Fungicidal Activity in the Presence of Keratin as an Important Factor Contributing to In Vivo Efficacy: A Comparison of Efinaconazole, Tavaborole, and Ciclopirox
Abstract
:1. Introduction
2. Materials and Methods
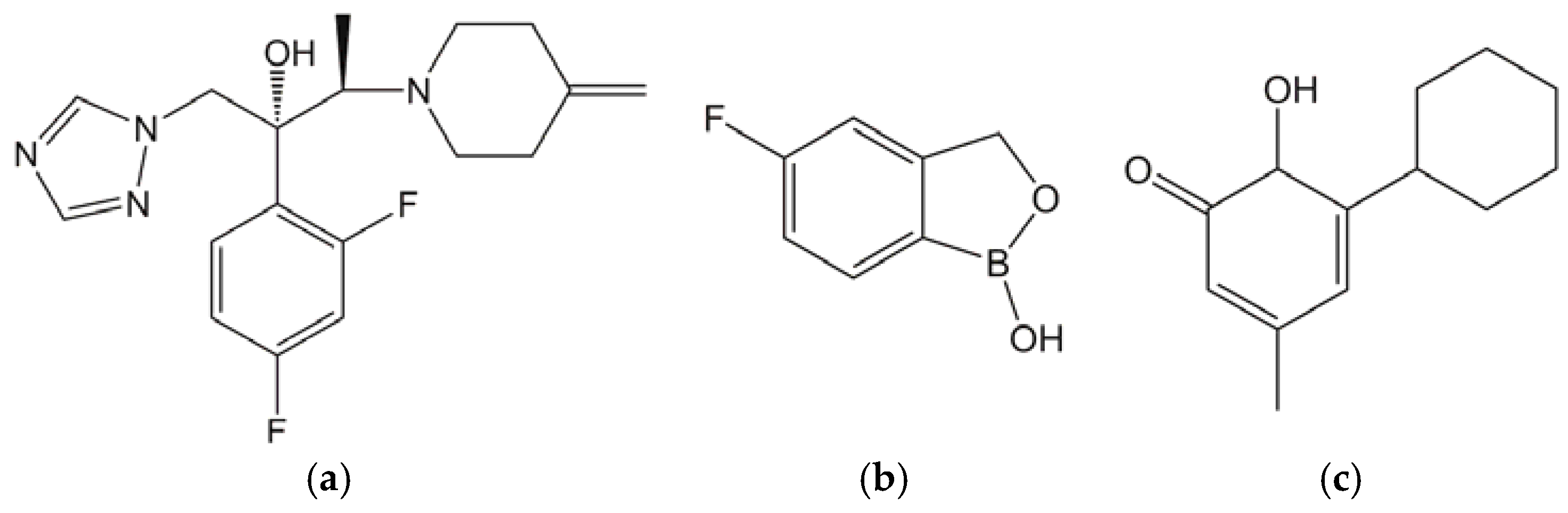
2.1. Test Substances
2.2. Media and Keratin
2.3. Test Organisms
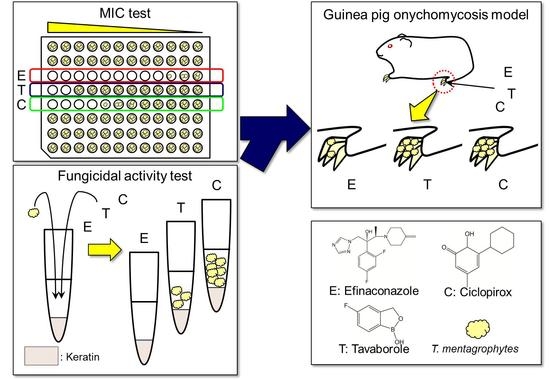
2.4. MIC Study of Antifungals for T. rubrum and T. mentagrophytes
2.5. Time-Kill Study of Antifungals against T. mentagrophytes in the Presence of Keratin
2.6. Therapeutic Efficacy of Topically Applied Antifungals in a Guinea Pig Onychomycosis Model
3. Results
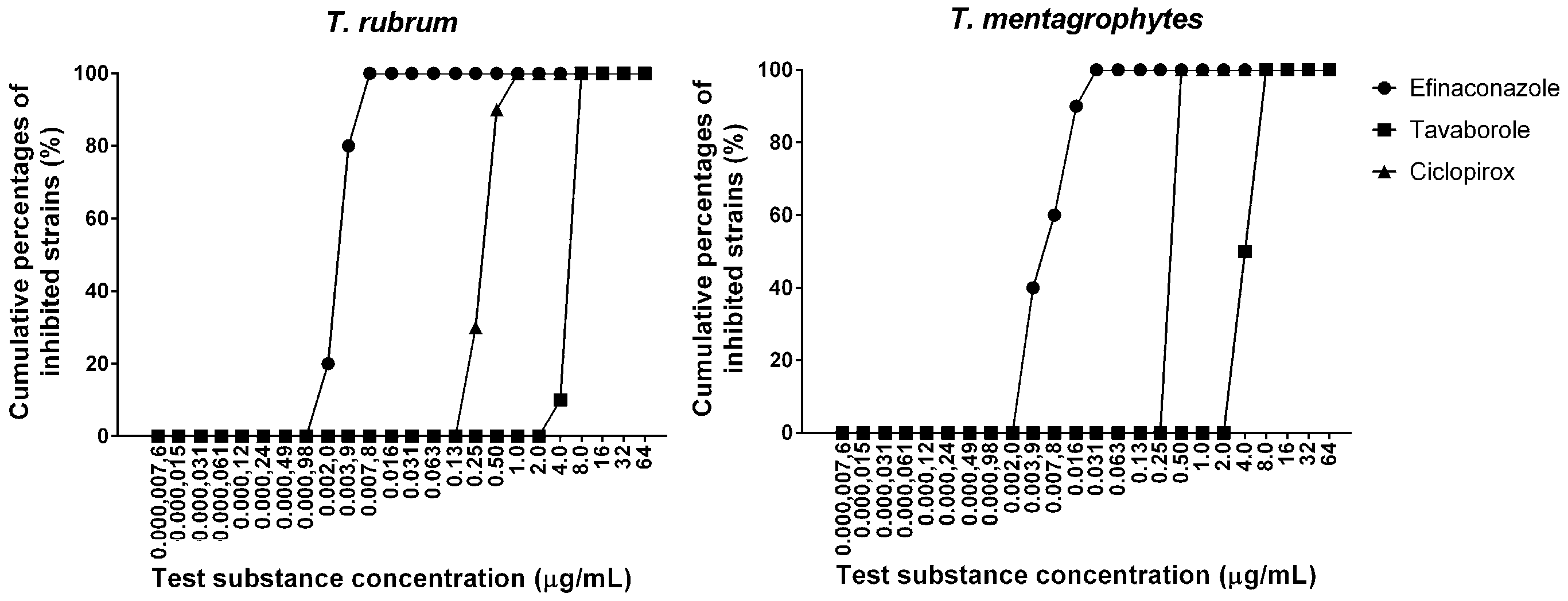
3.1. MIC Study of Antifungals for T. rubrum and T. mentagrophytes
3.2. Time-Kill Study of Antifungals against T. mentagrophytes in the Presence of Keratin
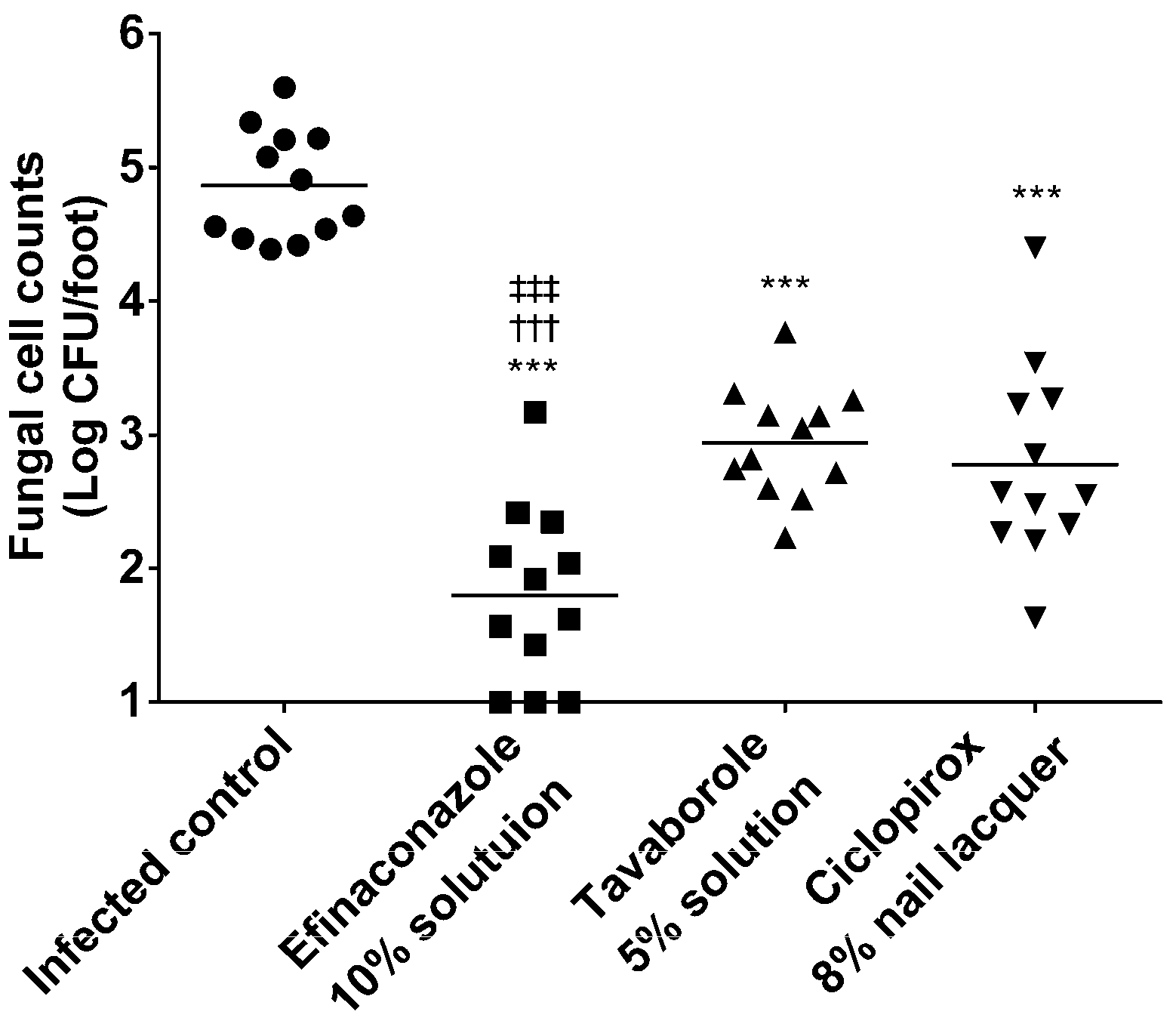
3.3. Therapeutic Efficacy in a Guinea Pig Onychomycosis Model
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, A.K.; Jain, H.C.; Lynde, C.W.; Macdonald, P.; Cooper, E.A.; Summerbell, R.C. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: A multicenter Canadian survey of 15,000 patients. J. Am. Acad. Dermatol. 2000, 43, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Harada, T.; Hiruma, M.; Iozumi, K.; Katoh, T.; Mochizuki, T.; Naka, W. Japan Foot Week Group Epidemiological survey of foot diseases in Japan: Results of 30,000 foot checks by dermatologists. J. Dermatol. 2010, 37, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.G.; Sigurgeirsson, B. Double blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. BMJ 1999, 318, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Niewerth, M.; Korting, H.C. Management of onychomycoses. Drugs 1999, 58, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E.; Ghannoum, M.A.; Mayser, P.; Gupta, A.K.; Korting, H.C.; Shouey, R.J.; Baker, D.R.; Rich, P.A.; Ling, M.; Hugot, S.; et al. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild-to-moderate toenail onychomycosis: Results from three randomized studies using double-blind vehicle-controlled and open-label active-controlled designs. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Fleckman, P.; Baran, R. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J. Am. Acad. Dermatol. 2000, 43, S70–S80. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E.; Rich, P.; Pollak, R.; Pariser, D.M.; Watanabe, S.; Senda, H.; Ieda, C.; Smith, K.; Pillai, R.; Ramakrishna, T.; et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: Two phase III multicenter, randomized, double-blind studies. J. Am. Acad. Dermatol. 2013, 68, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E.; Aly, R.; Baldwin, S.L.; González Soto, R.F.; Rich, P.; Weisfeld, M.; Wiltz, H.; Zane, L.T.; Pollak, R. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: Results from 2 randomized phase-III studies. J. Am. Acad. Dermatol. 2015, 73, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Simpson, F.C. Efinaconazole: A new topical treatment for onychomycosis. Skin Therapy Lett. 2014, 19, 1–4. [Google Scholar] [PubMed]
- Sugiura, K.; Sugimoto, N.; Hosaka, S.; Katafuchi-Nagashima, M.; Arakawa, Y.; Tatsumi, Y.; Jo, S.W.; Pillai, R. The low keratin affinity of efinaconazole contributes to its nail penetration and fungicidal activity in topical onychomycosis treatment. Antimicrob. Agents Chemother. 2014, 58, 3837–3842. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.W.; Tatsumi, Y.; Senda, H.; Pillai, R.; Nakamura, T.; Sone, D.; Fothergill, A. Comparison of in vitro antifungal activities of efinaconazole and currently available antifungal agents against a variety of pathogenic fungi associated with onychomycosis. Antimicrob. Agents Chemother. 2013, 57, 1610–1616. [Google Scholar]
- Coronado, D.; Merchant, T.; Chanda, S.; Zane, L.T. In vitro nail penetration and antifungal activity of tavaborole, a boron-based pharmaceutical. J. Drugs Dermatol. 2015, 14, 609–614. [Google Scholar] [PubMed]
- Zane, L.T.; Chanda, S.; Coronado, D.; Del Rosso, J. Antifungal agents for onychomycosis: New treatment strategies to improve safety. Dermatol. Online J. 2016, 22, doj_30383. [Google Scholar]
- Matsuda, Y.; Sugiura, K.; Hashimoto, T.; Ueda, A.; Konno, Y.; Tatsumi, Y. Efficacy coefficient determined using nail permeability and antifungal activity in keratin-containing media are useful for predicting clinical efficacies of topical drugs for onychomycosis. PLoS ONE 2016, 11, e0159661. [Google Scholar] [CrossRef] [PubMed]
- Hasuko, M.; Toga, T.; Tsunemitsu, T.; Matsumoto, T.; Koga, H.; Hirano, H.; Tsuboi, R. Affinity of Luliconazole to Keratin Prepared from Healthy Human Nail and Porcine Hoof. Med. Mycol. J. 2016, 57, J7–J12. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Matsuyama, T. Experimental tinea pedis induced by non-abrasive inoculation of Trichophyton mentagrophytes arthrospores on the plantar part of a guinea pig foot. J. Med. Vet. Mycol. 1987, 25, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, Y.; Yokoo, M.; Senda, H.; Kakehi, K. Therapeutic Efficacy of Topically Applied KP-103 against Experimental Tinea Unguium in Guinea Pigs in Comparison with Amorolfine and Terbinafine. Antimicrob. Agents Chemother. 2002, 46, 3797–3801. [Google Scholar] [CrossRef] [PubMed]
- Narasimha Murthy, S.; Wiskirchen, D.E.; Bowers, C.P. Iontophoretic drug delivery across human nail. J. Pharm. Sci. 2007, 96, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E.; Tosti, A. Tavaborole for the treatment of onychomycosis. Expert Opin. Pharmacother. 2014, 15, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Kaken Pharmaceutical Co., Ltd., Kyoto, Japan. Personal communication, 2017.

| Test Substances | MIC (μg/mL) | |||||
|---|---|---|---|---|---|---|
| T. rubrum (10 Strains) | T. mentagrophytes (10 Strains) | |||||
| Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | |
| Efinaconazole | 0.0020–0.0078 | 0.0039 | 0.0078 | 0.0039–0.031 | 0.0078 | 0.016 |
| Tavaborole | 4.0–8.0 | 8.0 | 8.0 | 4.0–8.0 | 4.0 | 8.0 |
| Ciclopirox | 0.25–1.0 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Drugs | Mycological Cure Rate (%) | Complete Cure Rate (%) | Reference |
|---|---|---|---|
| Efinaconazole 10% solution | 55.2, 53.4 | 17.8, 15.2 | [7] |
| Tavaborole 5% solution | 31.1, 35.9 | 6.5, 9.1 | [8] |
| Ciclopirox 8% nail lacquer | 29, 36 | 5.5, 8.5 | [6] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachibana, H.; Kumagai, N.; Tatsumi, Y. Fungicidal Activity in the Presence of Keratin as an Important Factor Contributing to In Vivo Efficacy: A Comparison of Efinaconazole, Tavaborole, and Ciclopirox. J. Fungi 2017, 3, 58. https://doi.org/10.3390/jof3040058
Tachibana H, Kumagai N, Tatsumi Y. Fungicidal Activity in the Presence of Keratin as an Important Factor Contributing to In Vivo Efficacy: A Comparison of Efinaconazole, Tavaborole, and Ciclopirox. Journal of Fungi. 2017; 3(4):58. https://doi.org/10.3390/jof3040058
Chicago/Turabian StyleTachibana, Haruki, Naomichi Kumagai, and Yoshiyuki Tatsumi. 2017. "Fungicidal Activity in the Presence of Keratin as an Important Factor Contributing to In Vivo Efficacy: A Comparison of Efinaconazole, Tavaborole, and Ciclopirox" Journal of Fungi 3, no. 4: 58. https://doi.org/10.3390/jof3040058