Isolation of Ovicidal Fungi from Fecal Samples of Captive Animals Maintained in a Zoological Park
Abstract
:1. Introduction
2. Material and Methods
2.1. Marcelle Natureza Zoological Park
2.2. Collection and Analysis of Fecal Samples
2.3. Isolation of Fungi from the Feces
2.4. Identification of Fungal Species
2.5. Obtaining Parasites
2.6. Parasiticide Activity Testing Assays
2.7. Evaluation of the Fungal Parasiticide Activity
- -
- Type 0: Eggs are viable and damage or alterations are not observed.
- -
- Type 1: Hyphae attached to the eggshell but morphological damage was not provoked.
- -
- Type 2: The eggshell and embryo show damage without penetration.
- -
- Type 3: Fungal hyphae enter the egg, grow, and destroy the embryo.
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sanchís, J.; Sánchez-Andrade, R.; Macchi, M.I.; Piñeiro, P.; Suárez, J.L.; Cazapal-Monteiro, C.; Maldini, G.; Venzal, J.M.; Paz-Silva, A.; Arias, M.S. Infection by Paramphistomidae trematodes in cattle from two agricultural regions in NW Uruguay and NW Spain. Vet. Parasitol. 2013, 191, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.R.; Araújo, J.V.; Silva, A.R.; Carvalho, R.O.; Araujo, J.M.; Ferreira, S.R.; Carvalho, G.R. Viability of the nematophagous fungus Pochonia chlamydosporia after passage through the gastrointestinal tract of horses. Vet. Parasitol. 2010, 168, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Corning, S. Equine cyathostomins: A review of biology, clinical significance and therapy. Parasites Vectors 2009, 2, S1. [Google Scholar] [CrossRef] [PubMed]
- Saumell, C.A.; Fernández, A.S. Hongos nematófagos para el control biológico de nematodos parásitos de rumiantes. Rev. Med. Vet. 2000, 81, 270–273. [Google Scholar]
- Arias, M.; Cazapal-Monteiro, C.; Valderrábano, E.; Miguélez, S.; Rois, J.L.; López-Arellano, M.E.; Madeira de Carvalho, L.; Mendoza de Gives, P.; Sánchez-Andrade, R.; Paz-Silva, A. A preliminary study of the biological control of strongyles affecting equids in a zoological park. J. Equine Vet. Sci. 2013, 33, 1115–1120. [Google Scholar] [CrossRef]
- Barron, G.L. Fungal parasites and predators of rotifers, nematodes, and other invertebrates. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G.M., Bill, G.F., Foster, M.S., Eds.; Academic Press: New York, NY, USA, 2004; pp. 435–450. [Google Scholar]
- Cortiñas, F.J.; Cazapal-Monteiro, C.F.; Hernández, J.A.; Arroyo, F.L.; Miguélez, S.; Suárez, J.; López de Arellano, M.E.; Sánchez-Andrade, R.; Mendoza de Gives, P.; Paz-Silva, A.; et al. Potential use of Mucor circinelloides for the biological control of certain helminths affecting livestock reared in a care farm. Biocontrol Sci. Technol. 2015, 25, 1443–1452. [Google Scholar] [CrossRef]
- Mendoza de Gives, P.; Crespo, J.F.; Rodriguez, D.H.; Prats, V.V.; Hernandez, E.L.; Fernandez, G.E.O. Biological control of Haemonchus contortus infective larvae in ovine faeces by administering an oral suspension of Duddingtonia flagrans chlamydospores to sheep. J. Helminthol. 1998, 72, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Knox, M.R.; Faedo, M. The potential of nematophagous fungi to control the free-living stages of nematode parasites of sheep: Feeding and block studies with Duddingtonia flagrans. Vet. Parasitol. 2001, 102, 321–330. [Google Scholar] [CrossRef]
- Chandrawathani, P.; Jamnah, O.; Waller, P.J.; Hoglund, J.; Larsen, M.; Zahari, W.M. Nematophagous fungi as a biological control agent for nematode parasites of small ruminants in Malaysia: A special emphasis on Duddingtonia flagrans. Vet. Parasitol. 2002, 33, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Durand, D.T.; Boshoff, H.M.; Michael, L.M.; Krecek, R.C. Survey of nematophagous fungi in South Africa. Onderstepoort. J. Vet. Res. 2005, 72, 185–187. [Google Scholar]
- Ghahfarokhi, S.M.; Abyaneh, M.R.; Bahadori, S.R.; Eslami, A.; Zare, A.; Ebrahimi, M. Screening of soil and sheep samples for predacious fungi: Isolation and characterisation of the nematode-trapping fungus Arthrobotrys oligospora. Iran. Biomed. J. 2004, 8, 135–142. [Google Scholar]
- Kelly, P.; Good, B.; Hanrahan, J.P.; Fitzpatrick, R.; de Waal, T. Screening for the presence of nematophagous fungi collected from Irish sheep pastures. Vet. Parasitol. 2009, 165, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Faedo, M.; Waller, P.J. The potential of nematophagous fungi to control the free-living stages of nematode parasites of sheep: Survey for the presence of fungi in fresh faeces of grazing livestock in Australia. Vet. Parasitol. 1994, 53, 275–281. [Google Scholar] [CrossRef]
- Manueli, P.R.; Waller, P.J.; Faedo, M.; Mahommed, F. Biological control of nematode parasites of livestock in Fiji: Screening of fresh dung of small ruminants for the presence of nematophagous fungi. Vet. Parasitol. 1999, 81, 39–45. [Google Scholar] [CrossRef]
- McEwen, H. Nematophagous Fungi from Farm Soils in the Lower North Island New Zealand. Master’s Thesis, Victoria University of Wellington, Wellington, New Zealand, July 2000. [Google Scholar]
- Skipp, R.A.; Yeates, G.W.; Chen, L.Y.; Glare, T.R. Occurrence, morphological characteristic and ribotyping of New Zealand isolate of Duddingtonia flagrans, a candidate for biocontrol of animal parasitic nematodes. N. Z. J. Agric. Res. 2002, 45, 187–196. [Google Scholar] [CrossRef]
- Arroyo, F.L.; Arias, M.S.; Cazapal-Monteiro, C.F.; Hernández, J.A.; Suárez, J.; Miguélez, S.; Romasanta, A.; Sánchez-Andrade, R.; Paz-Silva, A. The capability of the fungus Mucor circinelloides to maintain parasiticidal activity after the industrial feed pelleting enhances the possibilities of biological control of livestock parasites. Biol. Control 2016, 92, 38–44. [Google Scholar] [CrossRef]
- De Carvalho, L.M.; Braga, F.R.; Domingues, R.R.; Araujo, J.M.; Lelis, R.T.; de Paula, A.T.; da Silveira, W.F.; de Araújo, J.V. Interaction of the nematophagous fungus Pochonia chlamydosporia and Parascaris equorum eggs in different culture media. J. Basic Microbiol. 2014, 54, S109–S114. [Google Scholar] [CrossRef] [PubMed]
- Hiura, E.; del Carmen García Lopes, A.; da Paz, J.S.; Gava, M.G.; Flecher, M.C.; Colares, M.; de Freitas Soares, F.E.; da Fonseca, L.A.; Lacerda, T.; de Araújo, J.V.; et al. Fungi predatory activity on embryonated Toxocara canis eggs inoculated in domestic chickens (Gallus gallus domesticus) and destruction of second stage larvae. Parasitol. Res. 2015, 114, 3301–3308. [Google Scholar] [CrossRef] [PubMed]
- Cazapal-Monteiro, C.F.; Hernández, J.A.; Arroyo, F.L.; Miguélez, S.; Romasanta, A.; Paz-Silva, A.; Sánchez-Andrade, R.; Arias, M.S. Analysis of the effect of soil saprophytic fungi on the eggs of Baylisascaris procyonis. Parasitol. Res. 2015, 114, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Lýsek, H.; Kraícjk, D. Penetration of ovicidal fungus Verticillium chlamydosporium through the Ascaris lumbricoides egg shells. Folia Parasitol. 1987, 34, 57–60. [Google Scholar]
- Rodríguez-González, Á.; Frontela, A.; Lorenzana, A.; Henrique da Silva, P.; Mayo, S.; González-López, Ó.; Gutiérrez, S.; Casquero, P.A. Insecticidal activity of Trichoderma harzianum against Xylotrechus arvicola and Acanthoscelides obtectus inmature stages. Planta Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Mayo, S.; González-López, Ó.; Reinoso, B.; Gutierrez, S.; Casquero, P.A. Inhibitory activity of Beauveria bassiana and Trichoderma spp. on the insect pests Xylotrechus arvicola (Coleoptera: Cerambycidae) and Acanthoscelides obtectus (Coleoptera: Chrisomelidae: Bruchinae). Environ. Monit. Assess. 2017, 189, 12. [Google Scholar] [CrossRef] [PubMed]
- Francisco, I.; Arias, M.; Cortiñas, F.J.; Francisco, R.; Mochales, E.; Sánchez, J.A.; Uriarte, J.; Suárez, J.L.; Morrondo, P.; Sánchez-Andrade, R.; et al. Silvopastoralism and autochthonous equine livestock: Analysis of the infection by endoparasites. Vet. Parasitol. 2009, 164, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Llerandi-Juárez, J.R.D.; Mendoza de Gives, P. Resistance of nematophagous fungi chlamydospores to the digestive processes of sheep in Mexico. J. Helminthol. 1998, 72, 155–158. [Google Scholar] [CrossRef]
- Bills, G.F.; Foster, M.S. Formulae for selected materials used to isolate and study fungi and fungal allies. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G.M., Bill, G.F., Foster, M.S., Eds.; Academic Press: New York, NY, USA, 2004; pp. 595–618. [Google Scholar]
- Davet, P.; Rouxel, F. Détection et Isolement des Champignons du sol; INRA: Paris, France, 1997. [Google Scholar]
- Arx, J.A. The Genera of Fungi Sporulating in Pure Culture; Cramer, J.: Vaduz, Liechtenstein, 1974; pp. 1–315. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi; MacMilllan Publishing Co.: New York, NY, USA, 1987. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi; Academic Press: London, UK, 1980; Volume 2. [Google Scholar]
- Seifert, K.; Morgan-Jones, G.; Gams, W.; Kendrick, B. The Genera of Hyphomycetes; CBS Biodiversity Series; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2011; Volume 9, pp. 1–997. [Google Scholar]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Lýsek, H.; Fassatiová, O.; Cuervo Pineda, N.; Lorenzo Hernández, N. Ovicidal fungi in soils of Cuba. Folia Parasitol. 1982, 29, 265–270. [Google Scholar] [PubMed]
- Siddiqui, Z.A.; Mahmood, I. Biological control of Heterodera cajani and Fusarium udum on pigeonpea by Glomus mosseae, Trichoderma harzianum and Verticillium chlamydosporium. Isr. J. Plant Sci. 1996, 44, 49–56. [Google Scholar] [CrossRef]
- Vargas Gil, S.; Pastor, S.; March, G.J. Quantitative isolation of biocontrol agents Trichoderma spp., Gliocladium spp. and actinomycetes from soil with culture media. Microbiol. Res. 2009, 164, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Papavizas, G.C. Characterization of alginate pellets formulated with Trichoderma and Gliocladium and their effect on the proliferation of the fungi in soil. Plant Pathol. 1985, 34, 571–577. [Google Scholar] [CrossRef]
- Mcquilken, M.P.; Gemmell, J.; Lahdenperä, M.I. Gliocladium catenulatum as a potential biological control agent of damping-off in bedding plants. J. Phytopathol. 2001, 149, 171–178. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Futai, K. Biocontrol of Meloidogyne incognita on tomato using antagonistic fungi, plant-growth-promoting rhizobacteria and cattle manure. Pest Manag. Sci. 2009, 65, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Bojanich, M.V.; Sarmiento, M.M.; Guisiano, G.; Mangiaterra, M.; Basualdo, J.A. Huevos de Toxocara canis como anzuelo para hongos géofilos en una ciudad subtropical. Rev. Iber. Micol. 2015, 32, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, M.L.; Arambarri, A.M.; Basualdo, J.A.; Minvielle, M.C. Effect of saprotrophic soil fungi on Toxocara canis eggs. Malays. J. Microbiol. 2010, 6, 75–80. [Google Scholar] [CrossRef]
- De Souza Maia Filho, F.; Nunes Vieira, J.; Aires Berne, M.E.; Stoll, F.E.; Da Silva Nascente, P.; Pötter, L.; Brayer Pereira, D.I. Fungal ovicidal activity on Toxocara canis eggs. Rev. Iber. Micol. 2013, 30, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Lelis, R.T.; Braga, F.R.; de Carvalho, L.M.; de Paula, A.T.; Araujo, J.M.; Fausto, M.C.; Junior, A.M.; Rodrigues, J.V.; de Freitas Soares, F.E.; Garcia, J.S.; et al. Effect of the fungus Pochonia chlamydosporia on Echinostoma paraensei (Trematoda: Echinostomatidae). Acta Trop. 2014, 139, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Colvin, A.F.; Walkden-Brown, S.W.; Knox, M.R.; Scott, J.M. Intensive rotational grazing assists control of gastrointestinal Nematodirus of sheep in a cool temperate environment with summer-dominant rainfall. Vet. Parasitol. 2008, 153, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, B.; Rohn, K.; Schnieder, T.; von Samson-Himmelstjerna, G. Endoparasite control management on horse farms-lessons from worm prevalence and questionnaire data. Equine Vet. J. 2010, 42, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Geurden, T.; Van Doorn, D.; Claerebout, E.; Kooyman, F.; de Keersmaecker, S.; Vercruysse, J.; Besognet, B.; Vanimisetti, B.; di Regalbono, A.F.; Beraldo, P.; et al. Decreased strongyle egg re-appearance period after treatment with ivermectin and moxidectin in horses in Belgium, Italy and The Netherlands. Vet. Parasitol. 2014, 204, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Stirling, G.R. Biological Control of Plant-Parasitic Nematodes; CAB International: Wallingford, UK, 1991. [Google Scholar]
- Kim, D.G.; Riggs, R.D. Biological Control; American Phytopathological Society: Washington, DC, USA, 1992; pp. 133–142. [Google Scholar]
- Ojeda-Robertos, N.F.; Torres-Acosta, J.F.; Aguilar-Caballero, A.J.; Ayala-Burgos, A.; Cob-Galera, L.A.; Sandoval-Castro, C.A.; Barrientos-Medina, R.C.; de Gives, P.M. Assessing the efficacy of Duddingtonia flagrans chlamydospores per gram of faeces to control Haemonchus contortus larvae. Vet. Parasitol. 2008, 158, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.E.; Braga, F.R.; Borges, L.A.; Oliveira, J.M.; Lima Wdos, S.; Guimarães, M.P.; Araújo, J.V. Evaluation of the effectiveness of Duddingtonia flagrans and Monacrosporium thaumasium in the biological control of gastrointestinal nematodes in female bovines bred in the semiarid region. Vet. Res. Commun. 2014, 38, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.Á.; Arroyo, F.L.; Suárez, J.; Cazapal-Monteiro, C.F.; Romasanta, Á.; López-Arellano, M.E.; Pedreira, J.; de Carvalho, L.M.; Sánchez-Andrade, R.; Arias, M.S.; et al. Feeding horses with industrially manufactured pellets with fungal spores to promote nematode integrated control. Vet. Parasitol. 2016, 229, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Criado, P.R.; Belda, W., Jr.; Vasconcellos, C.; Silva, C.S. Cutaneous Larva migrans: A bad souvenir from the vacation. Dermatol. Online. J. 2012, 18, 11. [Google Scholar] [PubMed]
- Traub, R.J. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int. J. Parasitol. 2013, 43, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Saumell, C.A.; Padilha, T.; Santos, C.; de, P.; Roque, M.V.C. Nematophagous fungi in fresh feces of cattle in the Mata region of Minas Gerais state, Brazil. Vet. Parasitol. 1999, 82, 217–220. [Google Scholar] [CrossRef]
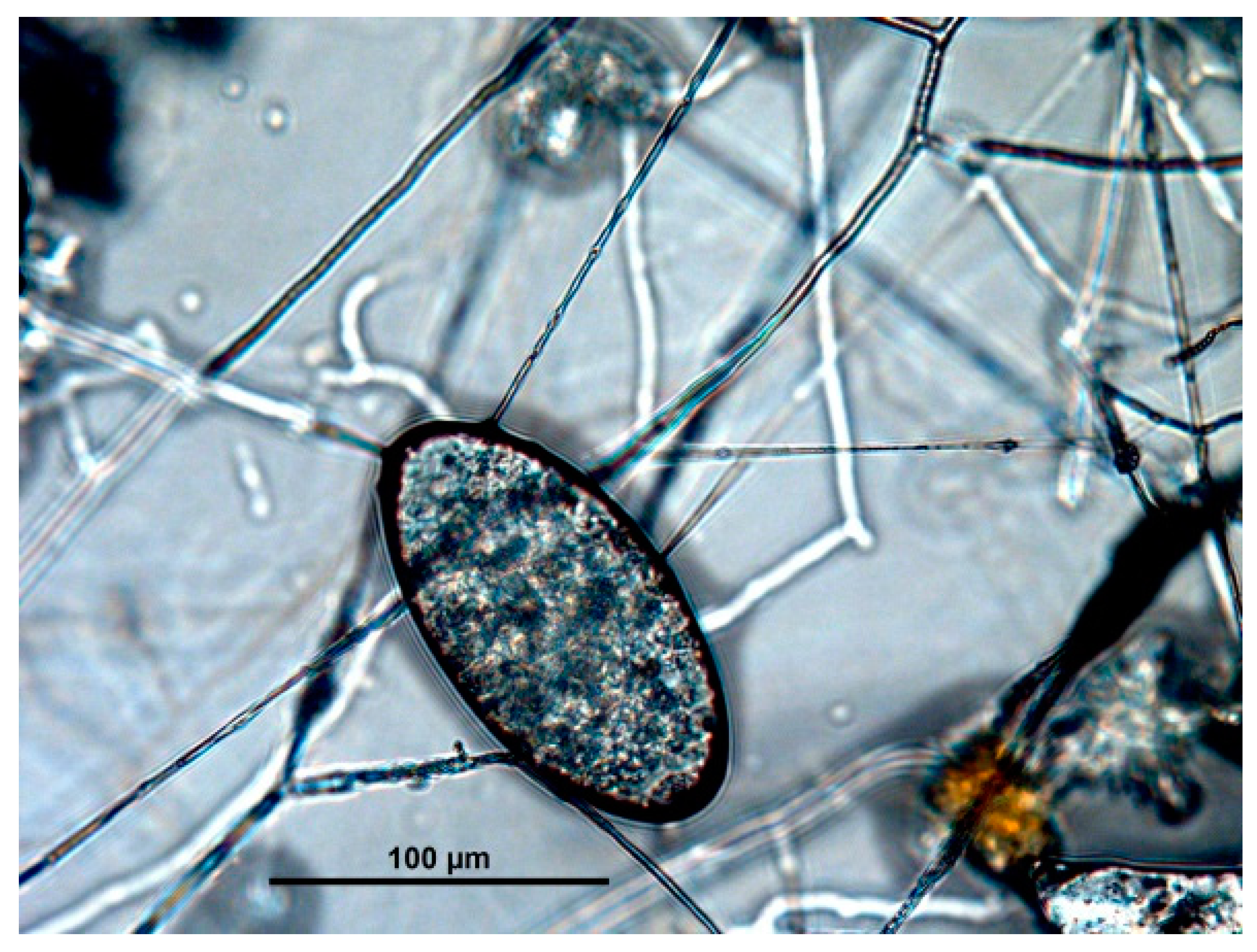
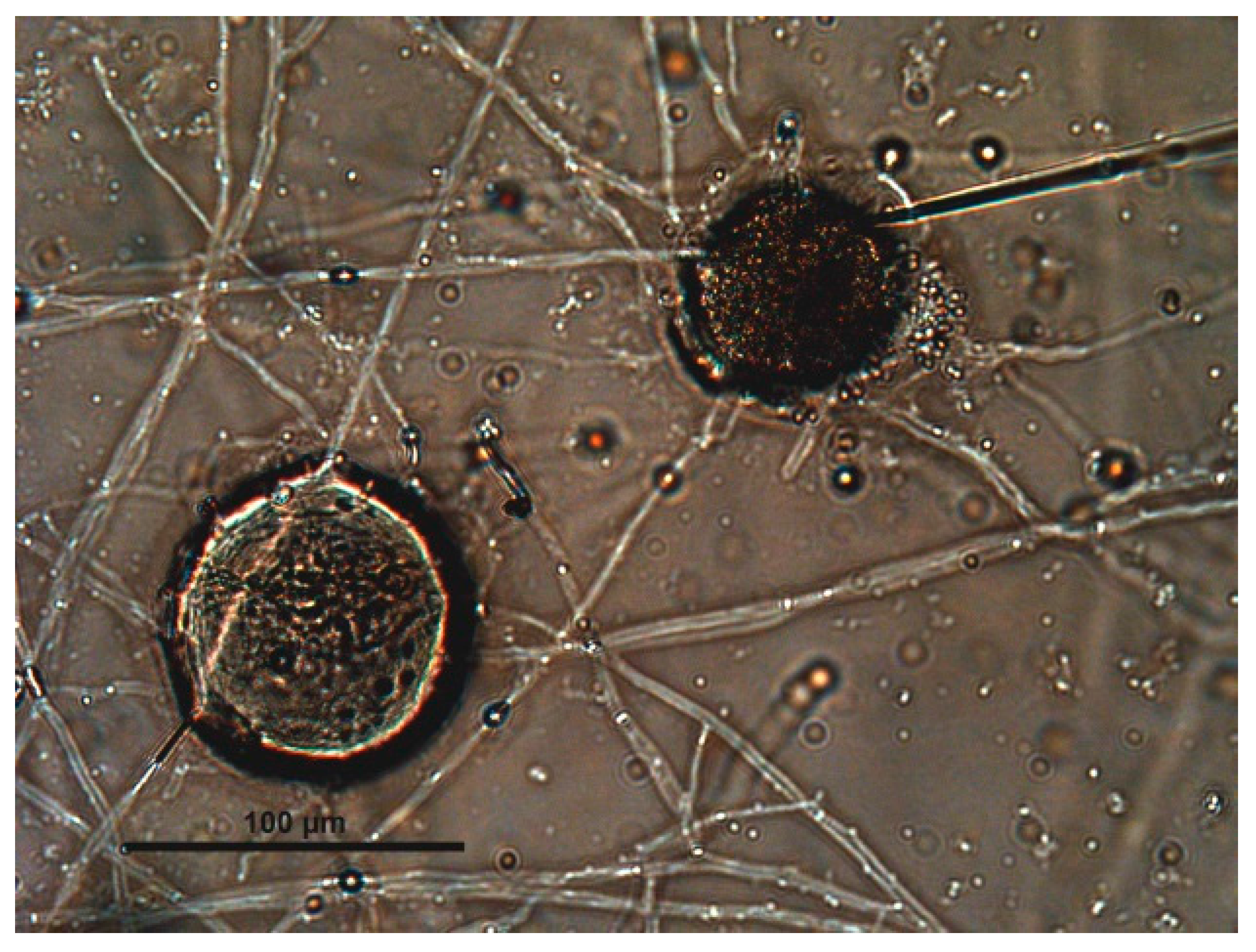

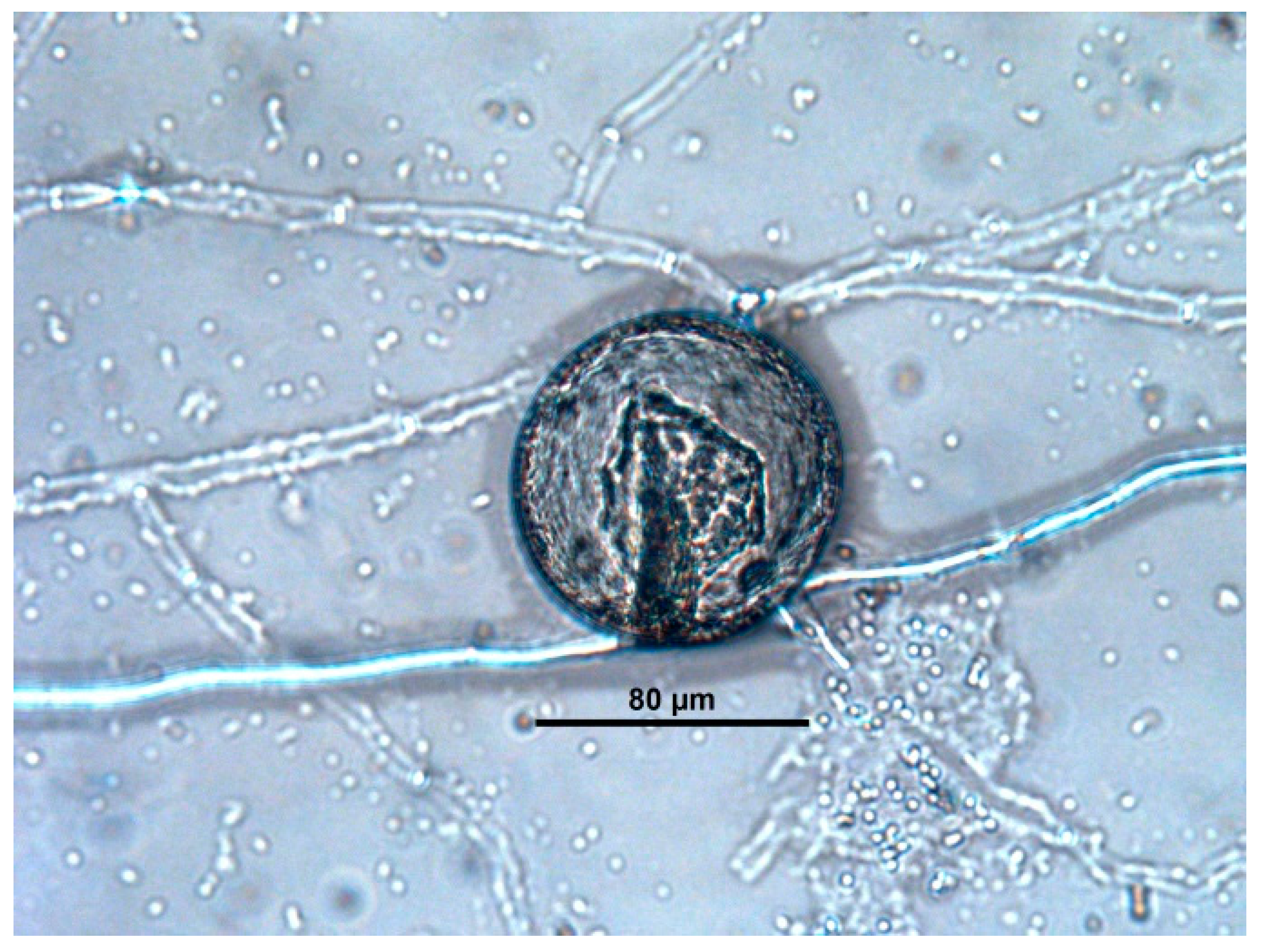
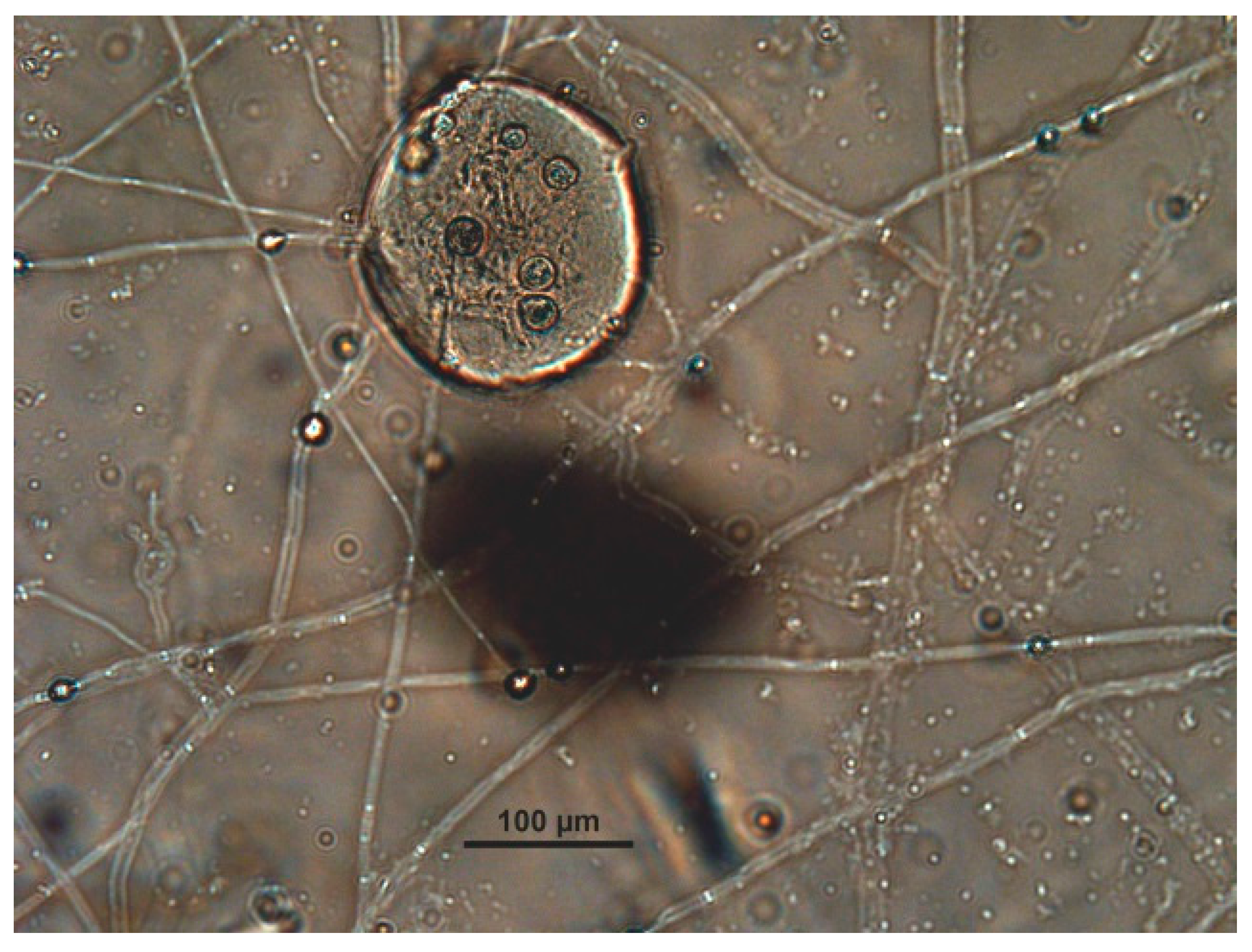
| Captive Animals | Fungal Isolation | |||||
|---|---|---|---|---|---|---|
| Common Name | Scientific Name | Parasites Diagnosed | Culture | Subculture 1 | Subculture 2 | Genera Identified |
| Coati | Nasua nasua | Nematodes: Strongyles | WA | MEA | Trichoderma | |
| CMA | Trichoderma | |||||
| Verticillium | ||||||
| Raccoon | Procyon lottor | Nematodes: Strongyles | WA | MEA | Mucor | |
| CMA | Mucor | |||||
| Eurasian lynx | Lynx lynx | Nematodes: Strongyles | WA | MEA | PGA | Fusarium |
| CMA | Gliocladium | |||||
| Brown bear | Ursus arctos | Nematodes: Strongyles | WA | MEA | Trichoderma | |
| CMA | Trichoderma | |||||
| Goat | Capra hircus spp. | Coccidia | WA | MEA | PGA | Verticillium |
| Nematodes: Strongyles | CMA | Verticillium | ||||
| Mouflon | Ovis musimon | Coccidia | WA | MEA | Fusarium | |
| Penicillium | ||||||
| Nematodes: Strongyles | CMA | Fusarium | ||||
| Gazelle | Gazella cuvieri | Coccidia | WA | MEA | Mucor | |
| Nematodes: Strongyles | CMA | Mucor | ||||
| Penicillium | ||||||
| Axis | Axis axis | Nematodes: Strongyles | WA/AT | MEA | Verticillium | |
| Lecanicillium | ||||||
| CMA | Verticillium | |||||
| Lecanicillium | ||||||
| Bison | Bison bison | Coccidia | WA | MEA | Trichoderma | |
| Nematodes: Strongyles | CMA | Trichoderma | ||||
| Dromedary | Camelus dromedarius | Nematodes: Strongyles | WA | MEA | PGA | Trichoderma |
| CMA | Trichoderma | |||||
| Verticillium | ||||||
| Guanaco | Lama guanicoe | Coccidia | WA | MEA | PGA | Gliocladium |
| Nematodes: Strongyles | CMA | Gliocladium | ||||
| Falabella | Equus caballus | Coccidia | WA | MEA | Fusarium | |
| CMA | Fusarium | |||||
| Penicillium | ||||||
| Wallaby | Macropus rufogriseus | - | WA | MEA | Mucor | |
| CMA | (Sordariaceae) | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, J.A.; Vázquez-Ruiz, R.A.; Cazapal-Monteiro, C.F.; Valderrábano, E.; Arroyo, F.L.; Francisco, I.; Miguélez, S.; Sánchez-Andrade, R.; Paz-Silva, A.; Arias, M.S. Isolation of Ovicidal Fungi from Fecal Samples of Captive Animals Maintained in a Zoological Park. J. Fungi 2017, 3, 29. https://doi.org/10.3390/jof3020029
Hernández JA, Vázquez-Ruiz RA, Cazapal-Monteiro CF, Valderrábano E, Arroyo FL, Francisco I, Miguélez S, Sánchez-Andrade R, Paz-Silva A, Arias MS. Isolation of Ovicidal Fungi from Fecal Samples of Captive Animals Maintained in a Zoological Park. Journal of Fungi. 2017; 3(2):29. https://doi.org/10.3390/jof3020029
Chicago/Turabian StyleHernández, José A., Rosa A. Vázquez-Ruiz, Cristiana F. Cazapal-Monteiro, Esther Valderrábano, Fabián L. Arroyo, Iván Francisco, Silvia Miguélez, Rita Sánchez-Andrade, Adolfo Paz-Silva, and María S. Arias. 2017. "Isolation of Ovicidal Fungi from Fecal Samples of Captive Animals Maintained in a Zoological Park" Journal of Fungi 3, no. 2: 29. https://doi.org/10.3390/jof3020029







