The Effect of Novel Heterocyclic Compounds on Cryptococcal Biofilm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cryptococcus Strains and Growth Conditions
2.2. Determination of Antifungal Susceptibility
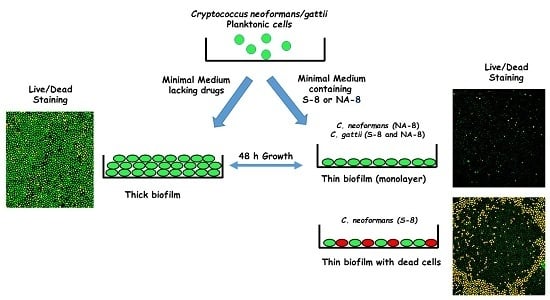
2.3. Biofilm Formation
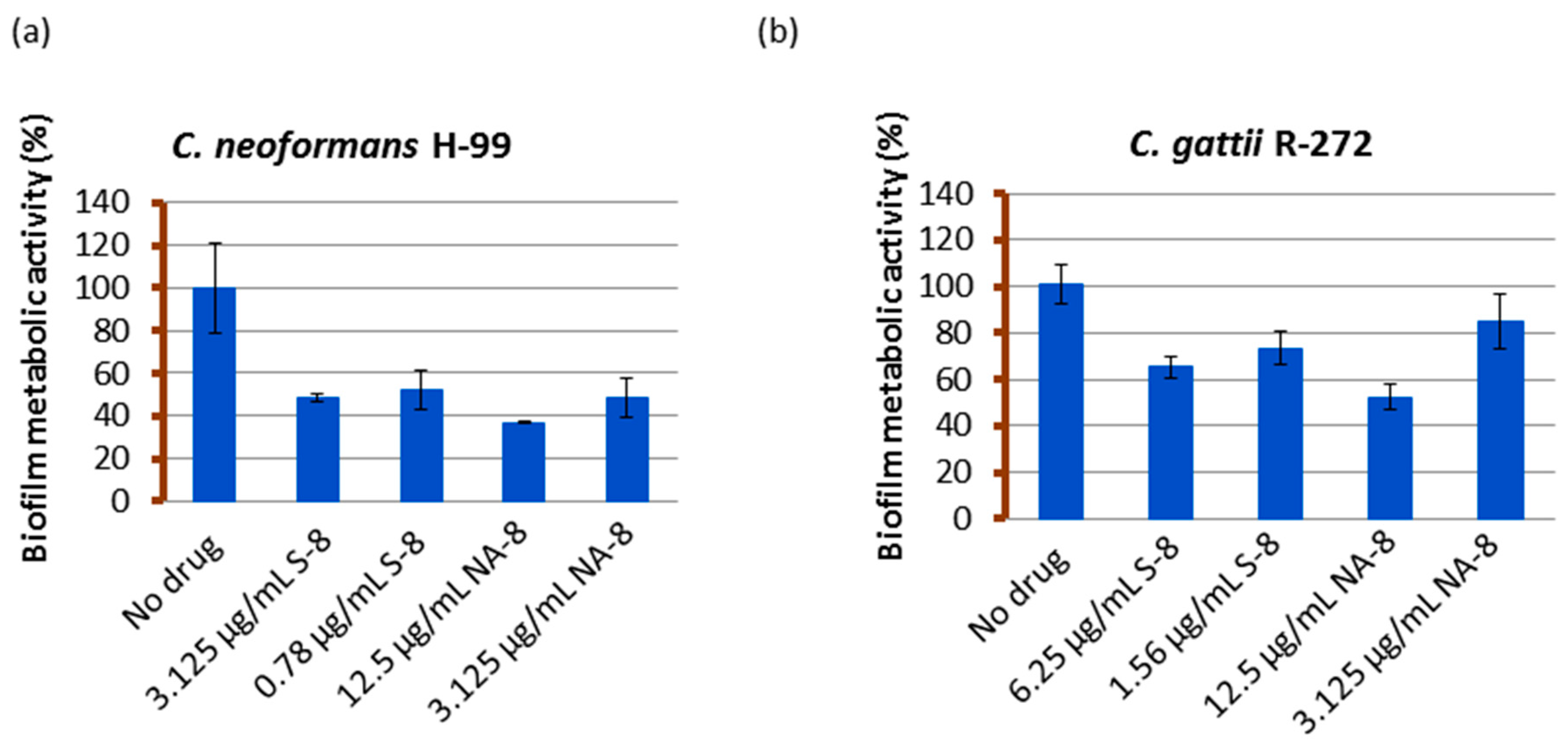
2.4. Measuring of Biofilm Metabolic Activity by XTT Reduction Assay
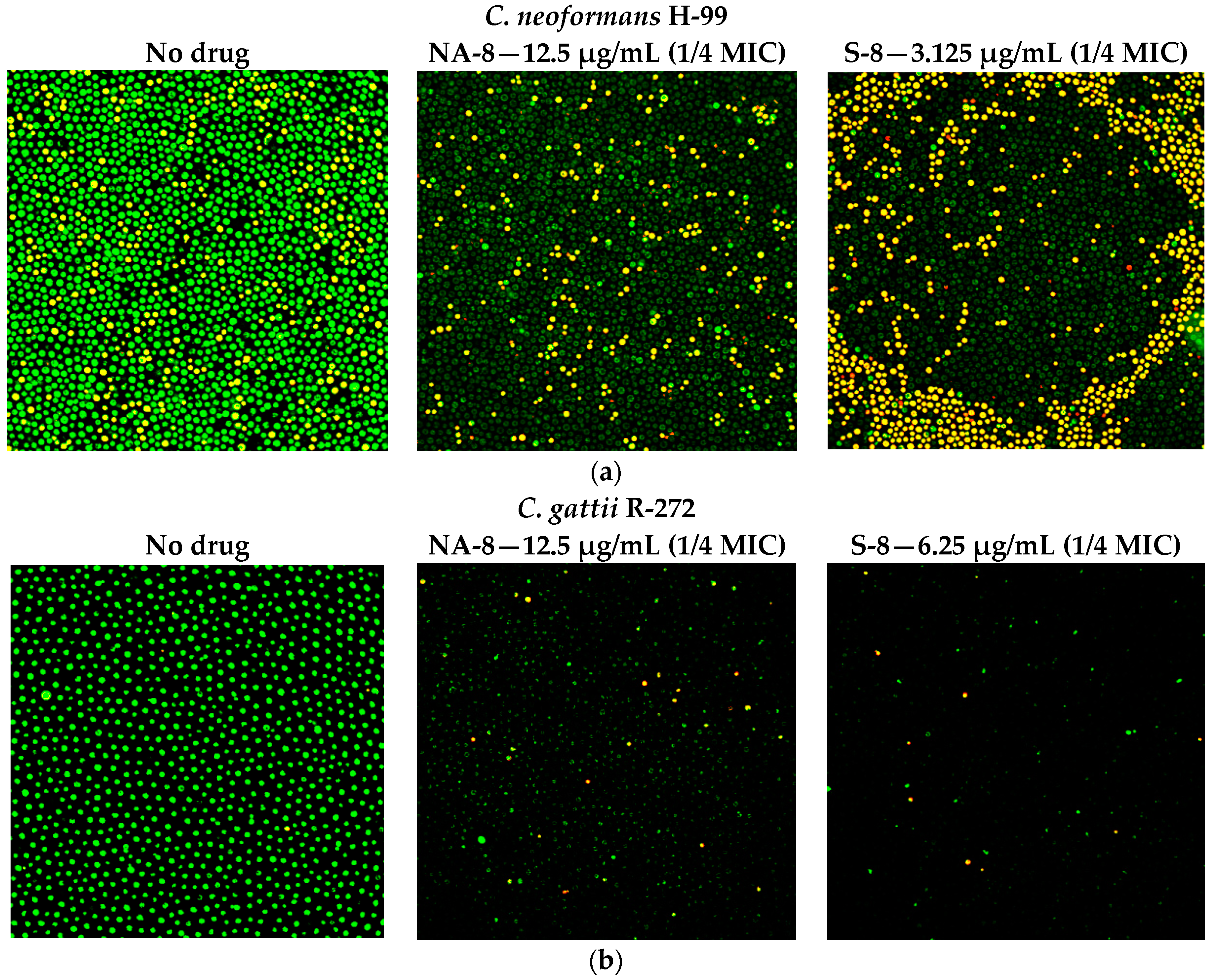
2.5. Fluorescent Stains
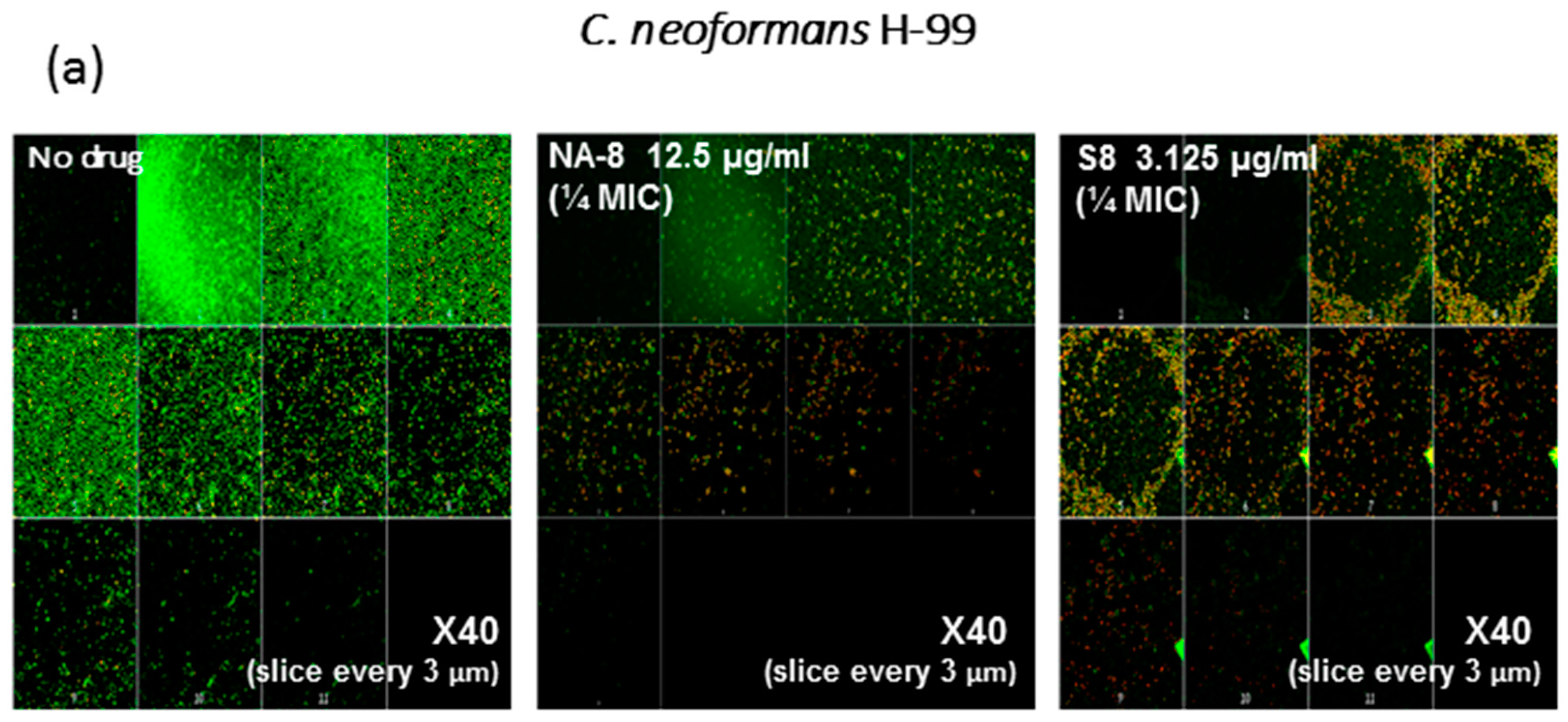
2.6. Confocal Microscopy
2.7. Statistical Analysis
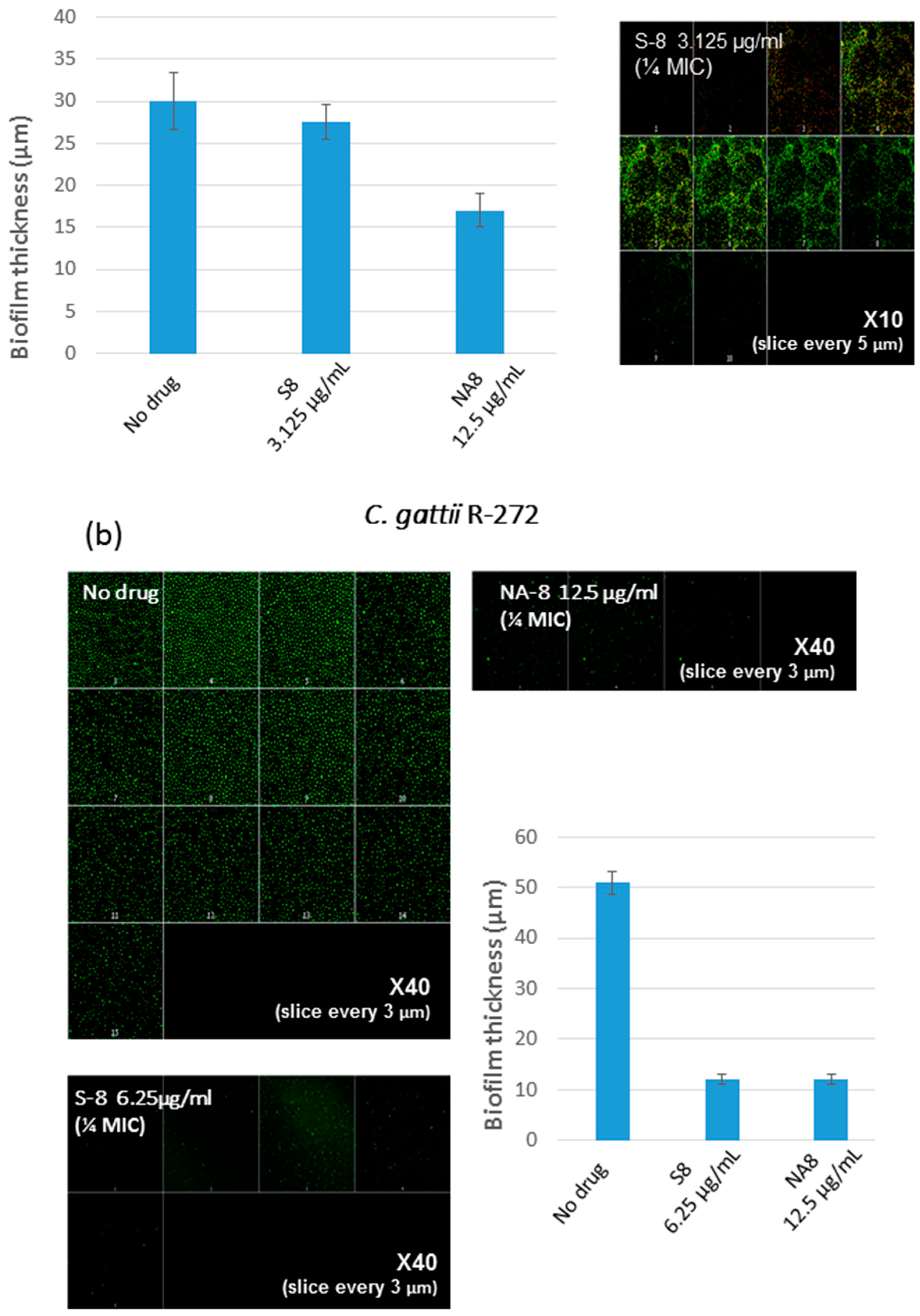
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mitchell, T.G.; Perfect, J.R. Cryptococcosis in the ERA of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 1995, 8, 515–548. [Google Scholar]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009, 23, 525–530. [Google Scholar] [CrossRef]
- Armstrong-James, D.; Meintjes, G.; Brown, G.D. A neglected epidemic: Fungal infections in HIV/AIDS. Trends Microbiol. 2014, 22, 120–127. [Google Scholar] [CrossRef]
- Albuquerque, P.; Nicola, A.M.; Nieves, E.; Paes, H.C.; Williamson, P.R.; Silva-Pereira, I.; Casadevall, A. Quorum sensing-mediated, cell density-dependent regulation of growth and virulence in Cryptococcus neoformans. mBio 2013, 5, e00986-13. [Google Scholar] [CrossRef]
- Martinez, L.R.; Casadevall, A. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect. Immun. 2005, 73, 6350–6362. [Google Scholar] [CrossRef]
- Walsh, T.J.; Schlegel, R.; Moody, M.M.; Costerton, J.W.; Salcman, M. Ventriculoatrial shunt infection due to Cryptococcus neoformans: An ultrastructural and quantitative microbiological study. Neurosurgery 1986, 18, 373–375. [Google Scholar] [CrossRef]
- Banerjee, U.; Gupta, K.; Venugopal, P. A case of prosthetic valve endocarditis caused by Cryptococcus neoformans var. neoformans. J. Med. Vet. Mycol. 1997, 35, 139–141. [Google Scholar] [CrossRef]
- Braun, D.K.; Janssen, D.A.; Marcus, J.R.; Kauffman, C.A. Cryptococcal infection of a prosthetic dialysis fistula. Am. J. Kidney Dis. 1994, 24, 864–867. [Google Scholar] [CrossRef]
- Penk, A.; Pittrow, L. Role of fluconazole in the long-term suppressive therapy of fungal infections in patients with artificial implants. Mycoses 1999, 42, S91–S96. [Google Scholar]
- Martinez, L.R.; Casadevall, A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 2006, 50, 1021–1033. [Google Scholar] [CrossRef]
- Martinez, L.R.; Casadevall, A. Biofilm formation by Cryptococcus neoformans. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Al-Fattani, M.A.; Douglas, L.J. Penetration of Candida biofilms by antifungal agents. Antimicrob. Agents Chemother. 2004, 48, 3291–3297. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Vande Walle, K.; Wickes, B.L.; López-Ribot, J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D. Resistance of human fungal pathogens to antifungal drugs. Curr. Opin. Microbiol. 2002, 5, 379–385. [Google Scholar] [CrossRef]
- Albuquerque, P.; Casadevall, A. Quorum sensing in fungi—A review. Med. Mycol. 2012, 50, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L. How bacteria talk to each other: Regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999, 2, 582–587. [Google Scholar] [CrossRef]
- De Kievit, T.R.; Iglewski, B.H. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 2000, 68, 4839–4849. [Google Scholar] [CrossRef] [PubMed]
- Deveau, A.; Hogan, D.A. Linking quorum sensing regulation and biofilm formation by Candida albicans. Methods Mol. Biol. 2011, 692, 219–233. [Google Scholar] [PubMed]
- Han, T.L.; Cannon, R.D.; Villas-Bôas, S.G. The metabolic basis of Candida albicans morphogenesis and quorum sensing. Fungal Genet. Biol. 2011, 48, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Saville, S.P.; Wickes, B.L.; López-Ribot, J.L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 2002, 68, 5459–5463. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Schulz, B.; Ruhnke, M. The quorum-sensing molecule e,e-farnesol—Its variable secretion and its impact on the growth and metabolism of candida species. Yeast 2010, 27, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Quntar, A.A.; Enk, C.D.; Karalic, I.; Nelis, H.J.; Calenbergh, S.V.; Srebnik, M.; Coenye, T. Synthesis and evaluation of thiazolidinedione and dioxazaborocane analogues as inhibitors of AI-2 quorum sensing in Vibrio harveyi. Bioorg. Med. Chem. 2013, 21, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Kagan, S.; Jabbour, A.; Sionov, E.; Alquntar, A.A.; Steinberg, D.; Srebnik, M.; Nir-Paz, R.; Weiss, A.; Polacheck, I. Anti-Candida albicans biofilm effect of novel heterocyclic compounds. J. Antimicrob. Chemother. 2014, 69, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.J.; Douglas, L.M.; Konopka, J.B. The Sur7 protein resides in punctate membrane subdomains and mediates spatial regulation of cell wall synthesis in Candida albicans. Commun. Integr. Biol. 2009, 2, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.J.; Douglas, L.M.; Rosebrock, A.; Konopka, J.B. The Sur7 protein regulates plasma membrane organization and prevents intracellular cell wall growth in Candida albicans. Mol. Biol. Cell 2008, 19, 5214–5225. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.M.; Wang, H.X.; Keppler-Ross, S.; Dean, N.; Konopka, J.B. Sur7 promotes plasma membrane organization and is needed for resistance to stressful conditions and to the invasive growth and virulence of Candida albicans. mBio 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Nelson, J.; VanderSluis, B.; Deshpande, R.; Butts, A.; Kagan, S.; Polacheck, I.; Krysan, D.J.; Myers, C.L.; Madhani, H.D. Unraveling the biology of a fungal meningitis pathogen using chemical genetics. Cell 2014, 159, 1168–1187. [Google Scholar] [CrossRef] [PubMed]
- Vecchiarelli, A. Immunoregulation by capsular components of Cryptococcus neoformans. Med. Mycol. 2000, 38, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Soustre, J.; Rodier, M.H.; Imbert-Bouyer, S.; Daniault, G.; Imbert, C. Caspofungin modulates in vitro adherence of Candida albicans to plastic coated with extracellular matrix proteins. J. Antimicrob. Chemother. 2004, 53, 522–525. [Google Scholar] [CrossRef] [PubMed]
- De Sordi, L.; Mühlschlegel, F.A. Quorum sensing and fungal-bacterial interactions in Candida albicans: A communicative network regulating microbial coexistence and virulence. FEMS Yeast Res. 2009, 9, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.; Cheung, B.P.; Watt, R.M.; Jin, L.J.; Samaranayake, L.P. Secretory products of Escherichia coli biofilm modulate candida biofilm formation and hyphal development. J. Investig. Clin. Dent. 2013, 4, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.R.; Graham, C.E.; Gagliano, B.C.; Lorenz, M.C.; Garsin, D.A. Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans. Infect. Immun. 2013, 81, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.K.; Grahl, N.; Okegbe, C.; Dietrich, L.E.; Jacobs, N.J.; Hogan, D.A. Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines. mBio 2013, 4, e00526-12. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Al-Quntar, A.; Polacheck, I.; Friedman, M.; Steinberg, D. Therapeutic potential of thiazolidinedione-8 as an antibiofilm agent against Candida albicans. PLoS ONE 2014, 9, e93225. [Google Scholar] [CrossRef] [PubMed]
| Compound and Structure | MIC a (µg/mL) | |
|---|---|---|
| C. neoformans H-99 | C. gattii R-272 | |
S-8 | 12.5 | 25 |
NA-8 | 50 | 50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korem, M.; Kagan, S.; Polacheck, I. The Effect of Novel Heterocyclic Compounds on Cryptococcal Biofilm. J. Fungi 2017, 3, 42. https://doi.org/10.3390/jof3030042
Korem M, Kagan S, Polacheck I. The Effect of Novel Heterocyclic Compounds on Cryptococcal Biofilm. Journal of Fungi. 2017; 3(3):42. https://doi.org/10.3390/jof3030042
Chicago/Turabian StyleKorem, Maya, Sarah Kagan, and Itzhack Polacheck. 2017. "The Effect of Novel Heterocyclic Compounds on Cryptococcal Biofilm" Journal of Fungi 3, no. 3: 42. https://doi.org/10.3390/jof3030042