Neuro-Immune Mechanisms of Anti-Cryptococcal Protection
Abstract
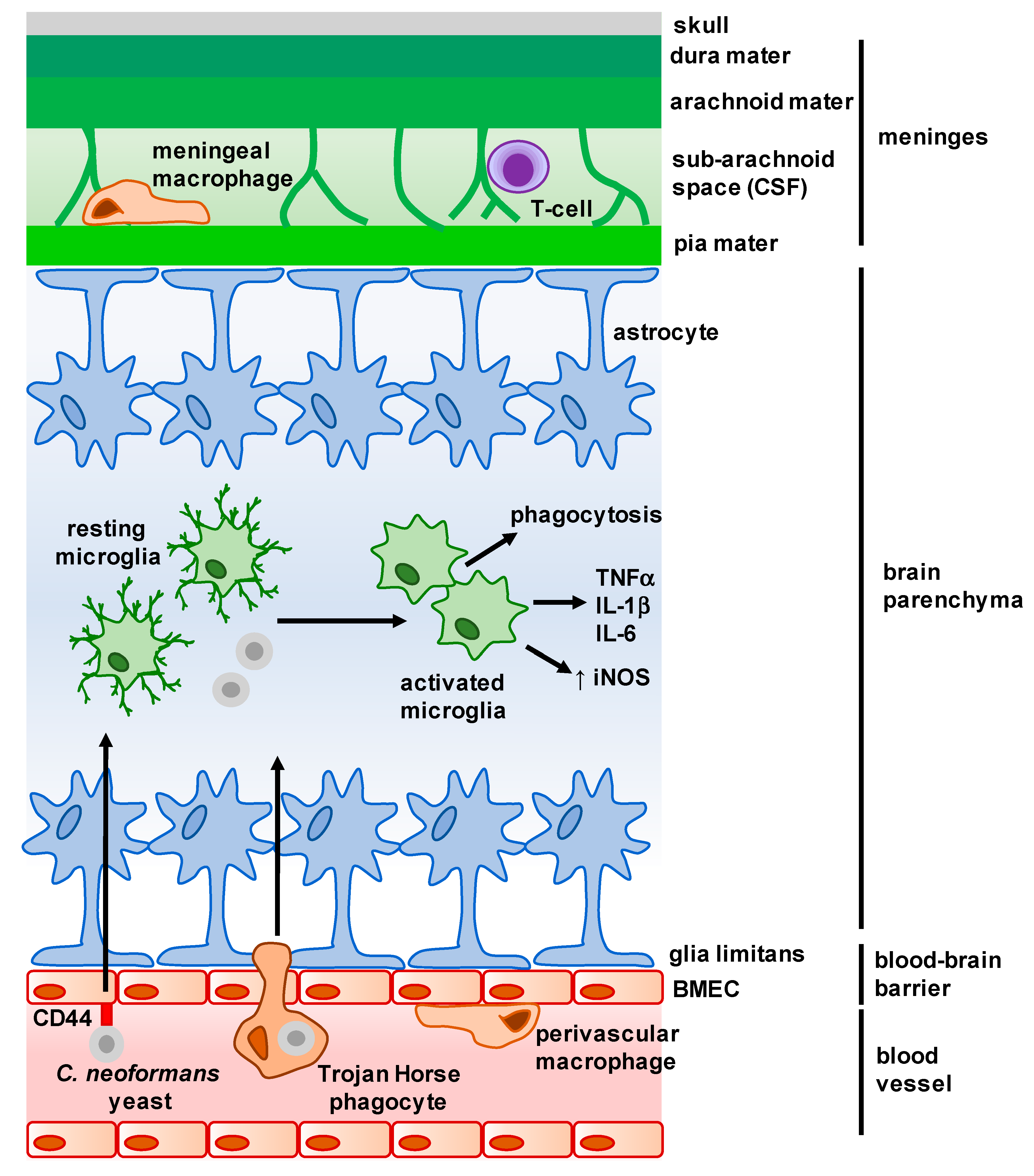
:1. Introduction
2. Microglia
3. Non-Parenchymal Macrophages
4. Macroglia: Astrocytes and Oligodendrocytes
5. Neurons
6. Brain Microvascular Endothelial Cells (BMECs)
7. T-cells
8. Other Lymphocytes
9. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Dromer, F.; Casadevall, A.; Perfect, J.; Sorrell, T. Cryptococcus neoformans: Latency and Disease; American Society of Microbiology: Washington, DC, USA, 2011. [Google Scholar]
- Pasquier, E.; Kunda, J.; de Beaudrap, P.; Loyse, A.; Temfack, E.; Molloy, S.F.; Harrison, T.S.; Lortholary, O. Long term mortality and disability in Cryptococcal Meningitis: A systematic literature review. Clin. Infect. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Khan, P.A.; Shujatullah, F.; Fatima, N.; Shameem, M.; Siddiqui, A. Rapid development of IRIS in the form of cryptococcal meningitis after beginning ART. Med. Mycol. Case Rep. 2012, 1, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.R.; Jarvis, J.N.; Panackal, A.A.; Fisher, M.C.; Molloy, S.F.; Loyse, A.; Harrison, T.S. Cryptococcal meningitis: Epidemiology, immunology, diagnosis and therapy. Nat. Rev. Neurol. 2017, 13, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hole, C.; Wormley, F.L. Innate host defenses against Cryptococcus neoformans. J. Microbiol. 2016, 54, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Bocca, A.L.; Casadevall, A. The Intracellular Life of Cryptococcus neoformans. Annu. Rev. Pathol. 2014, 9, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Dixon, E.F.; May, R.C. The fungal pathogen Cryptococcus neoformans manipulates macrophage phagosome maturation. Cell. Microbiol. 2015, 17, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Eastman, A.J.; Qiu, Y.; Gregorka, B.; Kozel, T.R.; Osterholzer, J.J.; Curtis, J.L.; Swanson, J.A.; Olszewski, M.A. Cryptococcus neoformans—Induced Macrophage Lysosome Damage Crucially Contributes to Fungal Virulence. J. Immunol. 2015, 194, 2219. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.A.; May, R.C. The Human Fungal Pathogen Cryptococcus neoformans Escapes Macrophages by a Phagosome Emptying Mechanism That Is Inhibited by Arp2/3 Complex-Mediated Actin Polymerisation. PLoS Pathog. 2010, 6, e1001041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, A.S.; Seoane, P.I.; Sephton-Clark, P.; Bojarczuk, A.; Hotham, R.; Giurisato, E.; Sarhan, A.R.; Hillen, A.; Velde, G.V.; Gray, N.S.; et al. Vomocytosis of live pathogens from macrophages is regulated by the atypical MAP kinase ERK5. Sci. Adv. 2017, 3, e1700898. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; de Angelis, A.L.H.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Prinz, M. Origin of Microglia: Current Concepts and Past Controversies. Cold Spring Harb. Perspect. Biol. 2015, 7, a020537. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Mai, D.; Dautzenberg, J.; Fernandez-Klett, F.; Lin, G.; Datta, M.; Drougard, A.; Stempfl, T.; Ardura-Fabregat, A.; Staszewski, O.; et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 2017, 20, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Réu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017, 20, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Saijo, K.; Glass, C.K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011, 11, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Biondo, C.; Midiri, A.; Messina, L.; Tomasello, F.; Garufi, G.; Catania, M.I.; Bombaci, M.; Beninati, C.; Teti, G.; Mancuso, G. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur. J. Immunol. 2005, 35, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kinjo, T.; Saijo, S.; Miyazato, A.; Adachi, Y.; Ohno, N.; Fujita, J.; Kaku, M.; Iwakura, Y.; Kawakami, K. Dectin-1 is not required for the host defense to Cryptococcus neoformans. Microbiol. Immunol. 2007, 51, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Giles, S.S.; Dagenais, T.R.T.; Botts, M.R.; Keller, N.P.; Hull, C.M. Elucidating the Pathogenesis of Spores from the Human Fungal Pathogen Cryptococcus neoformans. Infect. Immun. 2009, 77, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Barluzzi, R.; Brozzetti, A.; Delfino, D.; Bistoni, F.; Blasi, E. Role of the capsule in microglial cell-Cryptococcus neoformans interaction: Impairment of antifungal activity but not of secretory functions. Med. Mycol. 1998, 36, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Neal, L.M.; Xing, E.; Xu, J.; Kolbe, J.L.; Osterholzer, J.J.; Segal, B.M.; Williamson, P.R.; Olszewski, M.A. CD4+ T Cells Orchestrate Lethal Immune Pathology despite Fungal Clearance during Cryptococcus neoformans Meningoencephalitis. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Adami, C.; Sorci, G.; Blasi, E.; Agneletti, A.L.; Bistoni, F.; Donato, R. S100B expression in and effects on microglia. Glia 2001, 33, 131–142. [Google Scholar] [CrossRef]
- Song, X.Y.; Tanaka, S.; Cox, D.; Lee, S.C. Fc gamma receptor signaling in primary human microglia: Differential roles of PI-3K and Ras/ERK MAPK pathways in phagocytosis and chemokine induction. J. Leukoc. Biol. 2004, 75, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Preissler, J.; Grosche, A.; Lede, V.; le Duc, D.; Krugel, K.; Matyash, V.; Szulzewsky, F.; Kallendrusch, S.; Immig, K.; Kettenmann, H.; et al. Altered Microglial Phagocytosis in GPR34-Deficient Mice. Glia 2015, 63, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kress, Y.; Dickson, D.W.; Casadevall, A. Human Microglia Mediate Anti-Cryptococcus neoformans Activity in The Presence of Specific Antibody. J. Neuroimmunol. 1995, 62, 43–52. [Google Scholar] [CrossRef]
- Lee, S.H.C.; Kress, Y.; Zhao, M.L.; Dickson, D.W.; Casadevall, A. Cryptococcus neoformans survive and replicate in human microglia. Lab. Investig. 1995, 73, 871–879. [Google Scholar] [PubMed]
- Aguirre, K.; Crowe, J.; Haas, A.; Smith, J. Resistance to Cryptococcus neoformans infection in the absence of CD4(+) T cells. Med. Mycol. 2004, 42, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Yates, J.L.; Lipscomb, M.F. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J. Exp. Med. 1991, 173, 793–800. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, P.M.; Gottfried-Blackmore, A.; Anandasabapathy, N.; Bulloch, K. Brain dendritic cells: Biology and pathology. Acta Neuropathol. 2012, 124, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Panackal, A.A.; Wuest, S.C.; Lin, Y.-C.; Wu, T.; Zhang, N.; Kosa, P.; Komori, M.; Blake, A.; Browne, S.K.; Rosen, L.B.; et al. Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis. PLoS Pathog. 2015, 11, e1004884. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, T.; Wieghofer, P.; Jordao, M.J.C.; Prutek, F.; Hagemeyer, N.; Frenzel, K.; Amann, L.; Staszewski, O.; Kierdorf, K.; Krueger, M.; et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016, 17, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Erny, D.; Hagemeyer, N. Ontogeny and homeostasis of CNS myeloid cells. Nat. Immunol. 2017, 18, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Shinoe, T.; Wanaka, A.; Nikaido, T.; Kakuta, Y.; Masunaga, A.; Shimizu, J.; Duyckaerts, C.; Imaizumi, K.; Iwamoto, A.; Kanazawa, I. The pro-apoptotic human BH3-only peptide harakiri is expressed in cryptococcus-infected perivascular macrophages in HIV-1 encephalitis patients. Neurosci. Lett. 2006, 393, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, K.; Miller, S. MHC class II-positive perivascular microglial cells mediate resistance to Cryptococcus neoformans brain infection. Glia 2002, 39, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Schwerk, C.; Tenenbaum, T.; Kim, K.S.; Schroten, H. The choroid plexus—A multi-role player during infectious diseases of the CNS. Front. Cell. Neurosci. 2015, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Horng, S.; Therattil, A.; Moyon, S.; Gordon, A.; Kim, K.; Argaw, A.T.; Hara, Y.; Mariani, J.N.; Sawai, S.; Flodby, P.; et al. Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J. Clin. Investig. 2017, 127, 3136–3151. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Shibata, K.; Yoshida, K.; Shigetomi, E.; Gachet, C.; Ikenaka, K.; Tanaka, K.F.; Koizumi, S. Transformation of Astrocytes to a Neuroprotective Phenotype by Microglia via P2Y1 Receptor Downregulation. Cell Rep. 2017, 19, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Wu, C.-H.; Chang, Y.C.; Kwon-Chung, K.J.; Brown, R.J.; Jong, A. Cryptococcus neoformans-Derived Microvesicles Enhance the Pathogenesis of Fungal Brain Infection. PLoS ONE 2012, 7, e48570. [Google Scholar] [CrossRef] [PubMed]
- Olave, M.C.; Vargas-Zambrano, J.C.; Celis, A.M.; Castañeda, E.; González, J.M. Infective capacity of Cryptococcus neoformans and Cryptococcus gattii in a human astrocytoma cell line. Mycoses 2017, 60, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.S.; Kluttz, J.M.; Bost, K.L.; Marriott, I. Prophylactic and Therapeutic Targeting of the Neurokinin-1 Receptor Limits Neuroinflammation in a Murine Model of Pneumococcal Meningitis. J. Immunol. 2011, 186, 7255. [Google Scholar] [CrossRef] [PubMed]
- Rambach, G.; Maier, H.; Vago, G.; Mohsenipour, I.; Lass-Flörl, C.; Defant, A.; Würzner, R.; Dierich, M.P.; Speth, C. Complement induction and complement evasion in patients with cerebral aspergillosis. Microbes Infect. 2008, 10, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Kashem, S.W.; Riedl, M.S.; Yao, C.; Honda, C.N.; Vulchanova, L.; Kaplan, D.H. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity 2015, 43, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Ogra, Y.; Tejima, A.; Hatakeyama, N.; Shiraiwa, M.; Wu, S.; Ishikawa, T.; Yawata, A.; Anan, Y.; Suzuki, N. Changes in intracellular copper concentration and copper-regulating gene expression after PC12 differentiation into neurons. Sci. Rep. 2016, 6, 33007. [Google Scholar] [CrossRef] [PubMed]
- García-Santamarina, S.; Thiele, D.J. Copper at the Fungal Pathogen-Host Axis. J. Biol. Chem. 2015, 290, 18945–18953. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Hare, D.J.; Cottam, V.; Chen, N.; Hilgers, L.; Halliday, G.; Mercer, J.F.B.; Double, K.L. Localization of copper and copper transporters in the human brain. Metallomics 2013, 5, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.R.; Waterman, S.R.; Qiu, J.; Bleher, R.; Williamson, P.R.; O’Halloran, T.V. A Copper Hyperaccumulation Phenotype Correlates with Pathogenesis in Cryptococcus neoformans. Metallomics 2013, 5, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Davis, M.J.; Dayrit, J.K.; Hadd, Z.; Meister, D.L.; Osterholzer, J.J.; Williamson, P.R.; Olszewski, M.A. Immune Modulation Mediated by Cryptococcal Laccase Promotes Pulmonary Growth and Brain Dissemination of Virulent Cryptococcus neoformans in Mice. PLoS ONE 2012, 7, e47853. [Google Scholar] [CrossRef] [PubMed]
- Waterman, S.R.; Hacham, M.; Hu, G.; Zhu, X.; Park, Y.-D.; Shin, S.; Panepinto, J.; Valyi-Nagy, T.; Beam, C.; Husain, S.; et al. Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J. Clin. Investig. 2007, 117, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Festa, R.A.; Chen, Y.-L.; Espart, A.; Palacios, Ò.; Espín, J.; Capdevila, M.; Atrian, S.; Heitman, J.; Thiele, D.J. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe 2013, 13, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Masocha, W.; Kristensson, K. Passage of parasites across the blood-brain barrier. Virulence 2012, 3, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Iovino, F.; Seinen, J.; Henriques-Normark, B.; van Dijl, J.M. How Does Streptococcus pneumoniae Invade the Brain? Trends Microbiol. 2016, 24, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.M.; Stins, M.F.; Huang, S.-H.; Chen, Y.H.; Kwon-Chung, K.J.; Chang, Y.; Kim, K.S.; Suzuki, K.; Jong, A.Y. Cryptococcus neoformans induces alterations in the cytoskeleton of human brain microvascular endothelial cells. J. Med. Microbiol. 2003, 52, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Sabiiti, W.; May, R.C. Capsule Independent Uptake of the Fungal Pathogen Cryptococcus neoformans into Brain Microvascular Endothelial Cells. PLoS ONE 2012, 7, e35455. [Google Scholar] [CrossRef] [PubMed]
- Jong, A.; Wu, C.-H.; Shackleford, G.M.; Kwon-Chung, K.J.; Chang, Y.C.; Chen, H.-M.; Ouyang, Y.; Huang, S.-H. Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell. Microbiol. 2008, 10, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Jong, A.; Huang, S.; Zerfas, P.; Kwon-Chung, K.J. CPS1, a Homolog of the Streptococcus pneumoniae Type 3 Polysaccharide Synthase Gene, Is Important for the Pathobiology of Cryptococcus neoformans. Infect. Immun. 2006, 74, 3930–3938. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Long, M.; Wu, C.-H.; Kwon-Chung, K.J.; Chang, Y.C.; Chi, F.; Lee, S.; Jong, A. Invasion of Cryptococcus neoformans into Human Brain Microvascular Endothelial Cells Is Mediated through the Lipid Rafts-Endocytic Pathway via the Dual Specificity Tyrosine Phosphorylation-regulated Kinase 3 (DYRK3). J. Biol. Chem. 2011, 286, 34761–34769. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.; Tham, R.; Uhrig, J.P.; Thompson, G.R.; Pombejra, S.N.; Jamklang, M.; Bautos, J.M.; Gelli, A. Invasion of the Central Nervous System by Cryptococcus neoformans Requires a Secreted Fungal Metalloprotease. mBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Li, S.S.; Zheng, C.; Jones, G.J.; Kim, K.S.; Zhou, H.; Kubes, P.; Mody, C.H. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J. Clin. Investig. 2010, 120, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Nielsen, K.; Daou, S.; Brigitte, M.; Chretien, F.; Dromer, F. Evidence of a Role for Monocytes in Dissemination and Brain Invasion by Cryptococcus neoformans. Infect. Immun. 2009, 77, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Tirado, F.H.; Onken, M.D.; Cooper, J.A.; Klein, R.S.; Doering, T.L. Trojan Horse Transit Contributes to Blood-Brain Barrier Crossing of a Eukaryotic Pathogen. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Choudhury, G.R.; Winters, A.; Yang, S.-H.; Jin, K. Cerebral regulatory T cells restrain microglia/macrophage-mediated inflammatory responses via IL-10. Eur. J. Immunol. 2015, 45, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Uicker, W.C.; McCracken, J.P.; Buchanan, K.L. Role of CD4(+) T cells in a protective immune response against Cryptococcus neoformans in the central nervous system. Med. Mycol. 2006, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; McNeil, L.K. Dissemination of C-neoformans to the central nervous system: Role of chemokines, Th1 immunity and leukocyte recruitment. J. Neurovirol. 1999, 5, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Uicker, W.C.; Doyle, H.A.; McCracken, J.P.; Langlois, M.; Buchanan, K.L. Cytokine and chemokine expression in the central nervous system associated with protective cell-mediated immunity against Cryptococcus neoformans. Med. Mycol. 2005, 43, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, K.L.; Doyle, H.A. Requirement for CD4+ T Lymphocytes in Host Resistance against Cryptococcus neoformans in the Central Nervous System of Immunized Mice. Infect. Immun. 2000, 68, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Haddow, L.J.; Colebunders, R.; Meintjes, G.; Lawn, S.D.; Elliott, J.H.; Manabe, Y.C.; Bohjanen, P.R.; Sungkanuparph, S.; Easterbrook, P.J.; French, M.A.; et al. International Network for the Study of H.I.V.a.I. Cryptococcal Immune Reconstitution Inflammatory Syndrome in HIV-1–infected individuals: Literature Review and Proposed Clinical Case Definitions. Lancet Infect. Dis. 2010, 10, 791–802. [Google Scholar] [CrossRef]
- Eschke, M.; Piehler, D.; Schulze, B.; Richter, T.; Grahnert, A.; Protschka, M.; Müller, U.; Köhler, G.; Höfling, C.; Rossner, S.; et al. A novel experimental model of Cryptococcus neoformans-related immune reconstitution inflammatory syndrome (IRIS) provides insights into pathogenesis. Eur. J. Immunol. 2015, 45, 3339–3350. [Google Scholar] [CrossRef] [PubMed]
- Dufaud, C.; Rivera, J.; Rohatgi, S.; Pirofski, L.-A. Naïve B cells reduce fungal dissemination in Cryptococcus neoformans infected Rag1−/− mice. Virulence 2017. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, S.; Pirofski, L.A. Host immunity to Cryptococcus neoformans. Future Microbiol. 2015, 10, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Li, S.S.; Oykhman, P.; Timm-McCann, M.; Huston, S.M.; Stack, D.; Xiang, R.F.; Kelly, M.M.; Mody, C.H. An Acidic Microenvironment Increases NK Cell Killing of Cryptococcus neoformans and Cryptococcus gattii by Enhancing Perforin Degranulation. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sanai, N.; Jin, W.-N.; la Cava, A.; van Kaer, L.; Shi, F.-D. Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation. Nat. Neurosci. 2016, 19, 243–252. [Google Scholar] [CrossRef] [PubMed]
| Turnover Rate | Surface Markers | Developmental Transcription Factors | |
|---|---|---|---|
| Microglia | Long-lived | CD45int CD11b Cx3CR1 Iba-1 Tmem119 MHCIIlow | PU.1 IRF8 CSF1R Sall1 |
| Perivascular Macrophages | Long-lived | CD45hi CD11b Cx3CR1 Iba-1 CD163 MHCIIhi CD206 | PU.1 CSF1R |
| Choroid Plexus Macrophages | Partial turnover from monocytes | CD45hi CD11b Cx3CR1 Iba-1 MHCIIhi | PU.1 CSF1R |
| Meningeal Macrophages | Long-lived | CD45hi CD11b Cx3CR1 Iba-1 MHCIIhi CD206 | PU.1 IRF8 CSF1R |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drummond, R.A. Neuro-Immune Mechanisms of Anti-Cryptococcal Protection. J. Fungi 2018, 4, 4. https://doi.org/10.3390/jof4010004
Drummond RA. Neuro-Immune Mechanisms of Anti-Cryptococcal Protection. Journal of Fungi. 2018; 4(1):4. https://doi.org/10.3390/jof4010004
Chicago/Turabian StyleDrummond, Rebecca A. 2018. "Neuro-Immune Mechanisms of Anti-Cryptococcal Protection" Journal of Fungi 4, no. 1: 4. https://doi.org/10.3390/jof4010004





