Triazole Resistance in Aspergillus spp.: A Worldwide Problem?
Abstract
:1. Introduction
2. Antifungal Susceptibility Testing and Azole Resistance within Aspergillus fumigatus
3. Azole Resistance Development in Aspergillus fumigatus
4. Mechanism of Azole Resistance
5. Cyp51A Mutations
6. Azole Resistance Mechanisms are cyp51A Independent
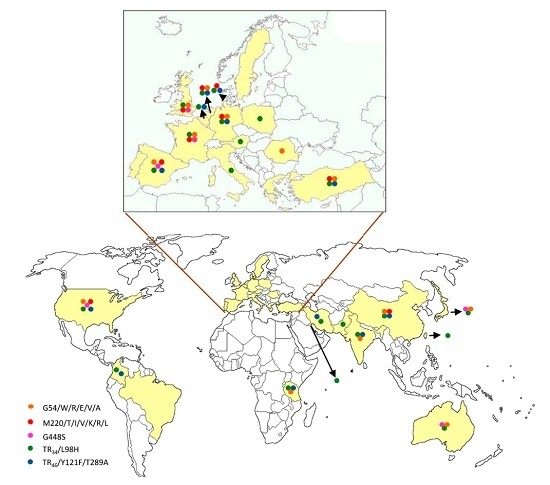
7. Prevalence of Azole Resistance in Aspergillus fumigatus throughout the World
8. Azole Resistance in Other Aspergillus Species
9. Treatment Options
10. Conclusions and Recommendations for Clinical Practice
Acknowledgments
Conflicts of Interest
References
- Chowdhary, A.; Sharma, C.; Hagen, F.; Meis, J.F. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future. Microbiol. 2014, 9, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Mavridou, E.; Mortensen, K.L.; Snelders, E.; Frimodt-Moller, N.; Khan, H.; Melchers, W.J.; Verweij, P.E. Development of azole resistance in Aspergillus fumigatus during azole therapy associated with change in virulence. PLoS ONE 2010, 5, e10080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhary, A.; Sharma, C.; Kathuria, S.; Hagen, F.; Meis, J.F. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Front. Microbiol. 2015, 6, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon-Chung, K.J.; Sugui, J.A. Aspergillus fumigatus—what makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 2013, 9, e1003743. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Kauffman, C.A. Isavuconazole: A new broad-spectrum triazole antifungal agent. Clin. Infect. Dis. 2015, 61, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Chowdhary, A.; Melchers, W.J.; Meis, J.F. Azole resistance in Aspergillus fumigatus: Can we retain the clinical use of mold-active antifungal azoles? Clin. Infect. Dis. 2016, 62, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, G.; Haas, A.; Cornely, O.A. Invasive aspergillosis: Epidemiology, diagnosis and management in immunocompromised patients. Drugs 2007, 67, 1567–1601. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Anaissie, E.J.; Denning, D.W.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Segal, B.H.; Steinbach, W.J.; Stevens, D.A.; et al. Treatment of aspergillosis: Clinical practice guidelines of the infectious diseases society of America. Clin. Infect. Dis. 2008, 46, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Pasqualotto, A.C.; Denning, D.W. Azole resistance in allergic bronchopulmonary aspergillosis and Aspergillus bronchitis. Clin. Microbiol. Infect. 2010, 16, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.T.; et al. Posaconazole vs. Fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007, 356, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A.; Diekema, D.J.; Fothergill, A.; Johnson, E.; Pelaez, T.; Pfaller, M.A.; Rinaldi, M.G.; Canton, E.; Turnidge, J. Wild-type MIC distributions and epidemiological cutoff values for the triazoles and six Aspergillus spp. For the CLSI broth microdilution method (M38-A2 document). J. Clin. Microbiol. 2010, 48, 3251–3257. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Tudela, J.L.; Alcazar-Fuoli, L.; Mellado, E.; Alastruey-Izquierdo, A.; Monzon, A.; Cuenca-Estrella, M. Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2008, 52, 2468–2472. [Google Scholar] [CrossRef] [PubMed]
- EUCAST European committee on antimicrobial susceptibility testing. Antifungal breakpoint tables for interpretation of MICs v 8.0. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/Antifungal_breakpoints_v_8.0_November_2015.pdf (accessed on 25 April 2016).
- Denning, D.W.; Venkateswarlu, K.; Oakley, K.L.; Anderson, M.J.; Manning, N.J.; Stevens, D.A.; Warnock, D.W.; Kelly, S.L. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 1997, 41, 1364–1368. [Google Scholar] [PubMed]
- Chowdhary, A.; Kathuria, S.; Xu, J.; Meis, J.F. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog. 2013, 9, e1003633. [Google Scholar] [CrossRef]
- Verweij, P.E.; Snelders, E.; Kema, G.H.; Mellado, E.; Melchers, W.J. Azole resistance in Aspergillus fumigatus: A side-effect of environmental fungicide use? Lancet Infect. Dis. 2009, 9, 789–795. [Google Scholar] [CrossRef]
- Howard, S.J.; Cerar, D.; Anderson, M.J.; Albarrag, A.; Fisher, M.C.; Pasqualotto, A.C.; Laverdiere, M.; Arendrup, M.C.; Perlin, D.S.; Denning, D.W. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 2009, 15, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Camps, S.M.; van der Linden, J.W.; Li, Y.; Kuijper, E.J.; van Dissel, J.T.; Verweij, P.E.; Melchers, W.J. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: A case study and review of the literature. Antimicrob. Agents Chemother. 2012, 56, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Dannaoui, E.; Borel, E.; Monier, M.F.; Piens, M.A.; Picot, S.; Persat, F. Acquired itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 2001, 47, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Snelders, E.; van der Lee, H.A.; Kuijpers, J.; Rijs, A.J.; Varga, J.; Samson, R.A.; Mellado, E.; Donders, A.R.; Melchers, W.J.; Verweij, P.E. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008, 5, e219. [Google Scholar] [CrossRef] [PubMed]
- Mellado, E.; Diaz-Guerra, T.M.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Identification of two different 14-alpha sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. J. Clin. Microbiol. 2001, 39, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Snelders, E.; Karawajczyk, A.; Schaftenaar, G.; Verweij, P.E.; Melchers, W.J. Azole resistance profile of amino acid changes in Aspergillus fumigatus cyp51A based on protein homology modeling. Antimicrob. Agents Chemother. 2010, 54, 2425–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Guerra, T.M.; Mellado, E.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. A point mutation in the 14alpha-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2003, 47, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.M.; Goldman, G.H.; Park, S.; Marras, S.A.; Delmas, G.; Oza, U.; Lolans, K.; Dudley, M.N.; Mann, P.A.; Perlin, D.S. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob. Agents Chemother. 2003, 47, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Mellado, E.; Garcia-Effron, G.; Alcazar-Fuoli, L.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Substitutions at methionine 220 in the 14alpha-sterol demethylase (cyp51A) of Aspergillus fumigatus are responsible for resistance in vitro to azole antifungal drugs. Antimicrob. Agents Chemother. 2004, 48, 2747–2750. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Webster, I.; Moore, C.B.; Gardiner, R.E.; Park, S.; Perlin, D.S.; Denning, D.W. Multi-azole resistance in Aspergillus fumigatus. Int. J. Antimicrob. Agents 2006, 28, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Bellete, B.; Raberin, H.; Morel, J.; Flori, P.; Hafid, J.; Manhsung, R.T. Acquired resistance to voriconazole and itraconazole in a patient with pulmonary aspergilloma. Med. Mycol. 2010, 48, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Manavathu, E.; Espinel-Ingroff, A.; Alangaden, G.; Chandrasekar, P. Molecular studies on voriconazole resistance in a clinical isolate of Aspergillus fumigatus. In Proceedings of 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 14–17 September 2003.
- Pelaez, T.; Gijon, P.; Bunsow, E.; Bouza, E.; Sanchez-Yebra, W.; Valerio, M.; Gama, B.; Cuenca-Estrella, M.; Mellado, E. Resistance to voriconazole due to a G448S substitution in Aspergillus fumigatus in a patient with cerebral aspergillosis. J. Clin. Microbiol. 2012, 50, 2531–2534. [Google Scholar] [CrossRef] [PubMed]
- Alanio, A.; Sitterle, E.; Liance, M.; Farrugia, C.; Foulet, F.; Botterel, F.; Hicheri, Y.; Cordonnier, C.; Costa, J.M.; Bretagne, S. Low prevalence of resistance to azoles in Aspergillus fumigatus in a French cohort of patients treated for haematological malignancies. J. Antimicrob. Chemother. 2011, 66, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Albarrag, A.M.; Anderson, M.J.; Howard, S.J.; Robson, G.D.; Warn, P.A.; Sanglard, D.; Denning, D.W. Interrogation of related clinical pan-azole-resistant Aspergillus fumigatus strains: G138C, Y431C, and G434C single nucleotide polymorphisms in cyp51A, upregulation of cyp51A, and integration and activation of transposon Atf1 in the cyp51a promoter. Antimicrob. Agents Chemother. 2011, 55, 5113–5121. [Google Scholar] [CrossRef] [PubMed]
- Bader, O.; Weig, M.; Reichard, U.; Lugert, R.; Kuhns, M.; Christner, M.; Held, J.; Peter, S.; Schumacher, U.; Buchheidt, D.; et al. cyp51A-based mechanisms of Aspergillus fumigatus azole drug resistance present in clinical samples from Germany. Antimicrob. Agents Chemother. 2013, 57, 3513–3517. [Google Scholar] [PubMed]
- Bueid, A.; Howard, S.J.; Moore, C.B.; Richardson, M.D.; Harrison, E.; Bowyer, P.; Denning, D.W. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J. Antimicrob. Chemother. 2010, 65, 2116–2118. [Google Scholar] [CrossRef] [PubMed]
- Escribano, P.; Recio, S.; Pelaez, T.; Bouza, E.; Guinea, J. Aspergillus fumigatus strains with mutations in the cyp51A gene do not always show phenotypic resistance to itraconazole, voriconazole, or posaconazole. Antimicrob. Agents Chemother. 2011, 55, 2460–2462. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.L.; Jensen, R.H.; Johansen, H.K.; Skov, M.; Pressler, T.; Howard, S.J.; Leatherbarrow, H.; Mellado, E.; Arendrup, M.C. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: A laboratory-based study with focus on Aspergillus fumigatus azole resistance. J. Clin. Microbiol. 2011, 49, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Prigitano, A.; Venier, V.; Cogliati, M.; De, L.G.; Esposto, M.C.; Tortorano, A.M. Azole-resistant Aspergillus fumigatus in the environment of Northern Italy, May 2011 to June 2012. Euro. Surveill 2014, 19, 20747. [Google Scholar] [CrossRef] [PubMed]
- Mellado, E.; Garcia-Effron, G.; Alcazar-Fuoli, L.; Melchers, W.J.; Verweij, P.E.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob. Agents Chemother. 2007, 51, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Mellado, E.; Melchers, W.J. Multiple-triazole-resistant aspergillosis. N. Engl. J. Med. 2007, 356, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.; Lagrou, K.; Verweij, P.E. Azole resistance in Aspergillus fumigatus: A growing public health concern. Curr. Opin. Infect. Dis. 2013, 26, 493–500. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, J.W.; Camps, S.M.; Kampinga, G.A.; Arends, J.P.; Debets-Ossenkopp, Y.J.; Haas, P.J.; Rijnders, B.J.; Kuijper, E.J.; van Tiel, F.H.; Varga, J.; et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin. Infect. Dis. 2013, 57, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Hodiamont, C.J.; Dolman, K.M.; Ten Berge, I.J.; Melchers, W.J.; Verweij, P.E.; Pajkrt, D. Multiple-azole-resistant Aspergillus fumigatus osteomyelitis in a patient with chronic granulomatous disease successfully treated with long-term oral posaconazole and surgery. Med. Mycol. 2009, 47, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Mellado, E.; Alcazar-Fuoli, L.; Pajkrt, D.; Verweij, P.E.; Melchers, W.J.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Alterations of the cyp51A gene promoter contribute to Aspergillus fumigatus multiple triazole resistance. In Proceedings of 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 17–20 September 2007.
- Mosquera, J.; Denning, D.W. Azole cross-resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2002, 46, 556–557. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Madison, V.; Chau, A.S.; Loebenberg, D.; Palermo, R.E.; McNicholas, P.M. Three-dimensional models of wild-type and mutated forms of cytochrome P450 14α-sterol demethylases from Aspergillus fumigatus and Candida albicans provide insights into posaconazole binding. Antimicrob. Agents Chemother. 2004, 48, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Gregson, L.; Goodwin, J.; Johnson, A.; McEntee, L.; Moore, C.B.; Richardson, M.; Hope, W.W.; Howard, S.J. In vitro susceptibility of Aspergillus fumigatus to isavuconazole: Correlation with itraconazole, voriconazole, and posaconazole. Antimicrob. Agents Chemother. 2013, 57, 5778–5780. [Google Scholar] [CrossRef] [PubMed]
- Snelders, E.; Camps, S.M.; Karawajczyk, A.; Schaftenaar, G.; Kema, G.H.; van der Lee, H.A.; Klaassen, C.H.; Melchers, W.J.; Verweij, P.E. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS ONE 2012, 7, e31801. [Google Scholar] [CrossRef] [PubMed]
- Bader, O.; Tunnermann, J.; Dudakova, A.; Tangwattanachuleeporn, M.; Weig, M.; Gross, U. Environmental isolates of azole-resistant Aspergillus fumigatus in Germany. Antimicrob. Agents Chemother. 2015, 59, 4356–4359. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.R.; Baixench, M.T.; Amsellem, M.; Audureau, E.; Chapron, J.; Kanaan, R.; Honore, I.; Dupouy-Camet, J.; Dusser, D.; Klaassen, C.H.; et al. High prevalence of azole-resistant Aspergillus fumigatus in adults with cystic fibrosis exposed to itraconazole. Antimicrob. Agents Chemother. 2012, 56, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Escribano, P.; Recio, S.; Pelaez, T.; Gonzalez-Rivera, M.; Bouza, E.; Guinea, J. In vitro acquisition of secondary azole resistance in Aspergillus fumigatus isolates after prolonged exposure to itraconazole: Presence of heteroresistant populations. Antimicrob. Agents Chemother. 2012, 56, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Morio, F.; Aubin, G.G.; Danner-Boucher, I.; Haloun, A.; Sacchetto, E.; Garcia-Hermoso, D.; Bretagne, S.; Miegeville, M.; Le, P.P. High prevalence of triazole resistance in Aspergillus fumigatus, especially mediated by TR34/L98H, in a French cohort of patients with cystic fibrosis. J. Antimicrob. Chemother. 2012, 67, 1870–1873. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Hamprecht, A.; Vehreschild, M.J.; Cornely, O.A.; Buchheidt, D.; Spiess, B.; Koldehoff, M.; Buer, J.; Meis, J.F.; Rath, P.M. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J. Antimicrob. Chemother. 2015, 70, 1522–1526. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, J.W.; Arendrup, M.C.; Warris, A.; Lagrou, K.; Pelloux, H.; Hauser, P.M.; Chryssanthou, E.; Mellado, E.; Kidd, S.E.; Tortorano, A.M.; et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg. Infect. Dis. 2015, 21, 1041–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Linden, J.W.; Snelders, E.; Kampinga, G.A.; Rijnders, B.J.; Mattsson, E.; Debets-Ossenkopp, Y.J.; Kuijper, E.J.; van Tiel, F.H.; Melchers, W.J.; Verweij, P.E. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg. Infect. Dis. 2011, 17, 1846–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Li, H.; Li, R.; Bu, D.; Wan, Z. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J. Antimicrob. Chemother. 2005, 55, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, M.; Izumikawa, K.; Hirano, K.; Ide, S.; Mihara, T.; Hosogaya, N.; Takazono, T.; Morinaga, Y.; Nakamura, S.; Kurihara, S.; et al. Correlation between triazole treatment history and susceptibility in clinically isolated Aspergillus fumigatus. Antimicrob. Agents Chemother. 2012, 56, 4870–4875. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, M.; Izumikawa, K.; Minematsu, A.; Hirano, K.; Iwanaga, N.; Ide, S.; Mihara, T.; Hosogaya, N.; Takazono, T.; Morinaga, Y.; et al. Antifungal susceptibilities of Aspergillus fumigatus clinical isolates obtained in Nagasaki, Japan. Antimicrob. Agents Chemother. 2012, 56, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, W.; Li, L.; Wan, Z.; Li, R.; Liu, W. Clinical itraconazole-resistant strains of Aspergillus fumigatus, isolated serially from a lung aspergilloma patient with pulmonary tuberculosis, can be detected with real-time PCR method. Mycopathologia 2010, 169, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Gil, V.G.; Gutierrez, F.; Lindner, J.R.; Albataineh, M.T.; McCarthy, D.I.; Sanders, C.; Fan, H.; Fothergill, A.W.; Sutton, D.A. First detection of TR34/L98H and TR46/Y121F/T289A cyp51 mutations in Aspergillus fumigatus isolates in the United States. J. Clin. Microbiol. 2016, 54, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.E.; Goeman, E.; Meis, J.F.; Slavin, M.A.; Verweij, P.E. Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses 2015, 58, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; van Koningsbruggen-Rietschel, S.; Rietschel, E.; Vehreschild, M.J.; Wisplinghoff, H.; Kronke, M.; Hamprecht, A. Prevalence and molecular characterization of azole resistance in Aspergillus spp. Isolates from German cystic fibrosis patients. J. Antimicrob. Chemother. 2014, 69, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Stensvold, C.R.; Perlin, D.S.; Arendrup, M.C. Azole resistance in Aspergillus fumigatus from bronchoalveolar lavage fluid samples of patients with chronic diseases. J. Antimicrob. Chemother. 2013, 68, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.D.; Reiss, E.; Hagen, F.; Meis, J.F.; Lockhart, S.R. Passive surveillance for azole-resistant Aspergillus fumigatus, United States, 2011–2013. Emerg. Infect. Dis. 2014, 20, 1498–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escribano, P.; Pelaez, T.; Munoz, P.; Bouza, E.; Guinea, J. Is azole resistance in Aspergillus fumigatus a problem in Spain? Antimicrob. Agents Chemother. 2013, 57, 2815–2820. [Google Scholar] [CrossRef] [PubMed]
- Toyotome, T.; Fujiwara, T.; Kida, H.; Matsumoto, M.; Wada, T.; Komatsu, R. Susceptibility to azoles in clinical isolates of Aspergillus fumigatus and A. tubingensis fron Obihiro, Japan. In Proceedings of 7th Advances Against Aspergillosis, Manchester, UK, 3–5 March 2016.
- Abdolrasouli, A.; Rhodes, J.; Beale, M.A.; Hagen, F.; Rogers, T.R.; Chowdhary, A.; Meis, J.F.; Armstrong-James, D.; Fisher, M.C. Genomic context of azole resistance mutations in Aspergillus fumigatus determined using whole-genome sequencing. MBio. 2015, 6, e00536. [Google Scholar] [PubMed]
- Astvad, K.M.; Jensen, R.H.; Hassan, T.M.; Mathiasen, E.G.; Thomsen, G.M.; Pedersen, U.G.; Christensen, M.; Hilberg, O.; Arendrup, M.C. First detection of TR46/Y121F/T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob. Agents Chemother. 2014, 58, 5096–5101. [Google Scholar] [CrossRef] [PubMed]
- Fuhren, J.; Voskuil, W.S.; Boel, C.H.; Haas, P.J.; Hagen, F.; Meis, J.F.; Kusters, J.G. High prevalence of azole resistance in Aspergillus fumigatus isolates from high-risk patients. J. Antimicrob. Chemother. 2015, 70, 2894–2898. [Google Scholar] [CrossRef] [PubMed]
- Jeurissen, A.; Cooreman, S.; Van, K.W.; Van, L.J.; Vanhove, P.; Lagrou, K.; Heytens, L. Invasive pulmonary aspergillosis due to a multi-azole resistant Aspergillus fumigatus. Acta Clin. Belg. 2012, 67, 46–48. [Google Scholar] [PubMed]
- Kurzyk, E.M.; Nawrot, U.; Mroczynska, M.; Wlodarczyk, K.; Ussowicz, M.; Zdziarski, P.; Arendrup, M.C.; Brillowska-Dabrowska, A. Detection of clinical Aspergillus fumigatus isolates resistant to triazoles. In Proceedings of the 7th Trends in Medical Mycology, Lisbon, Portugal, 9–12 October 2015; Volume 58, pp. 53–54.
- Mortensen, K.L.; Mellado, E.; Lass-Florl, C.; Rodriguez-Tudela, J.L.; Johansen, H.K.; Arendrup, M.C. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob. Agents Chemother. 2010, 54, 4545–4549. [Google Scholar] [CrossRef] [PubMed]
- Ozmerdiven, G.E.; Ak, S.; Ener, B.; Agca, H.; Cilo, B.D.; Tunca, B.; Akalin, H. First determination of azole resistance in Aspergillus fumigatus strains carrying the TR34/L98H mutations in Turkey. J. Infect. Chemother. 2015, 21, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Rath, P.M.; Buchheidt, D.; Spiess, B.; Arfanis, E.; Buer, J.; Steinmann, J. First reported case of azole-resistant Aspergillus fumigatus due to the TR34/L98H mutation in Germany. Antimicrob. Agents Chemother. 2012, 56, 6060–6061. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, S.; Daguindau, E.; Grenouillet, F.; Deconinck, E.; Bellanger, A.P.; Garcia-Hermoso, D.; Bretagne, S.; Reboux, G.; Millon, L. Azole-resistant Aspergillus fumigatus isolate with the TR34/L98H mutation in both a fungicide-sprayed field and the lung of a hematopoietic stem cell transplant recipient with invasive aspergillosis. J. Clin. Microbiol. 2014, 52, 1724–1726. [Google Scholar] [CrossRef] [PubMed]
- Snelders, E.; Huis In 't Veld, R.A.; Rijs, A.J.; Kema, G.H.; Melchers, W.J.; Verweij, P.E. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microbiol. 2009, 75, 4053–4057. [Google Scholar] [CrossRef] [PubMed]
- van Ingen, J.; van der Lee, H.A.; Rijs, T.A.; Zoll, J.; Leenstra, T.; Melchers, W.J.; Verweij, P.E. Azole, polyene and echinocandin MIC distributions for wild-type, TR34/L98H and TR46/Y121F/T289A Aspergillus fumigatus isolates in The Netherlands. J. Antimicrob. Chemother. 2015, 70, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.; Maertens, J.; De, B.A.; Nulens, E.; Boelens, J.; Surmont, I.; Mertens, A.; Boel, A.; Lagrou, K. Nationwide surveillance of azole resistance in Aspergillus diseases. Antimicrob. Agents Chemother. 2015, 59, 4569–4576. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Joseph, L.; Hagen, F.; Meis, J.F.; Khan, Z. Concomitant occurrence of itraconazole-resistant and -susceptible strains of Aspergillus fumigatus in routine cultures. J. Antimicrob. Chemother. 2015, 70, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, Z.; Hagen, F.; Meis, J.F. Occurrence of triazole-resistant Aspergillus fumigatus with TR34/L98H mutations in outdoor and hospital environment in Kuwait. Environ. Res. 2014, 133, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Badali, H.; Vaezi, A.; Haghani, I.; Yazdanparast, S.A.; Hedayati, M.T.; Mousavi, B.; Ansari, S.; Hagen, F.; Meis, J.F.; Chowdhary, A. Environmental study of azole-resistant Aspergillus fumigatus with TR34/L98H mutations in the cyp51A gene in Iran. Mycoses 2013, 56, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Kathuria, S.; Randhawa, H.S.; Gaur, S.N.; Klaassen, C.H.; Meis, J.F. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR34/L98H mutations in the cyp51A gene in India. J. Antimicrob. Chemother. 2012, 67, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Kathuria, S.; Xu, J.; Sharma, C.; Sundar, G.; Singh, P.K.; Gaur, S.N.; Hagen, F.; Klaassen, C.H.; Meis, J.F. Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR34/L98H mutations in the cyp51A gene in India. PLoS ONE 2012, 7, e52871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhary, A.; Sharma, C.; Kathuria, S.; Hagen, F.; Meis, J.F. Azole-resistant Aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J. Antimicrob. Chemother. 2014, 69, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, R.; Zhang, L.; Li, D.; Lv, G.; Shen, Y.; Zheng, H.; Zhang, Q.; Zhao, J.; Zheng, N.; et al. Multiple cyp51A-based mechanisms identified in azole-resistant isolates of Aspergillus fumigatus from China. Antimicrob. Agents Chemother. 2015, 59, 4321–4325. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Hashemi, S.J.; Zoll, J.; Melchers, W.J.; Rafati, H.; Dehghan, P.; Rezaie, S.; Tolooe, A.; Tamadon, Y.; van der Lee, H.A.; et al. Quantitative analysis of single-nucleotide polymorphism for rapid detection of TR34. Antimicrob. Agents Chemother. 2015, 60, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Perveen, I.; Sehar, S.; Naz, I.; Ahmed, S. Prospective evaluation of azole resistance in Aspergillus fumigatus clinical isolates in Pakistan. In Proceedings of 7th Advances Against Aspergillosis, Manchester, UK, 3–5 March 2016.
- Seyedmousavi, S.; Hashemi, S.J.; Zibafar, E.; Zoll, J.; Hedayati, M.T.; Mouton, J.W.; Melchers, W.J.; Verweij, P.E. Azole-resistant Aspergillus fumigatus, Iran. Emerg. Infect. Dis. 2013, 19, 832–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.J.; Wang, H.C.; Lee, J.C.; Lo, H.J.; Dai, C.T.; Chou, P.H.; Ko, W.C.; Chen, Y.C. Azole-resistant Aspergillus fumigatus isolates carrying TR34/L98H mutations in Taiwan. Mycoses 2015, 58, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Le Pape, P.; Lavergne, R.A.; Morio, F.; Alvarez-Moreno, C. Multiple fungicide-driven alterations in azole-resistant Aspergillus fumigatus, Colombia, 2015. Emerg. Infect. Dis. 2016, 22, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Garcia-Gil, V.; Lindner, J.R.; Sanders, C.; Fan, H.; Sutton, D.A.; Fothergill, A.W. Evaluation of cyp5A mechanisms of azole resistance in Aspergillus fumigatus isolates from the United States. In Proceedings of the 7th Trends in Medical Mycology, Lisbon, Portugal, 9–12 October 2015; Volume 58, p. 55.
- Chowdhary, A.; Sharma, C.; van den Boom, M.; Yntema, J.B.; Hagen, F.; Verweij, P.E.; Meis, J.F. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J. Antimicrob. Chemother. 2014, 69, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, R.A.; Morio, F.; Favennec, L.; Dominique, S.; Meis, J.F.; Gargala, G.; Verweij, P.E.; Le Pape, P. First description of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in France. Antimicrob. Agents Chemother. 2015, 59, 4331–4335. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, I.; Dodemont, M.; Lagrou, K.; Jacobs, F.; Etienne, I.; Denis, O. New case of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in Belgium. J. Antimicrob. Chemother. 2014, 69, 3439–3440. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, T.; Monteiro, M.C.; Garcia-Rubio, R.; Bouza, E.; Gomez-Lopez, A.; Mellado, E. First detection of Aspergillus fumigatus azole-resistant strain due to cyp51A TR46/Y121F/T289A in an azole-naive patient in Spain. New Microbes. New Infect. 2015, 6, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.; Maertens, J.; Schoemans, H.; Lagrou, K. Azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation emerging in Belgium, July 2012. Euro. Surveill 2012, 17, pii20326. [Google Scholar]
- Hagiwara, D.; Takahashi, H.; Fujimoto, M.; Sugahara, M.; Misawa, Y.; Gonoi, T.; Itoyama, S.; Watanabe, A.; Kamei, K. Multi-azole resistant Aspergillus fumigatus harboring cyp51A TR46/Y121F/T289A isolated in Japan. J. Infect. Chemother. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Lu, Z.; Li, P.; Zhang, Q.; Jia, T.; Zhao, J.; Tian, S.; Han, X.; Chen, F.; et al. Emergence of TR46/Y121F/T289A in an Aspergillus fumigatus isolate from a Chinese patient. Antimicrob Agents Chemother 2015, 59, 7148–7150. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, D.; Takahashi, H.; Watanabe, A.; Takahashi-Nakaguchi, A.; Kawamoto, S.; Kamei, K.; Gonoi, T. Whole-genome comparison of Aspergillus fumigatus strains serially isolated from patients with aspergillosis. J. Clin. Microbiol. 2014, 52, 4202–4209. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Park, S.; Lass-Florl, C.; Fraczek, M.G.; Kirwan, M.; Gore, R.; Smith, J.; Bueid, A.; Moore, C.B.; Bowyer, P.; et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin. Infect. Dis. 2011, 52, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Bueid, A.; Moore, C.B.; Denning, D.W.; Bowyer, P. High-level expression of cyp51B in azole-resistant clinical Aspergillus fumigatus isolates. J. Antimicrob. Chemother. 2013. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Kontoyiannis, D.P. Update on antifungal drug resistance mechanisms of Aspergillus fumigatus. Drug Resist. Updat. 2005, 8, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.B.; Peery, R.B.; Skatrud, P.L. Genes encoding multiple drug resistance-like proteins in Aspergillus fumigatus and Aspergillus flavus. Gene 1997, 200, 11–23. [Google Scholar] [CrossRef]
- Slaven, J.W.; Anderson, M.J.; Sanglard, D.; Dixon, G.K.; Bille, J.; Roberts, I.S.; Denning, D.W. Increased expression of a novel Aspergillus fumigatus ABC transporter gene, atrF, in the presence of itraconazole in an itraconazole resistant clinical isolate. Fungal. Genet. Biol. 2002, 36, 199–206. [Google Scholar] [CrossRef]
- Rajendran, R.; Mowat, E.; McCulloch, E.; Lappin, D.F.; Jones, B.; Lang, S.; Majithiya, J.B.; Warn, P.; Williams, C.; Ramage, G. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob. Agents Chemother. 2011, 55, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- da Silva Ferreira, M.E.; Malavazi, I.; Savoldi, M.; Brakhage, A.A.; Goldman, M.H.; Kim, H.S.; Nierman, W.C.; Goldman, G.H. Transcriptome analysis of Aspergillus fumigatus exposed to voriconazole. Curr. Genet. 2006, 50, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.G.; Bromley, M.; Buied, A.; Moore, C.B.; Rajendran, R.; Rautemaa, R.; Ramage, G.; Denning, D.W.; Bowyer, P. The cdr1B efflux transporter is associated with non-cyp51A-mediated itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 2013, 68, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Hassan, S.A.; Wilson, W.K.; Han, X.Y.; May, G.S.; Tarrand, J.J.; Matsuda, S.P. Cholesterol import by Aspergillus fumigatus and its influence on antifungal potency of sterol biosynthesis inhibitors. Antimicrob. Agents Chemother. 2005, 49, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Willger, S.D.; Puttikamonkul, S.; Kim, K.H.; Burritt, J.B.; Grahl, N.; Metzler, L.J.; Barbuch, R.; Bard, M.; Lawrence, C.B.; Cramer, R.A., Jr. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008, 4, e1000200. [Google Scholar] [CrossRef] [PubMed]
- Blatzer, M.; Barker, B.M.; Willger, S.D.; Beckmann, N.; Blosser, S.J.; Cornish, E.J.; Mazurie, A.; Grahl, N.; Haas, H.; Cramer, R.A. SREBP coordinates iron and ergosterol homeostasis to mediate triazole drug and hypoxia responses in the human fungal pathogen Aspergillus fumigatus. PLoS Genet. 2011, 7, e1002374. [Google Scholar] [CrossRef] [PubMed]
- Blosser, S.J.; Cramer, R.A. SREBP-dependent triazole susceptibility in Aspergillus fumigatus is mediated through direct transcriptional regulation of erg11A (cyp51A). Antimicrob. Agents Chemother. 2012, 56, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Juvvadi, P.R.; Fortwendel, J.R.; Steinbach, W.J. Heat shock protein 90 is required for conidiation and cell wall integrity in Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.E.; Lindquist, S. Hsp90 potentiates the rapid evolution of new traits: Drug resistance in diverse fungi. Science 2005, 309, 2185–2189. [Google Scholar] [CrossRef] [PubMed]
- Camps, S.M.; Dutilh, B.E.; Arendrup, M.C.; Rijs, A.J.; Snelders, E.; Huynen, M.A.; Verweij, P.E.; Melchers, W.J. Discovery of a hapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS ONE 2012, 7, e50034. [Google Scholar] [CrossRef] [PubMed]
- Lescar, J.; Meyer, I.; Akshita, K.; Srinivasaraghavan, K.; Verma, C.; Palous, M.; Mazier, D.; Datry, A.; Fekkar, A. Aspergillus fumigatus harbouring the sole Y121F mutation shows decreased susceptibility to voriconazole but maintained susceptibility to itraconazole and posaconazole. J. Antimicrob. Chemother. 2014, 69, 3244–3247. [Google Scholar] [CrossRef] [PubMed]
- Arabatzis, M.; Kambouris, M.; Kyprianou, M.; Chrysaki, A.; Foustoukou, M.; Kanellopoulou, M.; Kondyli, L.; Kouppari, G.; Koutsia-Karouzou, C.; Lebessi, E.; et al. Polyphasic identification and susceptibility to seven antifungals of 102 Aspergillus isolates recovered from immunocompromised hosts in Greece. Antimicrob. Agents Chemother. 2011, 55, 3025–3030. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, G.; Tokarzewski, S.; Nowakiewicz, A. Drug resistance of Aspergillus fumigatus strains isolated from flocks of domestic geese in Poland. Poult. Sci. 2014, 93, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.; Pina-Vaz, C.; Rodrigues, A.G. Susceptibility of environmental versus clinical strains of pathogenic Aspergillus. Int. J. Antimicrob. Agents 2007, 29, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Hagen, F.; Moroti, R.; Meis, J.F.; Chowdhary, A. Triazole-resistant Aspergillus fumigatus harbouring G54 mutation: Is it de novo or environmentally acquired? J. Glob. Antimicrob. Resist. 2015. [Google Scholar] [CrossRef]
- Alastruey-Izquierdo, A.; Mellado, E.; Pelaez, T.; Peman, J.; Zapico, S.; Alvarez, M.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Population-based survey of filamentous fungi and antifungal resistance in Spain (FILPOP study). Antimicrob. Agents Chemother. 2013, 57, 3380–3387. [Google Scholar] [CrossRef] [PubMed]
- Chryssanthou, E. In vitro susceptibility of respiratory isolates of Aspergillus species to itraconazole and amphotericin B. Acquired resistance to itraconazole. Scand. J. Infect. Dis. 1997, 29, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Watanabe, A.; Ito, J.; Oku, Y.; Wuren, T.; Taguchi, H.; Yarita, K.; Muraosa, Y.; Yahiro, M.; Yaguchi, T.; et al. Antifungal susceptibility of Aspergillus fumigatus clinical isolates collected from various areas in Japan. J. Infect. Chemother. 2014, 20, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W.; Marr, K.A.; Andes, D.R.; Walsh, T.J.; Kauffman, C.A.; Kontoyiannis, D.P.; Ito, J.I.; Balajee, S.A.; Pappas, P.G.; Moser, S.A. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the transplant-associated infection surveillance network. J. Clin. Microbiol. 2009, 47, 3271–3275. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Frade, J.P.; Etienne, K.A.; Pfaller, M.A.; Diekema, D.J.; Balajee, S.A. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR34/L98H mutation in the cyp51A gene. Antimicrob. Agents Chemother. 2011, 55, 4465–4468. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Messer, S.A.; Rhomberg, P.R.; Pfaller, M.A. Antifungal susceptibility patterns of a global collection of fungal isolates: Results of the sentry antifungal surveillance program (2013). Diagn Microbiol Infect. Dis 2016, 85, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, P.R.; Lau, Y.J.; Chuang, Y.C.; Wan, J.H.; Huang, W.K.; Shyr, J.M.; Yan, J.J.; Yu, K.W.; Wu, J.J.; Ko, W.C.; et al. Antifungal susceptibilities of clinical isolates of Candida species, Cryptococcus neoformans, and Aspergillus species from Taiwan: Surveillance of multicenter antimicrobial resistance in Taiwan program data from 2003. Antimicrob. Agents Chemother. 2005, 49, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Kano, R.; Makimura, K.; Hasegawa, A.; Kamata, H. Molecular typing and in vitro activity of azoles against clinical isolates of Aspergillus fumigatus and A. niger in Japan. J. Infect. Chemother. 2011, 17, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Kano, R.; Kohata, E.; Tateishi, A.; Murayama, S.Y.; Hirose, D.; Shibata, Y.; Kosuge, Y.; Inoue, H.; Kamata, H.; Hasegawa, A. Does farm fungicide use induce azole resistance in Aspergillus fumigatus? Med. Mycol. 2015, 53, 174–177. [Google Scholar] [CrossRef] [PubMed]
- European Commision Health & Consumer Protection Directorate-General. Opinion on azole antimycotic resistance. Available online: http://ec.europa.eu/food/fs/sc/ssc/out278_en.pdf (accessed on 25 April 2016).
- Lockhart, S.R. Azole resistance in the Americas: Not catching up with europe (yet). In Proceedings of the 7th Trends in Medical Mycology, Lisbon, Portugal, 9–12 October 2015; Volume 58, p. 20.
- Richardson, M.; Lass-Florl, C. Changing epidemiology of systemic fungal infections. Clin. Microbiol. Infect. 2008, 14, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Balajee, S.A.; Kano, R.; Baddley, J.W.; Moser, S.A.; Marr, K.A.; Alexander, B.D.; Andes, D.; Kontoyiannis, D.P.; Perrone, G.; Peterson, S.; et al. Molecular identification of Aspergillus species collected for the transplant-associated infection surveillance network. J. Clin. Microbiol. 2009, 47, 3138–3141. [Google Scholar] [CrossRef] [PubMed]
- Alastruey-Izquierdo, A.; Alcazar-Fuoli, L.; Cuenca-Estrella, M. Antifungal susceptibility profile of cryptic species of Aspergillus. Mycopathologia 2014, 178, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Fuoli, L.; Mellado, E.; Alastruey-Izquierdo, A.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Aspergillus section fumigati: Antifungal susceptibility patterns and sequence-based identification. Antimicrob. Agents Chemother. 2008, 52, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Balajee, S.A.; Gribskov, J.; Brandt, M.; Ito, J.; Fothergill, A.; Marr, K.A. Mistaken identity: Neosartorya pseudofischeri and its anamorph masquerading as Aspergillus fumigatus. J. Clin. Microbiol. 2005, 43, 5996–5999. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, G.; Sanchez, P.S.; Jewtuchowicz, V.M.; Pinoni, M.V.; Relloso, S.; Temporitti, E.; Iovannitti, C.A.; Mujica, M.T. Phenotypic and genotypic characterization of Aspergillus lentulus and Aspergillus fumigatus isolates in a patient with probable invasive aspergillosis. J. Med. Microbiol. 2009, 58, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Sugui, J.A.; Vinh, D.C.; Nardone, G.; Shea, Y.R.; Chang, Y.C.; Zelazny, A.M.; Marr, K.A.; Holland, S.M.; Kwon-Chung, K.J. Neosartorya udagawae (Aspergillus udagawae), an emerging agent of aspergillosis: How different is it from Aspergillus fumigatus? J. Clin. Microbiol. 2010, 48, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Vinh, D.C.; Shea, Y.R.; Jones, P.A.; Freeman, A.F.; Zelazny, A.; Holland, S.M. Chronic invasive aspergillosis caused by Aspergillus viridinutans. Emerg. Infect. Dis. 2009, 15, 1292–1294. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, M.; Beguin, H.; Detandt, M. Genetic re-identification and antifungal susceptibility testing of Aspergillus section nigri strains of the BCCM/IHEM collection. Mycoses 2012, 55, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; Espiritu, M.; Parmar, R. Paradoxical effect of caspofungin: Reduced activity against Candida albicans at high drug concentrations. Antimicrob Agents Chemother 2004, 48, 3407–3411. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Fuoli, L.; Mellado, E.; Alastruey-Izquierdo, A.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Species identification and antifungal susceptibility patterns of species belonging to Aspergillus section nigri. Antimicrob. Agents Chemother. 2009, 53, 4514–4517. [Google Scholar] [CrossRef] [PubMed]
- Szigeti, G.; Kocsube, S.; Doczi, I.; Bereczki, L.; Vagvolgyi, C.; Varga, J. Molecular identification and antifungal susceptibilities of black Aspergillus isolates from otomycosis cases in Hungary. Mycopathologia 2012, 174, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Manavathu, E.K.; Chandrasekar, P.H. Aspergillus flavus: An emerging non-fumigatus Aspergillus species of significance. Mycoses 2009, 52, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Balajee, S.A.; Lindsley, M.D.; Iqbal, N.; Ito, J.; Pappas, P.G.; Brandt, M.E. Nonsporulating clinical isolate identified as Petromyces alliaceus (anamorph Aspergillus alliaceus) by morphological and sequence-based methods. J. Clin. Microbiol. 2007, 45, 2701–2703. [Google Scholar] [CrossRef] [PubMed]
- Ozhak-Baysan, B.; Alastruey-Izquierdo, A.; Saba, R.; Ogunc, D.; Ongut, G.; Timuragaoglu, A.; Arslan, G.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Aspergillus alliaceus and Aspergillus flavus co-infection in an acute myeloid leukemia patient. Med. Mycol. 2010, 48, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, Y.; Chen, W.; Liu, W.; Wan, Z.; Bu, D.; Li, R. The T788G mutation in the cyp51C gene confers voriconazole resistance in Aspergillus flavus causing aspergillosis. Antimicrob. Agents Chemother. 2012, 56, 2598–2603. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.A.; Rudramurthy, S.M.; Meis, J.F.; Mouton, J.W.; Chakrabarti, A. A novel Y319H substitution in cyp51C associated with azole resistance in Aspergillus flavus. Antimicrob Agents Chemother 2015, 59, 6615–6619. [Google Scholar] [CrossRef] [PubMed]
- Lass-Florl, C.; Griff, K.; Mayr, A.; Petzer, A.; Gastl, G.; Bonatti, H.; Freund, M.; Kropshofer, G.; Dierich, M.P.; Nachbaur, D. Epidemiology and outcome of infections due to Aspergillus terreus: 10-year single centre experience. Br. J. Haematol. 2005, 131, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Perfect, J.R.; Schell, W.A.; Walsh, T.J.; Benjamin, D.K., Jr. In vitro analyses, animal models, and 60 clinical cases of invasive Aspergillus terreus infection. Antimicrob. Agents Chemother. 2004, 48, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Graybill, J.R.; Hernandez, S.; Bocanegra, R.; Najvar, L.K. Antifungal therapy of murine Aspergillus terreus infection. Antimicrob. Agents Chemother. 2004, 48, 3715–3719. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Jensen, R.H.; Grif, K.; Skov, M.; Pressler, T.; Johansen, H.K.; Lass-Florl, C. In vivo emergence of Aspergillus terreus with reduced azole susceptibility and a cyp51A M217I alteration. J. Infect. Dis. 2012, 206, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Peterson, S.W.; Frisvad, J.C.; Varga, J. New species in Aspergillus section terrei. Stud. Mycol. 2011, 69, 39–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinea, J.; Sandoval-Denis, M.; Escribano, P.; Pelaez, T.; Guarro, J.; Bouza, E. Aspergillus citrinoterreus, a new species of section terrei isolated from samples of patients with nonhematological predisposing conditions. J. Clin. Microbiol. 2015, 53, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, S.; Sharma, C.; Singh, P.K.; Agarwal, P.; Agarwal, K.; Hagen, F.; Meis, J.F.; Chowdhary, A. Molecular epidemiology and in vitro antifungal susceptibility of Aspergillus terreus species complex isolates in Delhi, India: Evidence of genetic diversity by amplified fragment length polymorphism and microsatellite typing. PLoS ONE 2015, 10, e0118997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, J.; Houbraken, J.; van der Lee, H.A.; Verweij, P.E.; Samson, R.A. Aspergillus calidoustus sp. nov., causative agent of human infections previously assigned to Aspergillus ustus. Eukaryot. Cell 2008, 7, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Alastruey-Izquierdo, A.; Cuesta, I.; Houbraken, J.; Cuenca-Estrella, M.; Monzon, A.; Rodriguez-Tudela, J.L. In vitro activity of nine antifungal agents against clinical isolates of Aspergillus calidoustus. Med. Mycol. 2010, 48, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Ananda-Rajah, M.; Andes, D.; Arendrup, M.C.; Bruggemann, R.J.; Chowdhary, A.; Cornely, O.A.; Denning, D.W.; Groll, A.H.; Izumikawa, K.; et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist. Updat. 2015, 21, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Melchers, W.J.; Mouton, J.W.; Verweij, P.E. Pharmacodynamics and dose-response relationships of liposomal amphotericin B against different azole-resistant Aspergillus fumigatus isolates in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 2013, 57, 1866–1871. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Sheehan, D.J.; Hitchcock, C.A.; Ghannoum, M.A. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 2005, 18, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Krishnan-Natesan, S.; Wu, W.; Chandrasekar, P.H. In vitro efficacy of the combination of voriconazole and anidulafungin against voriconazole-resistant cyp51A mutants of Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 2012, 73, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Schlamm, H.T.; Herbrecht, R.; Rottinghaus, S.T.; Bow, E.J.; Cornely, O.A.; Heinz, W.J.; Jagannatha, S.; Koh, L.P.; Kontoyiannis, D.P.; et al. Combination antifungal therapy for invasive aspergillosis: A randomized trial. Ann. Intern. Med. 2015, 162, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Bruggemann, R.J.; Melchers, W.J.; Verweij, P.E.; Mouton, J.W. Pharmacodynamics of anidulafungin against clinical Aspergillus fumigatus isolates in a nonneutropenic murine model of disseminated aspergillosis. Antimicrob. Agents Chemother 2013, 57, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Bowyer, P. Voriconazole resistance in Aspergillus fumigatus: Should we be concerned? Clin. Infect. Dis. 2013, 57, 521–523. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Risk assessment on the impact of environmental usage of triazoles on the development and spread of resistance to medical triazoles in Aspergillus species. Available online: http://ecdc.europa.eu/en/publications/Publications/risk-assessment-impact-environmental-usage-of-triazoles-on-Aspergillus-spp-resistance-to-medical-triazoles.pdf (accessed on 1 May 2016).

| cyp51A Amino Acid No./Change | Continents | References |
|---|---|---|
| Described in resistant strains with a known mechanism | ||
| G54/W/R/E/V/A | Europe | [12,17,18,23,32,47,48,49,50,51,52,53] |
| Asia | [3,54,55,56,57] | |
| America | [58] | |
| Oceania | [59] | |
| M220/T/V/I/K/R/L | Europe | [12,17,20,25,32,33,35,47,48,50,52,60,61] |
| Asia | [54,57] | |
| America | [58,62] | |
| G448S | Europe | [17,27,29,63] |
| Asia | [64] | |
| America | [58] | |
| Oceania | [59] | |
| Promoter tandem insertion + cyp51A amino acid No./change | ||
| TR34/L98H with or without S297T/F497I | Europe | [12,17,20,32,35,36,37,38,40,47,48,50,51,52,53,60,65,66,67,68,69,70,71,72,73,74,75,76] |
| Asia | [3,77,78,79,80,81,82,83,84,85,86,87] | |
| America | [58,88,89] | |
| Africa | [90] | |
| Oceania | [59] | |
| TR46/Y121F/T289A with or without S297T/F497I | Europe | [40,47,51,52,60,66,67,75,76,91,92,93,94] |
| Asia | [82,95,96] | |
| America | [58,88,89] | |
| Africa | [90] | |
| TR53 | Europe | [41] |
| America | [88] | |
| Described in resistant strains with an unknown mechanism | ||
| G138/C/S | Europe | [17,26,31] |
| America | [58] | |
| Described both in resistant and susceptible strains | ||
| F46Y/M172V/N248T/D255E/E42 7K or some other combinations | Europe | [17,33,34,36,53,61,65,71] |
| Asia | [3] | |
| Oceania | [59] | |
| F46Y/M172V/E427K | Europe | [12,17,33,34,74,75] |
| Occasionally described in susceptible or resistant strains | ||
| P216L | [17,18,53,61,75,97] | |
| F219/S/C/I | [18,32,53,58] | |
| I242V | [12,62] | |
| N248K | [12,34,83] | |
| Y431/S/C | [17,31,35,59] | |
| G432/S/A | [30,83] | |
| G434C | [17,31] | |
| Continent/Country | % Resistance | Source of the Isolates | References |
|---|---|---|---|
| Europe | |||
| Belgium | 5.7 | C | [76] |
| France | 0.85–10.6 | C | [30,48,50] |
| Germany | 1.1–12 | C and E | [32,47,60] |
| Netherlands | 2.1–20 | C and E | [20,53,67,74] |
| Poland | 2.25 | C | [69] |
| Spain | 1.8 | C | [63] |
| Turkey | 10.2 | C | [71] |
| United Kingdom | 6.6–28 | C | [17,33] |
| Other continents | |||
| Asia * | 1.9–11.1 | C and E | [55,77,78,80,81,82,83,84,85,86,121] |
| Africa (Tanzania) | 13.9 | E | [90] |
| America (USA) | 0.6–11.8 | C | [58,122] |
| Oceania (Australia) | 2.6 | C | [59] |
| International surveillance studies | |||
| America-Asia-Australia-Europe | 1.4–5.8 | C and E | [52,70,123,124] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero-Menendez, O.; Alastruey-Izquierdo, A.; Mellado, E.; Cuenca-Estrella, M. Triazole Resistance in Aspergillus spp.: A Worldwide Problem? J. Fungi 2016, 2, 21. https://doi.org/10.3390/jof2030021
Rivero-Menendez O, Alastruey-Izquierdo A, Mellado E, Cuenca-Estrella M. Triazole Resistance in Aspergillus spp.: A Worldwide Problem? Journal of Fungi. 2016; 2(3):21. https://doi.org/10.3390/jof2030021
Chicago/Turabian StyleRivero-Menendez, Olga, Ana Alastruey-Izquierdo, Emilia Mellado, and Manuel Cuenca-Estrella. 2016. "Triazole Resistance in Aspergillus spp.: A Worldwide Problem?" Journal of Fungi 2, no. 3: 21. https://doi.org/10.3390/jof2030021