 15 | 37 |
| Up to 50% | 7 | 1 |
| More than 50% | 10 | 0 |
| Total Number | 32 | 38 |
| Requirement for Post-Operative Endoscopic Debridement | |||
|---|---|---|---|
| Occurrence | Total Patient | % Incidence | |
| Control | 9 | 16 | 56 |
| Chitosan | 2 | 19 | 11 |
3.2. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yan, M.; Zheng, D.; Li, Y.; Zheng, Q.; Chen, J.; Yang, B. Biodegradable nasal Packings for Endoscopic Sinus Surgery: A Systematic Review and Meta-Analysis. PLoS One 2014, 9, e115458. [Google Scholar] [CrossRef] [PubMed]
- Cote, D.W.; Wright, E.D. Triamcinolone-Impregnated Nasal Dressing Following Endoscopic Sinus Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. Laryngoscope 2010, 120, 1269–1273. [Google Scholar] [PubMed]
- Catalano, P.J.; Payne, S.; Thong, M. Clinical Evaluation of a Fully Synthetic Middle Meatal Stent for Safety and Tolerability. Otolaryngol.–Head Neck Surg. 2011, 144, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.J.; Roffman, E. Evaluation of middle meatal stenting after minimally invasive sinus techniques (MIST). Otolaryngol.–Head Neck Surg. 2003, 128, 875–881. [Google Scholar] [CrossRef]
- Weitzel, E.K.; Wormald, P.J. A scientific review of middle meatal packing/stents. Am. J. Rhinol. 2008, 22, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Kheirabadi, B.S.; Acheson, E.M.; Deguzman, R.; Sondeen, J.L.; Ryan, K.L.; Delgado, A.; Dick, E.J., Jr.; Holcomb, J.B. Hemostatic efficacy of two advanced dressings in an aortic hemorrhage model in Swine. J. Trauma. 2005, 59, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Pusateri, A.E.; McCarthy, S.J.; Gregory, K.W.; Harris, R.A.; Cardenas, L.; McManus, A.T.; Goodwin, C.W. Effect of a Chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J. Trauma 2003, 54, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.M.; Piccirillo, J.F.; Chandrasekhar, S.S.; Brook, I.; Kumar, K.A.; Kramper, M.; Orlandi, R.R.; Palmer, J.N.; Patel, Z.M.; Peters, A.; et al. Clinical Practice Guideline (Update): Adult Sinusitis. Otolaryngol.-Head Neck Surg. 2015, 152, s1–s39. [Google Scholar] [CrossRef] [PubMed]
- Kastl, K.G.; Reichert, M.; Scheithauer, M.O.; Sommer, F.; Kisser, U.; Braun, T.; Havel, M.; Leunig, A. Patient comfort following FESS and NasoPore Packing, A double blind, prospective, randomized trial. Int. J. Rhinol. 2014, 52, 60–65. [Google Scholar] [CrossRef]
- Chandra, R.K.; Conley, D.; Kern, R. The effect of FloSeal on mucosal healing after endoscopic sinus surgery: A Comparison with Thrombin-Soaked Gelatin Foam. Am. J. Rhinol. 2003, 17, 51–55. [Google Scholar] [PubMed]
- Shashoua, A.R.; Gill, D.; Barajas, R.; Dini, M.; August, C.; Kirschenbaum, G.L.; Escuardo, L. Caseating granulomata caused by hemostatic agent posing as metastatic leiomyosarcoma. J. Soc. Laparoendsc. Surg. 2009, 13, 226–228. [Google Scholar]
- Ong, S.Y.; Wu, J.; Moochhala, S.M.; Tang, M.H.; Lu, J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, Y.F.; Nie, J.; Lu, W.; Yang, L.; Zhang, Z.; Yin, H.; Wang, Z.; Hu, Q. Using absorbable chitosan hemostatic sponges as a promising surgical dressing. Int. J. Biol. Macromol. 2015, 75, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Wijewickrama, R.; Catalano, P.; Gupta, R.; Willen, S.; More, Y.; Jonnalagadda, S.; Warman, M. Efficacy of targeted middle metal antibiotics and endoscopic sinus surgery. Am. J. Rhinol. Allergy 2013, 27, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.L.; Shyu, S.S.; Wu, Y.B.; Lee, S.T.; Shyong, J.Y.; Huang, R.N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef]
- More, Y.; Willen, S.; Catalano, P. Management of early nasal polyposis using a steroid-impregnated nasal dressing. Int. Forum Allergy Rhinol. 2011, 1, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.J.; Strouch, M. The minimally invasive sinus technique: Theory and practice. Otolaryn. Clin. N. Am. 2004, 37, 401–409. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, K.; Ericksen, M.; Catalano, P. Effect of a Chitosan-Based Biodegradable Middle Meatal Dressing after Endoscopic Sinus Surgery: A Prospective Randomized Comparative Study. Sinusitis 2016, 1, 3-12. https://doi.org/10.3390/sinusitis1010003
Hsu K, Ericksen M, Catalano P. Effect of a Chitosan-Based Biodegradable Middle Meatal Dressing after Endoscopic Sinus Surgery: A Prospective Randomized Comparative Study. Sinusitis. 2016; 1(1):3-12. https://doi.org/10.3390/sinusitis1010003
Chicago/Turabian StyleHsu, Kevin, Matthew Ericksen, and Peter Catalano. 2016. "Effect of a Chitosan-Based Biodegradable Middle Meatal Dressing after Endoscopic Sinus Surgery: A Prospective Randomized Comparative Study" Sinusitis 1, no. 1: 3-12. https://doi.org/10.3390/sinusitis1010003





