Assessment of the Dyeing Properties of the Pigments Produced by Talaromyces spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Inoculum Preparation
2.2. Culture Media
2.3. Cultivation Conditions for Pigment Production
2.4. Pigment Extraction
2.5. Wool Dyeing Process
2.5.1. Fabric Scouring
2.5.2. Fabric Mordanting
2.6. Dyeing
2.7. Data Analysis
3. Results and Discussion
Dying Properties of Pigments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Shahid, M.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Kostick, R.; Wang, S.; Wang, J. Color Cosmetics—Coloring/Staining the Skin from Fruit and Plant Color Pigments. Patent US20060280762, 2006. [Google Scholar]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial pigments and their applications. Process Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Arai, T.; Kojima, R.; Motegi, Y.; Kato, J.; Kasumi, T.; Ogihara, J. PP-O and PP-V, Monascus pigment homologs, production and phylogenetic analysis in Penicillium purpurogenum. Fungal Biol. 2015, 119, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.S.; Hansen, M.E.; Meyer, A.S.; Thrane, U. Computerized screening for novel producers of Monascus-like food pigments in Penicillium species. J. Agric. Food Chem. 2008, 56, 9981–9989. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ebinuma, V.C.; Roberto, I.C.; Teixeira, M.F.S.; Pessoa, A., Jr. Improvement of submerged culture conditions to produce colorants by Penicillium purpurogenum. Braz. J. Microbiol. 2014, 45, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Morales-Oyervides, L.; Oliveira, J.C.; Sousa-Gallagher, M.J.; Méndez-Zavala, A.; Montañez, J.C. Selection of best conditions of inoculum preparation for optimum performance of the pigment production process by Talaromyces spp. using the Taguchi method. Biotechnol. Prog. 2017. [Google Scholar] [CrossRef] [PubMed]
- Padmapriya, C.; Murugesan, R. Optimization of physical parameters on polyketide red pigment production from Penicillium purpurogenum. Trends Biosci. 2014, 7, 1664–1667. [Google Scholar]
- Sudha; Gupta, C.; Aggarwal, S. Optimization and extraction of extra and intracellular color from Penicillium minioluteum for application on protein fibers. Fibers Polym. 2017, 18, 741–748. [Google Scholar] [CrossRef]
- Velmurugan, P.; Kim, M.J.; Park, J.S.; Karthikeyan, K.; Lakshmanaperumalsamy, P.; Lee, K.J.; Park, Y.J.; Oh, B.T. Dyeing of cotton yarn with five water soluble fungal pigments obtained from five fungi. Fibers Polym. 2010, 11, 598–605. [Google Scholar] [CrossRef]
- Dhale, M.A.; Vijay-Raj, A.S. Pigment and amylase production in Penicillium sp. NIOM-02 and its radical scavenging activity. Int. J. Food Sci. Technol. 2009, 44, 2424–2430. [Google Scholar] [CrossRef]
- Morales-Oyervides, L.; Oliveira, J.C.; Sousa-Gallagher, M.J.; Méndez-Zavala, A.; Montañez, J.C. Effect of heat exposure on the colour intensity of red pigments produced by Penicillium purpurogenum GH2. J. Food Eng. 2015, 164, 21–29. [Google Scholar] [CrossRef]
- Arora, S. Textile Dyes: It’s impact on environment and its treatment. J. Bioremediation Biodegrad. 2014, 5, 1. [Google Scholar] [CrossRef]
- Borges, M.E.; Tejera, R.L.; Díaz, L.; Esparza, P.; Ibáñez, E. Natural dyes extraction from cochineal (Dactylopius coccus). New extraction methods. Food Chem. 2012, 132, 1855–1860. [Google Scholar] [CrossRef]
- Patil, S.; Sivanandhan, G.; Thakare, D. Effect of physical and chemical parameters on the production of Red exopigment from Penicillium purpurogenum isolated from spoilt onion and study of its antimicrobial activity. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 599–609. [Google Scholar]
- Chadni, Z.; Rahaman, M.H.; Jerin, I.; Hoque, K.M.; Reza, M.A. Extraction and optimisation of red pigment production as secondary metabolites from Talaromyces verruculosus and its potential use in textile industries. Mycology 2017, 8, 48–57. [Google Scholar] [CrossRef]
- Sopandi, T.; Wardah, W. Sub-Acute toxicity of pigment derived from Penicillium resticulosum in mice. Microbiol. Indones. 2012, 6, 35–41. [Google Scholar] [CrossRef]
- Cruz-Hernández, M.; Rodráguez, R. Aislamiento y caracterización morfológica de cepas microbianas degradadotas de taninos. In Proceedings of the XXII Annual Meeting of the Mexican Academy of Chemical Engineering (AMIDIQ), Mazatlan, Mexico, 9 September 2001. [Google Scholar]
- Espinoza-Hernández, T. Caracterización Morfológica, Fisiológica y Molecular de tres Cepas Fúngicas Productoras de Pigmentos. Bachelor’s Thesis, Universidad Autónoma de Coahuila, Saltillo, México, 2004. [Google Scholar]
- Frisvad, J.C.; Yilmaz, N.; Thrane, U.; Rasmussen, K.B.; Houbraken, J.; Samson, R.A. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE 2013, 8, e84102. [Google Scholar] [CrossRef] [PubMed]
- Morales-Oyervides, L.; Oliveira, J.C.; Sousa-Gallagher, M.J.; Méndez-Zavala, A.; Montañez, J.C. Quantitative assessment of the impact of the type of inoculum on the kinetics of cell growth, substrate consumption and pigment productivity by Penicillium purpurogenum GH2 in liquid culture with an integrated stochastic approach. Food Bioprod. Process. 2015, 96, 221–231. [Google Scholar] [CrossRef]
- Méndez-Zavala, A. Identificación de Factores que Afectan la Producción de Pigmentos por Penicillium purpurogenum GH2 y Obtención de Perfiles Cromatográficos. Master’s Thesis, Universidad Autónoma de Coahuila, Saltillo, México, 2011. [Google Scholar]
- Méndez-Zavala, A.; Pérez, C.; Montañez, J.C.; Martínez, G.; Aguilar, C.N. Red pigment production by Penicillium purpurogenum GH2 is influenced by pH and temperature. J. Zhejiang Univ. Sci. B 2011, 12, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Figueroa, G.; Ruiz-Aguilar, G.M.L.; Cuevas-Rodriguez, G.; Sanchez, G.G. Cotton fabric dyeing with cochineal extract: Influence of mordant concentration. Color. Technol. 2011, 127, 39–46. [Google Scholar] [CrossRef]
- Velmurugan, P. Studies on the Production and Dyeing Properties of Water Soluble Pigments from Filamentous Fungi. Ph.D. Thesis, Bharathiar University, Coimbatore, India, 2008. [Google Scholar]
- Tseng, R.-L.; Wu, F.-C.; Juang, R.-S. Characteristics and applications of the Lagergren’s first-order equation for adsorption kinetics. J. Taiwan Inst. Chem. Eng. 2010, 41, 661–669. [Google Scholar] [CrossRef]
- Hill, C.G.J.; Grieger-Block, R.A. Kinetic data: Generation, interpretation, and use. Food Technol. 1980, 34, 55–66. [Google Scholar]
- Gupta, C.S.; Aggarwal, S. Dyeing wet blue goat nappa skin with a microbial colorant obtained from Penicillium minioluteum. J. Clean. Prod. 2016, 127, 585–590. [Google Scholar]
- Bechtold, T.; Mahmudali, A.; Mussak, R. Natural dyes for textile dyeing: A comparison of methods to assess the quality of Canadian golden rod plant material. Dyes Pigments 2007, 75, 287–293. [Google Scholar] [CrossRef]
- Nagia, F.A.; El-Mohamedy, R.S.R. Dyeing of wool with natural anthraquinone dyes from Fusarium oxysporum. Dyes Pigment. 2007, 75, 550–555. [Google Scholar] [CrossRef]
| Mordant | Concentration, % w/w | Code |
|---|---|---|
| Ferric chloride | 10 | F1 |
| 20 | F2 | |
| 30 | F3 | |
| Alum | 5 | A1 |
| 10 | A2 | |
| 15 | A3 |
| Experiment | Colour Coordinates | |||||
|---|---|---|---|---|---|---|
| L* | a* | b* | Chroma | Hue | ∆E* | |
| Wool | 93.89 ± 1.16 | 0.44 ± 0.22 | 5.15 ± 1.47 | 5.17 ± 1.47 | 84.94 ± 2.63 | 0.00 |
| F1 | 31.39 ± 1.11 b | 24.35 ± 0.44 c | 21.01 ± 0.20 c | 32.16 ± 0.23 b | 40.79 ± 0.76 b | 68.78 ± 0.96 b |
| F2 | 28.20 ± 0.64 a | 27.62 ± 0.68 b | 20.61 ± 0.41 bc | 34.47 ± 0.64 a | 36.73 ± 0.78 a | 72.76 ± 1.70 a |
| F3 | 27.53 ± 0.65 a | 28.91 ± 0.35 a | 21.87 ± 0.81 b | 36.25 ± 0.25 a | 37.10 ± 1.34 a | 74.14 ± 0.90 a |
| A1 | 44.47 ± 0.68 e | 14.84 ± 0.15 e | 27.34 ± 0.32 a | 31.11 ± 0.34 d | 61.50 ± 0.21 d | 56.07 ± 0.71 d |
| A2 | 42.48 ± 0.43 d | 15.07 ± 0.24 e | 27.83 ± 0.25 a | 31.65 ± 0.13 d | 61.56 ± 0.57 d | 58.09 ± 1.65 d |
| A3 | 37.08 ± 0.56 c | 17.14 ± 0.35 d | 27.76 ± 0.14 a | 32.63 ± 0.18 d | 58.32 ± 0.57 c | 63.40 ± 1.52 c |
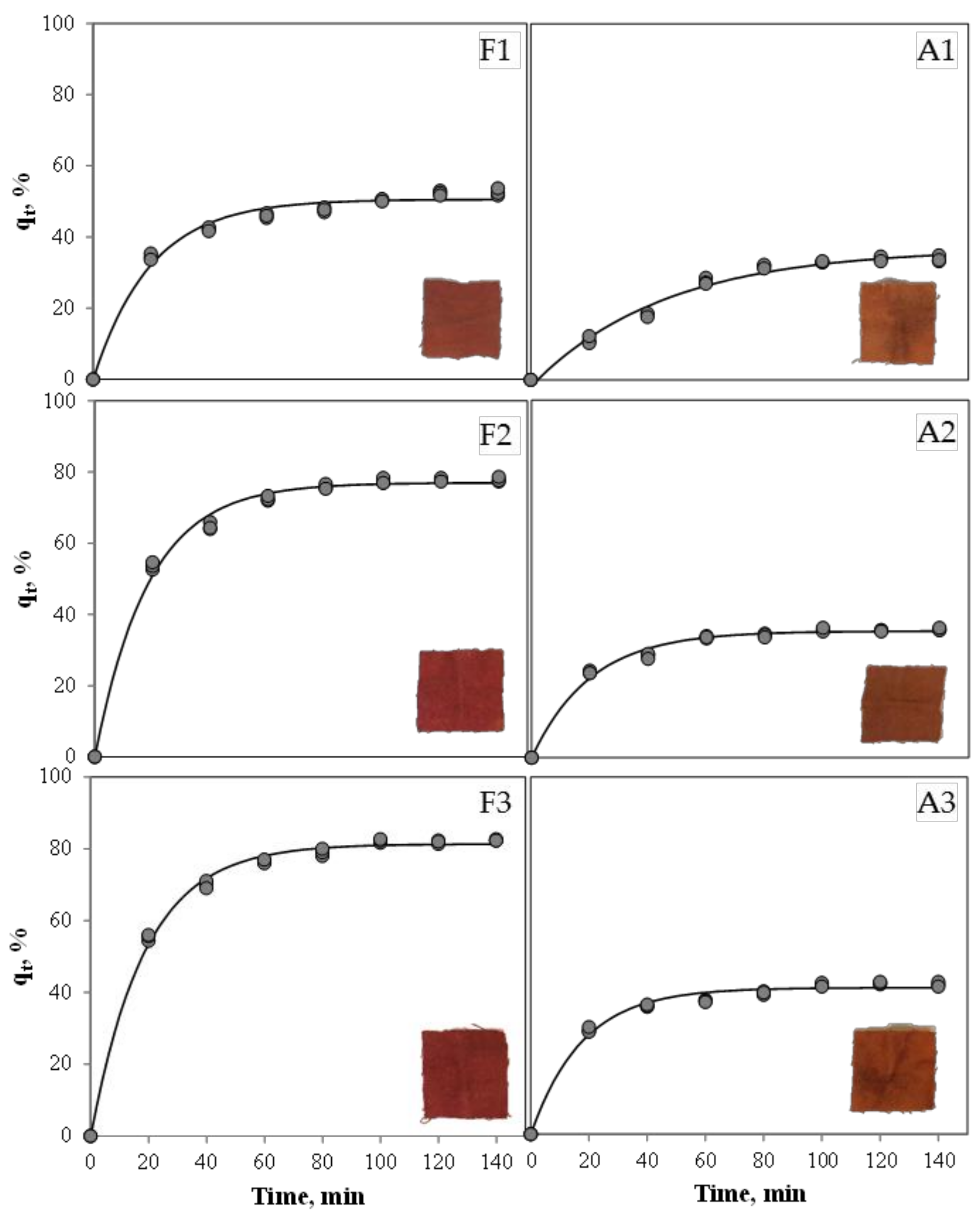
| Experiment | qr, % | k, min−1 | R2 |
|---|---|---|---|
| F1 | 50.56 ± 0.57 c | 0.0504 ± 0.0028 a | 0.98 |
| F2 | 76.99 ± 0.55 b | 0.0542 ± 0.0021 a | 0.99 |
| F3 | 81.33 ± 0.43 a | 0.0537 ± 0.0015 a | 0.99 |
| A1 | 36.91 ± 0.82 e | 0.0218 ± 0.0011 b | 0.98 |
| A2 | 35.40 ± 0.33 e | 0.0497 ± 0.0023 a | 0.99 |
| A3 | 41.02 ± 0.39 d | 0.0569 ± 0.0030 a | 0.99 |
| Experiment | ktr | t1/2, min | Zone |
|---|---|---|---|
| F1 | 7.06 | 13.74 | III |
| F2 | 7.58 | 12.79 | III |
| F3 | 7.51 | 12.92 | III |
| A1 | 3.04 | 31.84 | II |
| A2 | 6.95 | 13.95 | III |
| A3 | 7.97 | 12.17 | III |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Oyervides, L.; Oliveira, J.; Sousa-Gallagher, M.; Méndez-Zavala, A.; Montañez, J.C. Assessment of the Dyeing Properties of the Pigments Produced by Talaromyces spp. J. Fungi 2017, 3, 38. https://doi.org/10.3390/jof3030038
Morales-Oyervides L, Oliveira J, Sousa-Gallagher M, Méndez-Zavala A, Montañez JC. Assessment of the Dyeing Properties of the Pigments Produced by Talaromyces spp. Journal of Fungi. 2017; 3(3):38. https://doi.org/10.3390/jof3030038
Chicago/Turabian StyleMorales-Oyervides, Lourdes, Jorge Oliveira, Maria Sousa-Gallagher, Alejandro Méndez-Zavala, and Julio Cesar Montañez. 2017. "Assessment of the Dyeing Properties of the Pigments Produced by Talaromyces spp." Journal of Fungi 3, no. 3: 38. https://doi.org/10.3390/jof3030038