Mechanisms of Pulmonary Escape and Dissemination by Cryptococcus neoformans
Abstract
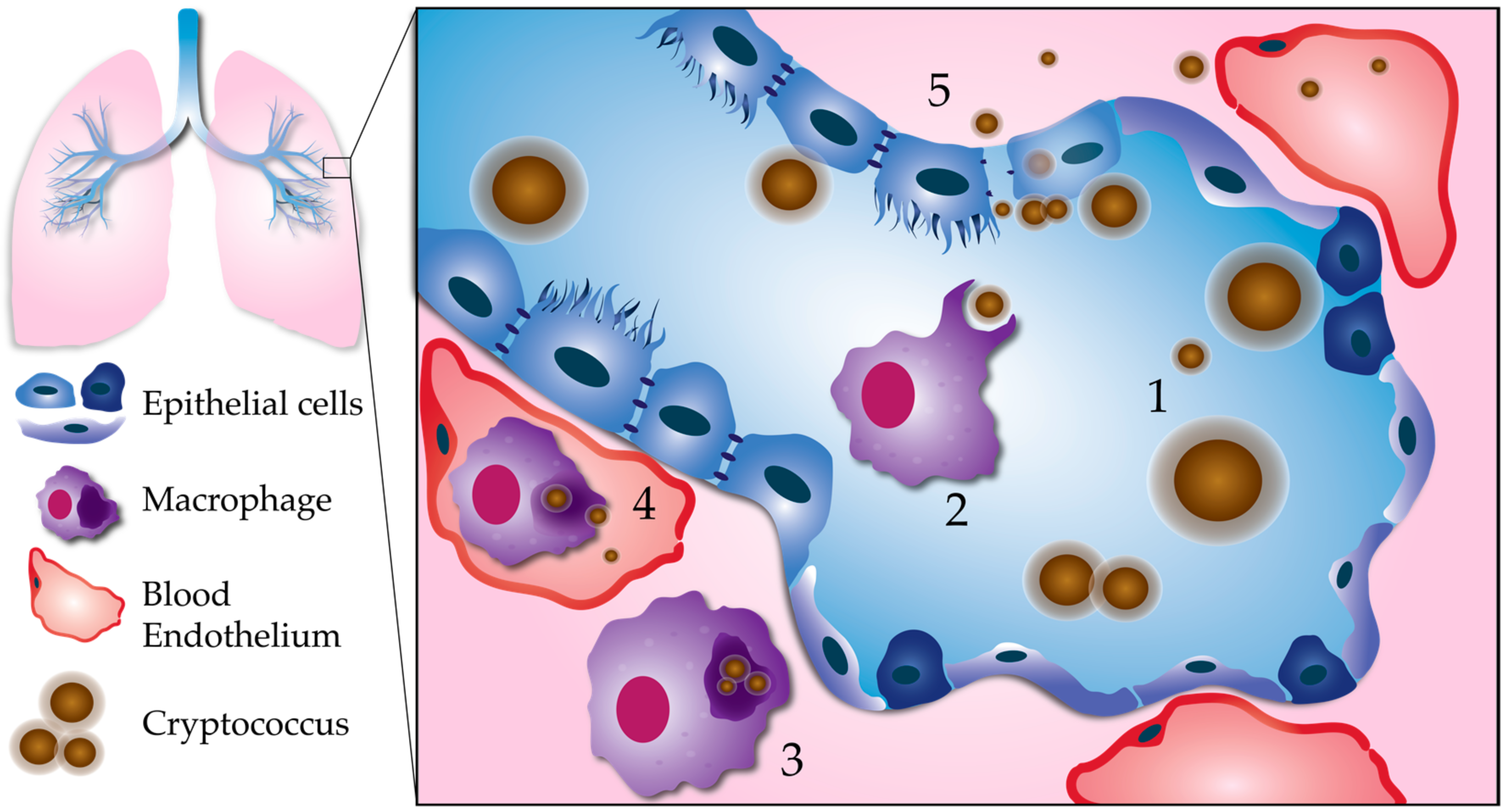
:1. Introduction
2. Routes of Escape
2.1. Intracellular Escape
2.1.1. Phagocytosis
2.1.2. Intracellular Survival
2.1.3. Escape from Macrophages
2.2. Extracellular Escape
3. Examples of Fungal Factors Influencing Pulmonary Escape
3.1. Cell Morphology
3.2. Age
3.3. Melanin
3.4. Phosphate Acquisition
3.5. Sphingolipids
4. Conclusions
5. Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fröhlich-Nowoisky, J.; Pickersgill, D.A.; Després, V.R.; Pöschl, U. High diversity of fungi in air particulate matter. Proc. Natl. Acad. Sci. USA 2009, 106, 12814–12819. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef] [PubMed]
- Lortholary, O.; Poizat, G.; Zeller, V.; Neuville, S.; Boibieux, A.; Alvarez, M.; Dellamonica, P.; Botterel, F.; Dromer, F.; Chêne, G.; et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS 2006, 20, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Wiesner, D.L.; Klein, B.S. Lung epithelium: Barrier immunity to inhaled fungi and driver of fungal-associated allergic asthma. Curr. Opin. Microbiol. 2017, 40, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2014, 16, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Weitnauer, M.; Mijošek, V.; Dalpke, A.H. Control of local immunity by airway epithelial cells. Mucosal Immunol. 2015, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.G.; Castaneda, E.; Nielsen, K.; Wanke, B.; Lazera, M.S. Environmental niches for Cryptococcus neoformans and Cryptococcus gattii. In Cryptococcus: From Human Pathogen to Model Yeast; ASM Press: Washington, DC, USA, 2011. [Google Scholar]
- Armstrong-James, D.; Meintjes, G.; Brown, G.D. A neglected epidemic: Fungal infections in HIV/AIDs. Trends Microbiol. 2014, 22, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.L.; Wang, Z.; Janbon, G.; Idnurm, A.; Bahn, Y.-S. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb. Perspect. Med. 2014, 4, a019760. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, E.J., III.; Li, W.; Lewit, Y.; Ma, H.; Voelz, K.; Ren, P.; Carter, D.A.; Chaturvedi, V.; Bildfell, R.J.; May, R.C.; et al. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest united states. PLoS Pathog. 2010, 6, e1000850. [Google Scholar] [CrossRef] [PubMed]
- May, R.C.; Stone, N.R.H.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2015, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Ballou, E.R.; Johnston, S.A. The cause and effect of Cryptococcus interactions with the host. Curr. Opin. Microbiol. 2017, 40, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D.L.; Khine, H.; Abadi, J.; Lindenberg, D.J.; Pirofski, L.-A.; Niang, R.; Casadevall, A. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 2001, 107, e66. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D.L.; Lee, S.C.; Mednick, A.J.; Montella, L.; Casadevall, A. Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect. Immun. 2000, 68, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hermoso, D.; Janbon, G.; Dromer, F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J. Clin. Microbiol. 1999, 37, 3204–3209. [Google Scholar] [PubMed]
- Gibson, J.F.; Johnston, S.A. Immunity to Cryptococcus neoformans and C. gattii during cryptococcosis. Fungal Genet. Biol. 2015, 78, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Caballero Van Dyke, M.C.; Wormley, F.L. A call to arms: Quest for a cryptococcal vaccine. Trends Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Tzao, C.; Hsu, H.-H.; Lee, S.-C.; Huang, K.-L.; Tung, H.-J.; Chen, C.-Y. Pulmonary cryptococcosis: Comparison of clinical and radiographic characteristics in immunocompetent and immunocompromised patients. Chest 2006, 129, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Shirley, R.M.; Baddley, J.W. Cryptococcal lung disease. Curr. Opin. Pulm. Med. 2009, 15, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-K.; Huang, T.-Y.; Wu, A.Y.-J.; Chen, H.-H.; Liu, C.-P.; Jong, A. How Cryptococcus interacts with the blood–brain barrier. Future Microbiol. 2015, 10, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Nielsen, K.; Daou, S.; Brigitte, M.; Chretien, F.; Dromer, F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect. Immun. 2009, 77, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Smith, L. Cryptococcus-epithelial interactions. J. Fungi 2017, 3, 53. [Google Scholar] [CrossRef] [PubMed]
- Feldmesser, M.; Kress, Y.; Novikoff, P.; Casadevall, A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 2000, 68, 4225–4237. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Stenzel, W.; Köhler, G.; Werner, C.; Polte, T.; Hansen, G.; Schütze, N.; Straubinger, R.K.; Blessing, M.; McKenzie, A.N.J.; et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 2007, 179, 5367. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Olszewski, M.A.; Tsang, T.M.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infect. Immun. 2011, 79, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Tirado, F.H.; Onken, M.D.; Cooper, J.A.; Klein, R.S.; Doering, T.L. Trojan horse transit contributes to blood–brain barrier crossing of a eukaryotic pathogen. mBio 2017, 8, e02183-16. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, B.; Mitchell, T.G. Phagocytosis and killing of Cryptococcus neoformans by rat alveolar macrophages in the absence of serum. J. Leukocyte Biol. 1989, 46, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Bojarczuk, A.; Miller, K.A.; Hotham, R.; Lewis, A.; Ogryzko, N.V.; Kamuyango, A.A.; Frost, H.; Gibson, R.H.; Stillman, E.; May, R.C.; et al. Cryptococcus neoformans intracellular proliferation and capsule size determines early macrophage control of infection. Sci. Rep. 2016, 6, 21489. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; Rodrigues, M.L.; De Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009, 68, 133–216. [Google Scholar] [PubMed]
- Doering, T.L. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Ann. Rev. Microbiol. 2009, 63, 223–247. [Google Scholar]
- O’Meara, T.R.; Alspaugh, J.A. The Cryptococcus neoformans capsule: A sword and a shield. Clin. Microbiol. Rev. 2012, 25, 387–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, O.W.; Chun, C.D.; Chow, E.D.; Chen, C.; Madhani, H.D.; Noble, S.M. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell 2008, 135, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.S.; Madhani, H.D. Approaching the functional annotation of fungal virulence factors using cross-species genetic interaction profiling. PLoS Genet. 2012, 8, e1003168. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.L.; Netski, D.; Thorkildson, P.; Kozel, T.R. Binding and internalization of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans, by murine peritoneal macrophages. Infect. Immun. 2006, 74, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Vecchiarelli, A.; Pericolini, E.; Gabrielli, E.; Kenno, S.; Perito, S.; Cenci, E.; Monari, C. Elucidating the immunological function of the Cryptococcus neoformans capsule. Future Microbiol. 2013, 8, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Chun, C.D.; Brown, J.C.S.; Madhani, H.D. A major role for capsule-independent phagocytosis-inhibitory mechanisms in mammalian infection by Cryptococcus neoformans. Cell Host Microbe 2011, 9, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Luberto, C.; Martinez-Mariño, B.; Taraskiewicz, D.; Bolaños, B.; Chitano, P.; Toffaletti, D.L.; Cox, G.M.; Perfect, J.R.; Hannun, Y.A.; Balish, E.; et al. Identification of app1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J. Clin. Investig. 2003, 112, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Stano, P.; Williams, V.; Villani, M.; Cymbalyuk, E.S.; Qureshi, A.; Huang, Y.; Morace, G.; Luberto, C.; Tomlinson, S.; Del Poeta, M. App1: An antiphagocytic protein that binds to complement receptors 3 and 2. J. Immunol. 2009, 182, 84. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.A. Primary isolation medium for Cryptococcus neoformans. Appl. Microbiol. 1969, 18, 1100. [Google Scholar] [PubMed]
- Diamond, R.D.; Bennett, J.E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect. Immun. 1973, 7, 231–236. [Google Scholar] [PubMed]
- Levitz, S.M.; Nong, S.-H.; Seetoo, K.F.; Harrison, T.S.; Speizer, R.A.; Simons, E.R. Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect. Immun. 1999, 67, 885–890. [Google Scholar] [PubMed]
- Smith, L.M.; Dixon, E.F.; May, R.C. The fungal pathogen Cryptococcus neoformans manipulates macrophage phagosome maturation. Cell. Microbiol. 2015, 17, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.A.; Maxfield, F.R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J. Cell Biol. 1984, 99, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Kronstad, J.W.; Hu, G.; Jung, W.H. An encapsulation of iron homeostasis and virulence in Cryptococcus neoformans. Trends Microbiol. 2013, 21, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Chayakulkeeree, M.; Johnston, S.A.; Oei, J.B.; Lev, S.; Williamson, P.R.; Wilson, C.F.; Zuo, X.; Leal, A.L.; Vainstein, M.H.; Meyer, W.; et al. Sec14 is a specific requirement for secretion of phospholipase B1 and pathogenicity of Cryptococcus neoformans. Mol. Microbiol. 2011, 80, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Wright, L.C.; Santangelo, R.T.; Muller, M.; Moran, V.R.; Kuchel, P.W.; Sorrell, T.C. Identification of extracellular phospholipase B, lysophospholipase, and acyltransferase produced by Cryptococcus neoformans. Infect. Immun. 1997, 65, 405–411. [Google Scholar] [PubMed]
- Chen, S.C.; Wright, L.C.; Golding, J.C.; Sorrell, T.C. Purification and characterization of secretory phospholipase B, lysophospholipase and lysophospholipase/transacylase from a virulent strain of the pathogenic fungus Cryptococcus neoformans. Biochem. J. 2000, 347, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.J.; Li, Z.; Hughes, W.S.; Djordjevic, J.T.; Nielsen, K.; May, R.C. Cryptococcal phospholipase B1 is required for intracellular proliferation and control of titan cell morphology during macrophage infection. Infect. Immun. 2015, 83, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, R.; Zoellner, H.; Sorrell, T.; Wilson, C.; Donald, C.; Djordjevic, J.; Shounan, Y.; Wright, L. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect. Immun. 2004, 72, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.C.; Casadevall, A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. USA 2002, 99, 3165–3170. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.A.; May, R.C. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog. 2010, 6, e1001041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.J.; Eastman, A.J.; Qiu, Y.; Gregorka, B.; Kozel, T.R.; Osterholzer, J.J.; Curtis, J.L.; Swanson, J.A.; Olszewski, M.A. Cryptococcus neoformans-induced macrophage lysosome damage crucially contributes to fungal virulence. J. Immunol. 2015, 194, 2219–2231. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; Chrisman, C.J.; Castelli, M.V.; Frases, S.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L.; Casadevall, A. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell. Microbiol. 2008, 10, 2043–2057. [Google Scholar] [CrossRef] [PubMed]
- Nordenfelt, P.; Tapper, H. Phagosome dynamics during phagocytosis by neutrophils. J. Leukocyte Biol. 2011, 90, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Denham, S.T.; Verma, S.; Reynolds, R.C.; Worne, C.L.; Daugherty, J.M.; Lane, T.E.; Brown, J.C.S. Regulated release of cryptococcal polysaccharide drives virulence and suppresses immune infiltration into the central nervous system. Infect. Immun. 2017. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B. Role of cytokines in T cell immunity to a pulmonary Cryptococcus neoformans infection. Neurosignals 1996, 5, 215–222. [Google Scholar] [CrossRef]
- Osterholzer, J.J.; Surana, R.; Milam, J.E.; Montano, G.T.; Chen, G.-H.; Sonstein, J.; Curtis, J.L.; Huffnagle, G.B.; Toews, G.B.; Olszewski, M.A. Cryptococcal urease promotes the accumulation of immature dendritic cells and a non-protective T2 immune response within the lung. Am. J. Pathol. 2009, 174, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Murdock, B.J.; Teitz-Tennenbaum, S.; Chen, G.-H.; Dils, A.J.; Malachowski, A.N.; Curtis, J.L.; Olszewski, M.A.; Osterholzer, J.J. Early or late IL-10 blockade enhances TH1 and TH17 effector reponses and promotes fungal clearance in mice with cryptococcal lung infection. J. Immunol. 2014, 193, 4107–4116. [Google Scholar] [CrossRef] [PubMed]
- Hardison, S.E.; Ravi, S.; Wozniak, K.L.; Young, M.L.; Olszewski, M.A.; Wormley, F.L. Pulmonary infection with an interferon-γ-producing Cryptococcus neoformans strain results in classical macrophage activation and protection. Am. J. Pathol. 2010, 176, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Eastman, A.J.; He, X.; Qiu, Y.; Davis, M.J.; Vedula, P.; Lyons, D.M.; Park, Y.-D.; Hardison, S.E.; Malachowski, A.N.; Osterholzer, J.J.; et al. Cryptococcal heat shock protein 70 homolog ssa1 contributes to pulmonary expansion of Cryptococcus neoformans during the afferent phase of the immune response by promoting macrophage M2 polarization. J. Immunol. 2015, 194, 5999–6010. [Google Scholar] [CrossRef] [PubMed]
- Nicola, A.M.; Robertson, E.J.; Albuquerque, P.; Derengowski, L.D.S.; Casadevall, A. Nonlytic exocytosis of Cryptococcus neoformans from macrophages occurs in vivo and is influenced by phagosomal PH. mBio 2011, 2, e00167-11. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.S.; Seoane, P.I.; Sephton-Clark, P.; Bojarczuk, A.; Hotham, R.; Giurisato, E.; Sarhan, A.R.; Hillen, A.; Velde, G.V.; Gray, N.S.; et al. Vomocytosis of live pathogens from macrophages is regulated by the atypical map kinase ERK5. Sci. Adv. 2017, 3, e1700898. [Google Scholar] [CrossRef] [PubMed]
- García-Rodas, R.; Zaragoza, O. Catch me if you can: Phagocytosis and killing avoidance by Cryptococcus neoformans. FEMS Immunol. Med. Microbiol. 2012, 64, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.A.; May, R.C. Cryptococcus interactions with macrophages: Evasion and manipulation of the phagosome by a fungal pathogen. Cell. Microbiol. 2013, 15, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Fonseca, F.L.; Frases, S.; Casadevall, A.; Nimrichter, L. The still obscure attributes of cryptococcal glucuronoxylomannan. Med. Mycol. 2009, 47, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.M.; Fonseca, F.L.; Holandino, C.; Alviano, C.S.; Nimrichter, L.; Rodrigues, M.L. Glucuronoxylomannan-mediated interaction of Cryptococcus neoformans with human alveolar cells results in fungal internalization and host cell damage. Microbes Infect. 2006, 8, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.M.; Fonseca, F.L.; Figueiredo, R.T.; Bozza, M.T.; Casadevall, A.; Nimrichter, L.; Rodrigues, M.L. Binding of glucuronoxylomannan to the CD14 receptor in human A549 alveolar cells induces interleukin-8 production. Clin. Vaccine Immunol. 2007, 14, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.A.C.; Penha, L.L.; Mendonça-Previato, L.; Previato, J.O. Mannoprotein MP84 mediates the adhesion of Cryptococcus neoformans to epithelial lung cells. Front. Cell. Infect. Microbiol. 2014, 4, 106. [Google Scholar] [CrossRef] [PubMed]
- Ganendren, R.; Carter, E.; Sorrell, T.; Widmer, F.; Wright, L. Phospholipase b activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect. 2006, 8, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Stukes, S.; Coelho, C.; Rivera, J.; Jedlicka, A.E.; Hajjar, K.A.; Casadevall, A. The membrane phospholipid binding protein annexin A2 promotes phagocytosis and non-lytic exocytosis of Cryptococcus neoformans and impacts survival in fungal infection. J. Immunol. 2016, 197, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Na Pombejra, S.; Salemi, M.; Phinney, B.S.; Gelli, A. The metalloprotease, Mpr1, engages annexin A2 to promote the transcytosis of fungal cells across the blood–brain barrier. Front. Cell. Infect. Microbiol. 2017, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Fa, Z.-Z.; Xie, Q.; Wang, G.-Z.; Yi, J.; Zhang, C.; Meng, G.-X.; Gu, J.-L.; Liao, W.-Q. Complex roles of annexin A2 in host blood–brain barrier invasion by Cryptococcus neoformans. CNS Neurosci. Ther. 2017, 23, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Aaron, P.A.; Jamklang, M.; Uhrig, J.P.; Gelli, A. The blood–brain barrier internalises Cryptococcus neoformans via the epha2-tyrosine kinase receptor. Cell. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef] [PubMed]
- Guillot, L.; Carroll, S.F.; Homer, R.; Qureshi, S.T. Enhanced innate immune responsiveness to pulmonary Cryptococcus neoformans infection is associated with resistance to progressive infection. Infect. Immun. 2008, 76, 4745–4756. [Google Scholar] [CrossRef] [PubMed]
- Guillot, L.; Carroll, S.F.; Badawy, M.; Qureshi, S.T. Cryptococcus neoformans induces IL-8 secretion and CXCl1 expression by human bronchial epithelial cells. Respir. Res. 2008, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Heyen, L.; Müller, U.; Siegemund, S.; Schulze, B.; Protschka, M.; Alber, G.; Piehler, D. Lung epithelium is the major source of IL-33 and is regulated by IL-33-dependent and IL-33-independent mechanisms in pulmonary cryptococcosis. Pathog. Dis. 2016, 74, ftw086. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Cherniak, R.; Kozel, T.R.; Granger, D.L.; Morris, L.C.; Weinhold, L.C.; Kwon-Chung, K.J. Structure and biological activities of acapsular Cryptococcus neoformans 602 complemented with the cap64 gene. Infect. Immun. 1997, 65, 1584–1592. [Google Scholar] [PubMed]
- Chang, Y.C.; Kwon-Chung, K.J. Isolation of the third capsule-associated gene, cap60, required for virulence in Cryptococcus neoformans. Infect. Immun. 1998, 66, 2230–2236. [Google Scholar] [PubMed]
- Mahajan, K.R.; Roberts, A.L.; Curtis, M.T.; Fortuna, D.; Dharia, R.; Sheehan, L. Diagnostic challenges of Cryptococcus neoformans in an immunocompetent individual masquerading as chronic hydrocephalus. Case Rep. Neurol. Med. 2016, 2016, 7. [Google Scholar]
- Cruickshank, J.G.; Cavill, R.; Jelbert, M. Cryptococcus neoformans of unusual morphology. Appl. Microbiol. 1973, 25, 309–312. [Google Scholar] [PubMed]
- Feldmesser, M.; Kress, Y.; Casadevall, A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 2001, 147, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; García-Rodas, R.; Nosanchuk, J.D.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L.; Casadevall, A. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010, 6, e1000945. [Google Scholar] [CrossRef]
- Okagaki, L.H.; Strain, A.K.; Nielsen, J.N.; Charlier, C.; Baltes, N.J.; Chrétien, F.; Heitman, J.; Dromer, F.; Nielsen, K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010, 6, e1000953. [Google Scholar] [CrossRef]
- Wang, J.-M.; Zhou, Q.; Cai, H.-R.; Zhuang, Y.; Zhang, Y.-F.; Xin, X.-Y.; Meng, F.-Q.; Wang, Y.-P. Clinicopathological features of pulmonary cryptococcosis with cryptococcal titan cells: A comparative analysis of 27 cases. Int. J. Clin. Exp. Pathol. 2014, 7, 4837–4846. [Google Scholar] [PubMed]
- Chrisman, C.J.; Albuquerque, P.; Guimaraes, A.J.; Nieves, E.; Casadevall, A. Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathog. 2011, 7, e1002047. [Google Scholar] [CrossRef] [PubMed]
- Okagaki, L.H.; Wang, Y.; Ballou, E.R.; O’Meara, T.R.; Bahn, Y.-S.; Alspaugh, J.A.; Xue, C.; Nielsen, K. Cryptococcal titan cell formation is regulated by G-protein signaling in response to multiple stimuli. Eukaryot. Cell 2011, 10, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.N.; Okagaki, L.H.; Wiesner, D.L.; Strain, A.K.; Nielsen, J.N.; Nielsen, K. Titan cell production enhances the virulence of Cryptococcus neoformans. Infect. Immun. 2012, 80, 3776–3785. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, T.R.; Holmer, S.M.; Selvig, K.; Dietrich, F.; Alspaugh, J.A. Cryptococcus neoformans rim101 is associated with cell wall remodeling and evasion of the host immune responses. mBio 2013, 4, e00522-12. [Google Scholar] [CrossRef] [PubMed]
- Ost, K.S.; O’Meara, T.R.; Huda, N.; Esher, S.K.; Alspaugh, J.A. The Cryptococcus neoformans alkaline response pathway: Identification of a novel rim pathway activator. PLoS Genet. 2015, 11, e1005159. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, A.C.; Fu, M.S.; Mukaremera, L.; Li, Z.; Ormerod, K.L.; Fraser, J.A.; Berman, J.; Nielsen, K. Polyploid titan cells produce haploid and aneuploid progeny to promote stress adaptation. mBio 2015, 6, e01340-15. [Google Scholar] [CrossRef] [PubMed]
- Okagaki, L.H.; Nielsen, K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot. Cell 2012, 11, 820–826. [Google Scholar] [CrossRef] [PubMed]
- García-Barbazán, I.; Trevijano-Contador, N.; Rueda, C.; de Andrés, B.; Pérez-Tavárez, R.; Herrero-Fernández, I.; Gaspar, M.L.; Zaragoza, O. The formation of titan cells in Cryptococcus neoformans depends on the mouse strain and correlates with induction of Th2-type responses. Cell. Microbiol. 2016, 18, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, D.L.; Specht, C.A.; Lee, C.K.; Smith, K.D.; Mukaremera, L.; Lee, S.T.; Lee, C.G.; Elias, J.A.; Nielsen, J.N.; Boulware, D.R.; et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 2015, 11, e1004701. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Sao, R.; Braun, A.; Bottone, E.J. Difference in Cryptococcus neoformans cellular and capsule size in sequential pulmonary and meningeal infection: A postmortem study. Diagn. Microbiol. Infect. Dis. 2012, 73, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Chrétien, F.; Baudrimont, M.; Mordelet, E.; Lortholary, O.; Dromer, F. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood–brain barrier. Am. J. Pathol. 2005, 166, 421–432. [Google Scholar] [CrossRef]
- Rivera, J.; Feldmesser, M.; Cammer, M.; Casadevall, A. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect. Immun. 1998, 66, 5027–5030. [Google Scholar] [PubMed]
- Bouklas, T.; Fries, B.C. Aging as an emergent factor that contributes to phenotypic variation in Cryptococcus neoformans. Fungal Genet. Biol. FG B 2015, 78, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.D.; Quain, D.E.; Smart, K.A. Chitin scar breaks in aged saccharomyces cerevisiae. Microbiology 2003, 149, 3129–3137. [Google Scholar] [CrossRef] [PubMed]
- Bouklas, T.; Pechuan, X.; Goldman, D.L.; Edelman, B.; Bergman, A.; Fries, B.C. Old Cryptococcus neoformans cells contribute to virulence in chronic cryptococcosis. mBio 2013, 4, e00455-13. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Cook, E.; Xess, I.; Hasan, F.; Fries, D.; Fries, B.C. Isolation and characterization of senescent Cryptococcus neoformans and implications for phenotypic switching and pathogenesis in chronic cryptococcosis. Eukaryot. Cell 2009, 8, 858–866. [Google Scholar] [CrossRef] [PubMed]
- García-Rodas, R.; Cordero, R.J.B.; Trevijano-Contador, N.; Janbon, G.; Moyrand, F.; Casadevall, A.; Zaragoza, O. Capsule growth in Cryptococcus neoformans is coordinated with cell cycle progression. mBio 2014, 5, e00945-14. [Google Scholar] [CrossRef] [PubMed]
- Bouklas, T.; Jain, N.; Fries, B.C. Modulation of replicative lifespan in Cryptococcus neoformans: Implications for virulence. Front. Microbiol. 2017, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Casadevall, A. Cryptococcal therapies and drug targets: The old, the new and the promising. Cell. Microbiol. 2016, 18, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Nizet, V. Color me bad: Microbial pigments as virulence factors. Trends Microbiol. 2009, 17, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Pukkila-Worley, R.; Gerrald, Q.D.; Kraus, P.R.; Boily, M.-J.; Davis, M.J.; Giles, S.S.; Cox, G.M.; Heitman, J.; Alspaugh, J.A. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic amp cascade. Eukaryot. Cell 2005, 4, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Noverr, M.C.; Williamson, P.R.; Fajardo, R.S.; Huffnagle, G.B. CNLAC1 is required for extrapulmonary dissemination of Cryptococcus neoformans but not pulmonary persistence. Infect. Immun. 2004, 72, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Salas, S.D.; Bennett, J.E.; Kwon-Chung, K.J.; Perfect, J.R.; Williamson, P.R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 1996, 184, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Sabiiti, W.; Robertson, E.; Beale, M.A.; Johnston, S.A.; Brouwer, A.E.; Loyse, A.; Jarvis, J.N.; Gilbert, A.S.; Fisher, M.C.; Harrison, T.S.; et al. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J. Clin. Investig. 2014, 124, 2000–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lev, S.; Li, C.; Desmarini, D.; Saiardi, A.; Fewings, N.L.; Schibeci, S.D.; Sharma, R.; Sorrell, T.C.; Djordjevic, J.T. Fungal inositol pyrophosphate IP7 is crucial for metabolic adaptation to the host environment and pathogenicity. mBio 2015, 6, e00531-15. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lev, S.; Desmarini, D.; Kaufman-Francis, K.; Saiardi, A.; Silva, A.P.G.; Mackay, J.P.; Thompson, P.E.; Sorrell, T.C.; Djordjevic, J.T. Ip3-4 kinase Arg1 regulates cell wall homeostasis and surface architecture to promote clearance of Cryptococcus neoformans infection in a mouse model. Virulence 2017, 8, 1833–1848. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lev, S.; Saiardi, A.; Desmarini, D.; Sorrell, T.C.; Djordjevic, J.T. Identification of a major IP5 kinase in Cryptococcus neoformans confirms that PP-IP5/IP7, not IP6, is essential for virulence. Sci. Rep. 2016, 6, 23927. [Google Scholar] [CrossRef] [PubMed]
- Lev, S.; Kaufman-Francis, K.; Desmarini, D.; Juillard, P.G.; Li, C.; Stifter, S.A.; Feng, C.G.; Sorrell, T.C.; Grau, G.E.R.; Bahn, Y.-S.; et al. Pho4 is essential for dissemination of Cryptococcus neoformans to the host brain by promoting phosphate uptake and growth at alkaline PH. mSphere 2017, 2, e00381-16. [Google Scholar] [CrossRef] [PubMed]
- Toh-e, A.; Ohkusu, M.; Li, H.-M.; Shimizu, K.; Takahashi-Nakaguchi, A.; Gonoi, T.; Kawamoto, S.; Kanesaki, Y.; Yoshikawa, H.; Nishizawa, M. Identification of genes involved in the phosphate metabolism in Cryptococcus neoformans. Fungal Genet. Biol. 2015, 80, 19–30. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, T.R.; Norton, D.; Price, M.S.; Hay, C.; Clements, M.F.; Nichols, C.B.; Alspaugh, J.A. Interaction of Cryptococcus neoformans rim101 and protein kinase a regulates capsule. PLoS Pathog. 2010, 6, e1000776. [Google Scholar] [CrossRef] [PubMed]
- Ost, K.S.; Esher, S.K.; Leopold Wager, C.M.; Walker, L.; Wagener, J.; Munro, C.; Wormley, F.L.; Alspaugh, J.A. Rim pathway-mediated alterations in the fungal cell wall influence immune recognition and inflammation. mBio 2017, 8, e02290-16. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; MacKenzie, A.; Girnun, G.; Del Poeta, M. Analysis of sphingolipids, sterols, and phospholipids in human pathogenic Cryptococcus strains. J. Lipid Res. 2017, 58, 2017–2036. [Google Scholar] [CrossRef] [PubMed]
- McQuiston, T.; Luberto, C.; Del Poeta, M. Role of sphingosine-1-phosphate (S1P) and S1P receptor 2 in the phagocytosis of Cryptococcus neoformans by alveolar macrophages. Microbiology 2011, 157, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- McQuiston, T.; Luberto, C.; Del Poeta, M. Role of host sphingosine kinase 1 in the lung response against cryptococcosis. Infect. Immun. 2010, 78, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Shea, J.M.; Kechichian, T.B.; Luberto, C.; Del Poeta, M. The cryptococcal enzyme inositol phosphosphingolipid-phospholipase c confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect. Immun. 2006, 74, 5977–5988. [Google Scholar] [CrossRef] [PubMed]
- Farnoud, A.M.; Bryan, A.M.; Kechichian, T.; Luberto, C.; Del Poeta, M. The granuloma response controlling cryptococcosis in mice depends on the sphingosine kinase 1–sphingosine 1-phosphate pathway. Infect. Immun. 2015, 83, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Rittershaus, P.C.; Kechichian, T.B.; Allegood, J.C.; Merrill, A.H.; Hennig, M.; Luberto, C.; Del Poeta, M. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Investig. 2006, 116, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Wang, H.; Silva, L.C.; Na, C.; Prieto, M.; Futerman, A.H.; Luberto, C.; Del Poeta, M. Methylation of glycosylated sphingolipid modulates membrane lipid topography and pathogenicity of Cryptococcus neoformans. Cell. Microbiol. 2012, 14, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Nazemidashtarjandi, S.; Kim, J.; Joffe, L.; Zhang, X.; Singh, A.; Mor, V.; Desmarini, D.; Djordjevic, J.; Raleigh, D.P.; et al. Changes in glucosylceramide structure affect virulence and membrane biophysical properties of Cryptococcus neoformans. Biochimica et Biophysica Acta (BBA) Biomembranes 2017, 1859, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Farnoud, A.M.; Mor, V.; Singh, A.; Del Poeta, M. Inositol phosphosphingolipid phospholipase c1 regulates plasma membrane atpase (pma1) stability in Cryptococcus neoformans. FEBS Lett. 2014, 588, 3932–3938. [Google Scholar] [CrossRef] [PubMed]
- Beattie, S.R.; Mark, K.M.K.; Thammahong, A.; Ries, L.N.A.; Dhingra, S.; Caffrey-Carr, A.K.; Cheng, C.; Black, C.C.; Bowyer, P.; Bromley, M.J.; et al. Filamentous fungal carbon catabolite repression supports metabolic plasticity and stress responses essential for disease progression. PLoS Pathog. 2017, 13, e1006340. [Google Scholar] [CrossRef] [PubMed]
- Pirofski, L.-A.; Casadevall, A. Immune-mediated damage completes the parabola: Cryptococcus neoformans pathogenesis can reflect the outcome of a weak or strong immune response. mBio 2017, 8, e02063-17. [Google Scholar] [CrossRef] [PubMed]
- Janbon, G.; Ormerod, K.L.; Paulet, D.; Byrnes, E.J., III.; Yadav, V.; Chatterjee, G.; Mullapudi, N.; Hon, C.-C.; Billmyre, R.B.; Brunel, F.; et al. Analysis of the genome and transcriptome of Cryptococcus neoformans var. Grubii reveals complex rna expression and microevolution leading to virulence attenuation. PLoS Genet. 2014, 10, e1004261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufaud, C.; Rivera, J.; Rohatgi, S.; Pirofski, L.-A. Naïve B cells reduce fungal dissemination in Cryptococcus neoformans infected Rag1−/− mice. Virulence 2018, 9, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, S.; Nakouzi, A.; Carreño, L.J.; Slosar-Cheah, M.; Kuniholm, M.H.; Wang, T.; Pappas, P.G.; Pirofski, L.-A. Antibody and B cell subset perturbations in human immunodeficiency virus-uninfected patients with cryptococcosis. Open Forum Infect. Dis. 2018, 5, ofx255. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, D.L.; Smith, K.D.; Kashem, S.W.; Bohjanen, P.R.; Nielsen, K. Different lymphocyte populations direct dichotomous eosinophil or neutrophil responses to pulmonary Cryptococcus infection. J. Immunol. 2017, 198, 1627. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denham, S.T.; Brown, J.C.S. Mechanisms of Pulmonary Escape and Dissemination by Cryptococcus neoformans. J. Fungi 2018, 4, 25. https://doi.org/10.3390/jof4010025
Denham ST, Brown JCS. Mechanisms of Pulmonary Escape and Dissemination by Cryptococcus neoformans. Journal of Fungi. 2018; 4(1):25. https://doi.org/10.3390/jof4010025
Chicago/Turabian StyleDenham, Steven T., and Jessica C. S. Brown. 2018. "Mechanisms of Pulmonary Escape and Dissemination by Cryptococcus neoformans" Journal of Fungi 4, no. 1: 25. https://doi.org/10.3390/jof4010025




