Innate and Adaptive Immunity to Mucorales
Abstract
:1. Introduction
2. The Innate Immune Response to Mucorales
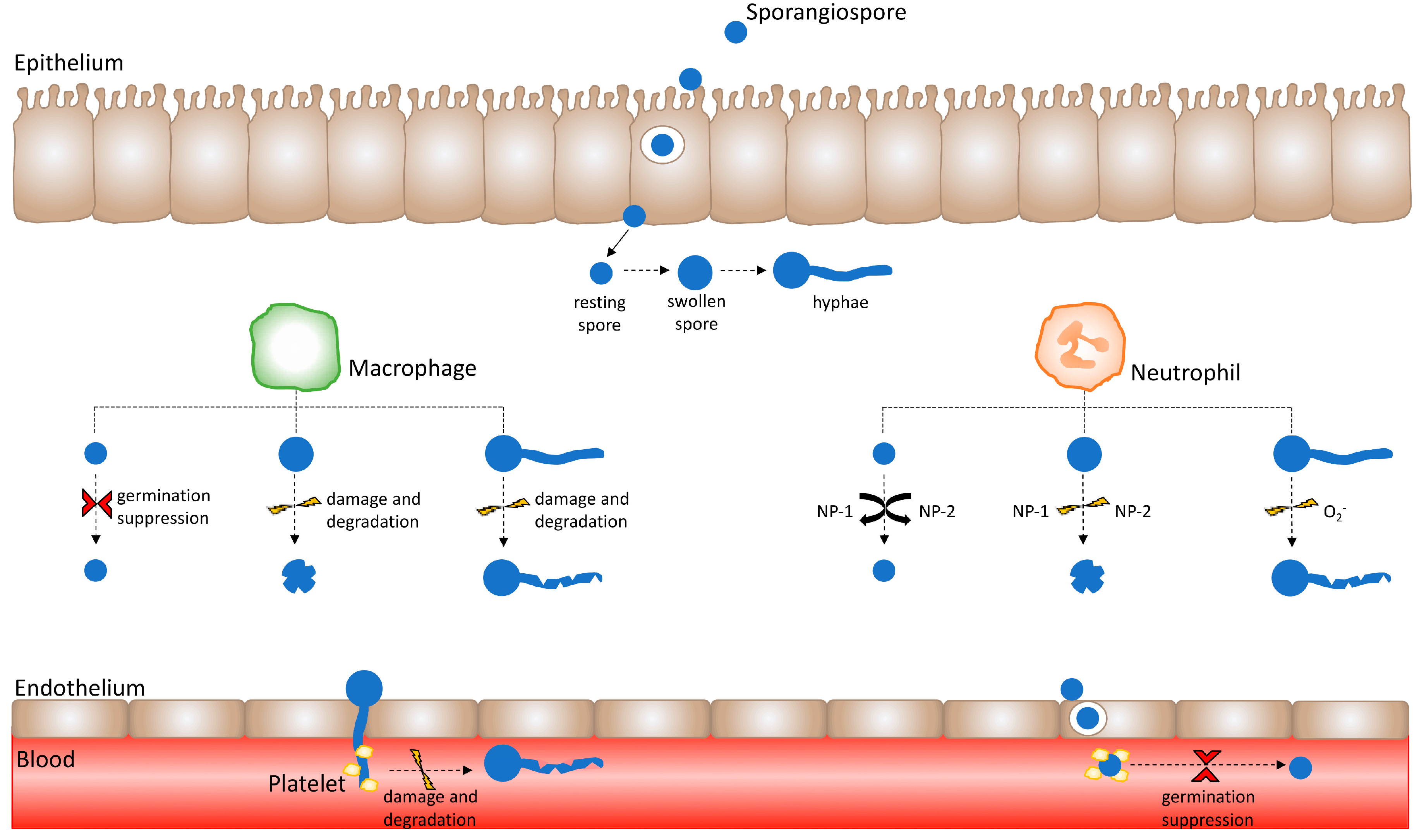
2.1. Epithelial Interaction with Mucorales
2.2. Macrophage Response to Mucorales
2.3. Neutrophil Response to Mucorales
2.4. Endothelial Interaction with Mucorales
2.5. Platelet-Mucorales Interaction
2.6. Dendritic Cell–Mucorales Interaction
2.7. Natural Killer Cell Response to Mucorales
3. The Adaptive Immune Response to Mucorales
T-Cell Responses to Mucorales
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and Clinical Manifestations of Mucormycosis. Clin. Infect. Dis. 2012, 54, S23–S34. [Google Scholar] [CrossRef] [PubMed]
- Chibucos, M.C.; Soliman, S.; Gebremariam, T.; Lee, H.; Daugherty, S.; Orvis, J.; Shetty, A.C.; Crabtree, J.; Hazen, T.H.; Etienne, K.A.; et al. An integrated genomic and transcriptomic survey of mucormycosis-causing fungi. Nat. Commun. 2016, 7, 12218. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lewis, R.E.; Hu, J.; Xiao, L.; Zal, T.; Gilliet, M.; Halder, G.; Kontoyiannis, D.P. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc. Natl. Acad. Sci. USA 2008, 105, 9367–9372. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of mucormycosis. Clin. Infect. Dis. 2012, 54, S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.Z.; Lewis, R.E.; Kontoyiannis, D.P. Mucormycosis Caused by Unusual Mucormycetes, Non-Rhizopus, -Mucor, and -Lichtheimia Species. Clin. Microbiol. Rev. 2011, 24, 411–445. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Edwards, J., Jr.; Ibrahim, A.S. Novel Perspectives on Mucormycosis: Pathophysiology, Presentation, and Management. Clin. Microbiol. Rev. 2005, 18, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Sarvestani, A.S.; Pishdad, G.; Bolandparvaz, S. Predisposing Factors for Mucormycosis in Patients with Diabetes Mellitus; An Experience of 21 Years in Southern Iran. Bull. Emerg. Trauma 2013, 1, 164–170. [Google Scholar]
- Nam, Y.; Jung, J.; Park, S.S.; Kim, S.J.; Shin, S.J.; Choi, J.H.; Kim, M.; Yoon, H.E. Disseminated mucormycosis with myocardial involvement in a renal transplant recipient. Transpl. Infect. Dis. 2015, 17, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Nateson, S.K.; Chandrasekar, P.H. Isavuconazole for the treatment of invasive aspergillosis and mucormycosis: Current evidence, safety, efficacy, and clinical recommendations. Infect. Drug Resist. 2016, 9, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Neblett Fanfair, R.; Benedict, K.; Bos, J.; Bennett, S.D.; Lo, Y.C.; Adebanjo, T.; Etienne, K.; Deak, E.; Derado, G.; Shieh, W.J.; et al. Necrotizing Cutaneous Mucormycosis after a Tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 2012, 367, 2214–2225. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Rigopoulos, D.; Larios, G.; Petrikkos, G.; Katsambas, A. Global epidemiology of cutaneous zygomycosis. Clin. Dermatol. 2012, 30, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B. Gastrointestinal Mucormycosis an Evolving Disease. Gastroenterol. Hepatol. 2012, 8, 140–142. [Google Scholar]
- Lee, S.C.; Billmyre, R.B.; Li, A.; Carson, S.; Sykes, S.M.; Huh, E.Y.; Mieczkowski, P.; Ko, D.C.; Cuomo, C.A.; Heitman, J. Analysis of a Food-Borne Fungal Pathogen Outbreak: Virulence and Genome of a Mucor circinelloides Isolate from Yogurt. mBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Waldorf, A.R.; Ruderman, N.; Diamond, R.D. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J. Clin. Investig. 1984, 74, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Voelz, K.; Gratacap, R.L.; Wheeler, R.T. A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides. Dis. Model. Mech. 2015, 8, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, W.H.; Bauer, H. Development of the acute inflammatory response to experimental cutaneous mucormycosis in normal and diabetic rabbits. J. Exp. Med. 1959, 110, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Bouchara, J.P.; Oumeziane, N.A.; Lissitzky, J.C.; Larcher, G.; Tronchin, G.; Chabasse, D. Attachment of spores of the human pathogenic fungus Rhizopus. oryzae to extracellular matrix components. Eur. J. Cell Biol. 1996, 70, 76–83. [Google Scholar] [PubMed]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.S.; Edwards, J.E., Jr.; Filler, S.G.; Ibrahim, A.S. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Investig. 2010, 120, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Spreer, A.; Ruchel, R.; Reichard, U. Characterization of an extracellular subtilisin protease of Rhizopus microsporus and evidence for its expression during invasive rhinoorbital mycosis. Med. Mycol. 2006, 44, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.V.; Rombouts, F.M.; Nout, M.J. Effect of individual amino acids and glucose on activation and germination of Rhizopus oligosporus sporangiospores in tempe starter. J. Appl. Microbiol. 2005, 99, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Kraibooj, K.; Park, H.R.; Dahse, H.M.; Skerka, C.; Voigt, K.; Figger, M.T. Virulent strain of Lichtheimia corymbifera shows increased phagocytosis by macrophages as revealed by automated microscopy image anaylsis. Mycoses 2014, 57, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Levitz, S.M.; Selsted, M.E.; Ganz, T.; Lehrer, R.I.; Diamond, R.D. In Vitro Killing of Spores and Hyphae of Aspergillus fumigatus and Rhizopus oryzae by Rabbit Neutrophil Cationic Peptides and Bronchoalveolar Macrophages. J. Infect. Dis. 1986, 154, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Waldorf, A.R.; Levitz, S.M.; Diamond, R.D. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol. Ser. 1989, 47, 243–271. [Google Scholar] [PubMed]
- Paduraru, M.; Moreno-Sanz, C.; Olalla Gallardo, J.M. Primary cutaneous mucormycosis in an immunocompetent patient. BMJ Case Rep. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Khiste, J.A.; Bolde, S.A.; Pandit, G.A.; Tamboli, N.A.S. Mucormycosis of Maxillary Sinus in Immunocompetent Patient Masquerading as Neoplasm: A Case Report. Int. J. Oral Maxillofac. Pathol. 2013, 4, 50–53. [Google Scholar]
- De Oliveira-Neto, M.P.; Da Silva, M.; Fialho Monteiro, P.C.; Lazera, M.; de Almeida Paes, R.; Novellino, A.B.; Cuzzi, T. Cutaneous mucormycosis in a young, immunocompetent girl. Med. Mycol. 2006, 44, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Waldorf, A.R.; Levitz, S.M.; Diamond, R.D. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J. Infect. Dis. 1984, 150, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D. Towards a comprehensive understanding of the role of neutrophils in innate immunity: A systems biology-level approach. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 309–333. [Google Scholar] [CrossRef] [PubMed]
- El Kebir, D.; Filep, J.G. Role of neutrophil apoptosis in the resolution of inflammation. Sci. World J. 2010, 10, 1731–1748. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Gamaletsou, M.N. Treatment of fungal disease in the setting of neutropenia. Hematol. Am. Soc. Hematol. Educ. Program. 2013, 2013, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Ricci, P.; Tonso, A.; Nosari, A.; Cudillo, L.; Montillo, M.; Cenacchi, A.; Pacilli, L.; Fabbiano, F.; Del Favero, A. Mucormycosis in patients with haematological malignancies: A retrospective clinical study of 37 cases. GIMEMA Infection Program (Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto). Br. J. Haemotol. 1997, 99, 331–336. [Google Scholar] [CrossRef]
- Waldorf, A.R.; Diamond, R.D. Neutrophil chemotactic responses induced by fresh and swollen Rhizopus oryzae spores and Aspergillus fumigatus conidia. Infect. Immun. 1985, 48, 458–463. [Google Scholar] [PubMed]
- Coutinho, H.D.M.; Lôbo, K.M.; Bezerra, D.A.C.; Lôbo, I. Peptides and proteins with antimicrobial activity. Indian J. Pharmacol. 2008, 40, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lewis, R.E.; Lamaris, G.; Walsh, T.J.; Kontoyiannis, D.P. Zygomycetes Hyphae Trigger an Early, Robust Proinflammatory Response in Human Polymorphonuclear Neutrophils through Toll-Like Receptor 2 Induction but Display Relative Resistance to Oxidative Damage. Antimicrob. Agents Chemother. 2008, 52, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Chinn, R.Y.; Diamond, R.D. Generation of Chemotactic Factors by Rhizopus oryzae in the Presence and Absence of Serum: Relationship to Hyphal Damage Mediated by Human Neutrophils and Effects of Hyperglycemia and Ketoacidosis. Infect. Immun. 1982, 38, 1123–1129. [Google Scholar] [PubMed]
- Lecube, A.; Pachón, G.; Petriz, J.; Hernández, C.; Simó, R. Phagocytic Activity Is Impaired in Type 2 Diabetes Mellitus and Increases after Metabolic Improvement. PLoS ONE 2011, 6, e23366. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.N.; Kerrigan, S.W.; Cox, D.; Henderson, I.R.; Watson, S.P.; Arman, M. Human platelet activation by Escherichia coli: Roles for FcgammaRIIA and integrin alphaIIbbeta3. Platelets 2016, 27, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Morrell, C.N.; Aggrey, A.A.; Chapman, L.M.; Modejski, K.L. Emerging roles for platelet as immune and inflammatory cells. Blood 2014, 123, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Perkhofer, S.; Kainzner, B.; Kehrel, B.E.; Dierich, M.P.; Nussbaumer, W.; Lass-Flörl, C. Potential Antifungal Effects of Human Platelets against Zygomycetes In Vitro. J. Infect. Dis. 2009, 200, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Noorifard, M.; Sekhavati, E.; Jalaei Khoo, H.; Hazraty, I.; Tabrizi, R. Epidemiology and clinical manifestation of fungal infection related to Mucormycosis in hematologic malignancies. J. Med. Life 2015, 8, 32–37. [Google Scholar] [PubMed]
- LeBlanc, D.M.; Barousse, M.M.; Fidel, P.L., Jr. Role for Dendritic Cells in Immunoregulation during Experimental Vaginal Candidiasis. Infect. Immun. 2006, 74, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.J.; Charleston, B.; Stephens, S.A.; Sopp, P.; Hope, J.C. The role of dendritic cells in the innate immune system. Anim. Health Res. Rev. 2004, 5, 191–195. [Google Scholar] [CrossRef] [PubMed]
- D’Ostiani, C.F.; Sero, G.D.; Bacci, A.; Montagnoli, C.; Spreca, A.; Mencacci, A.; Ricciardi-Castagnoli, P.; Romani, L. Dendritic Cells Discriminate between Yeasts and Hyphae of the Fungus Candida albicans: Implications for Initiation of T Helper Cell Immunity In Vitro and In Vivo. J. Exp. Med. 2000, 191, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Hejazi, A.S.; Wei, S.H.; Cahalan, M.D.; Parker, I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc. Natl. Acad. Sci. USA 2004, 101, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Ganguly, D.; Lande, R.; Gregorio, J.; Meller, S.; Goldman, W.E.; Gilliet, M.; Kontoyiannis, D.P. Generation of IL-23 Producing Dendritic Cells (DCs) by Airborne Fungi Regulates Fungal Pathogenicity via the Induction of TH-17 Responses. PLoS ONE 2010, 5, e12955. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Eastman, A.J.; Flaczyk, A.; Neal, L.M.; Zhao, G.; Carolan, J.; Malachowski, A.N.; Stolberg, V.R.; Yosri, M.; Chensue, S.W.; et al. Disruption of Early Tumor Necrosis Factor Alpha Signaling Prevents Classical Activation of Dendritic Cells in Lung-Associated Lymph Nodes and Development of Protective Immunity against Cryptococcal Infection. mBio 2016, 7, e00510-16. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Tramsen, L.; Perkhofer, S.; Lass-Florl, C.; Hanisch, M.; Röger, F.; Klingebiel, T.; Koehl, U.; Lehrnbecher, T. Rhizopus oryzae hyphae are damaged by human natural killer (NK) cells, but suppress NK cell mediated immunity. Immunobiology 2013, 218, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Kontoyiannis, D.P. Update on mucormycosis pathogenesis. Curr. Opin. Infect. Dis. 2013, 26, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Kasten, K.R.; Prakash, P.S.; Unsinger, J.; Goetzman, H.S.; England, L.G.; Cave, C.M.; Seitz, A.P.; Mazuski, C.N.; Zhou, T.T.; Morre, M.; et al. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infect. Immun. 2010, 78, 4714–4722. [Google Scholar] [CrossRef] [PubMed]
- Potenza, L.; Vallerini, D.; Barozzi, P.; Riva, G.; Forghieri, F.; Zanetti, E.; Quadrelli, C.; Candoni, A.; Maertens, J.; Rossi, G.; et al. Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic in high risk patients. Blood 2011, 118, 5416–5419. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghuman, H.; Voelz, K. Innate and Adaptive Immunity to Mucorales. J. Fungi 2017, 3, 48. https://doi.org/10.3390/jof3030048
Ghuman H, Voelz K. Innate and Adaptive Immunity to Mucorales. Journal of Fungi. 2017; 3(3):48. https://doi.org/10.3390/jof3030048
Chicago/Turabian StyleGhuman, Harlene, and Kerstin Voelz. 2017. "Innate and Adaptive Immunity to Mucorales" Journal of Fungi 3, no. 3: 48. https://doi.org/10.3390/jof3030048




