Thermoresponsive Gels
Abstract
:1. Introduction
2. Thermosensitivity, Thermoresponsiveness and Phase Transition Rheology
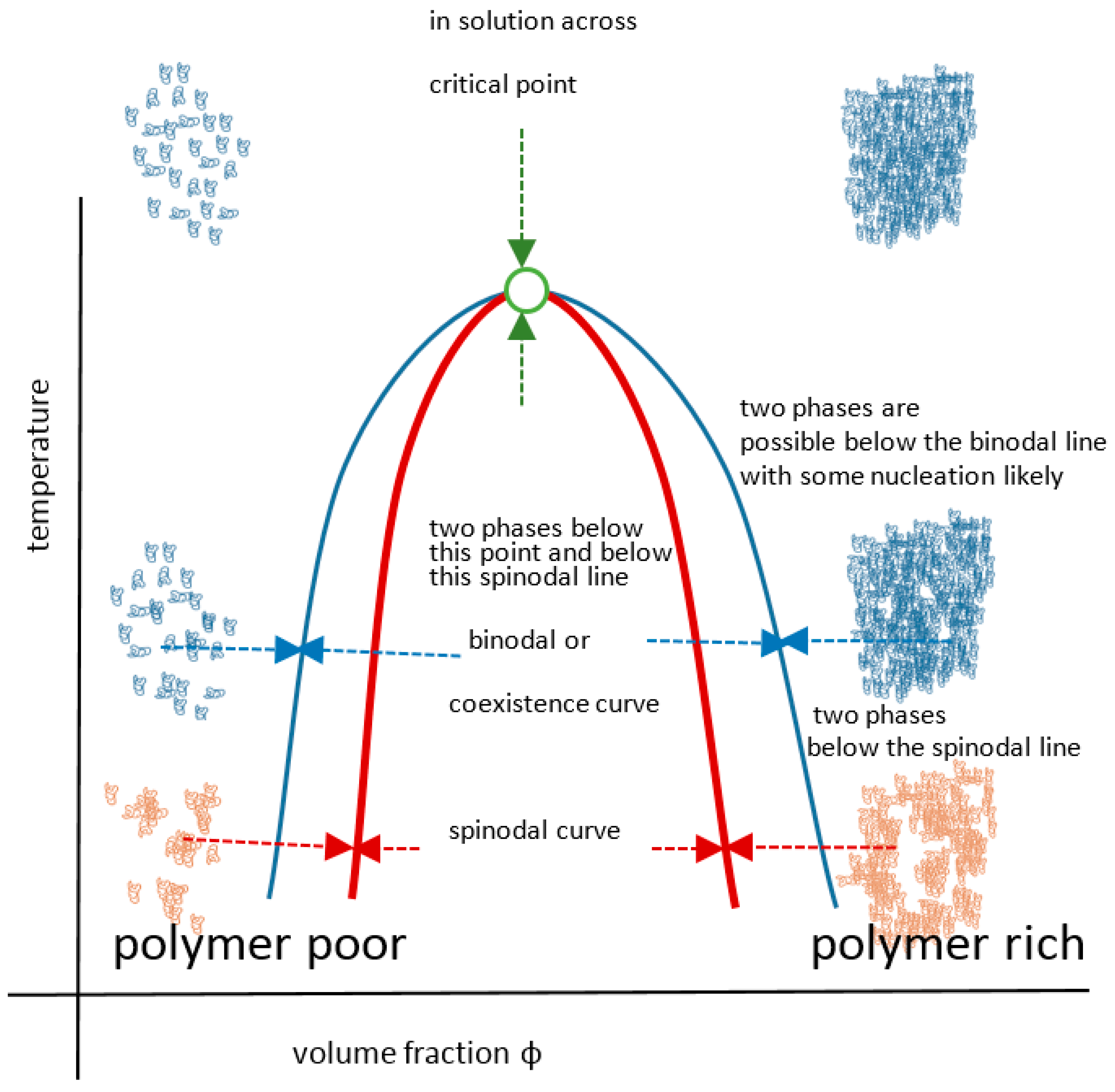
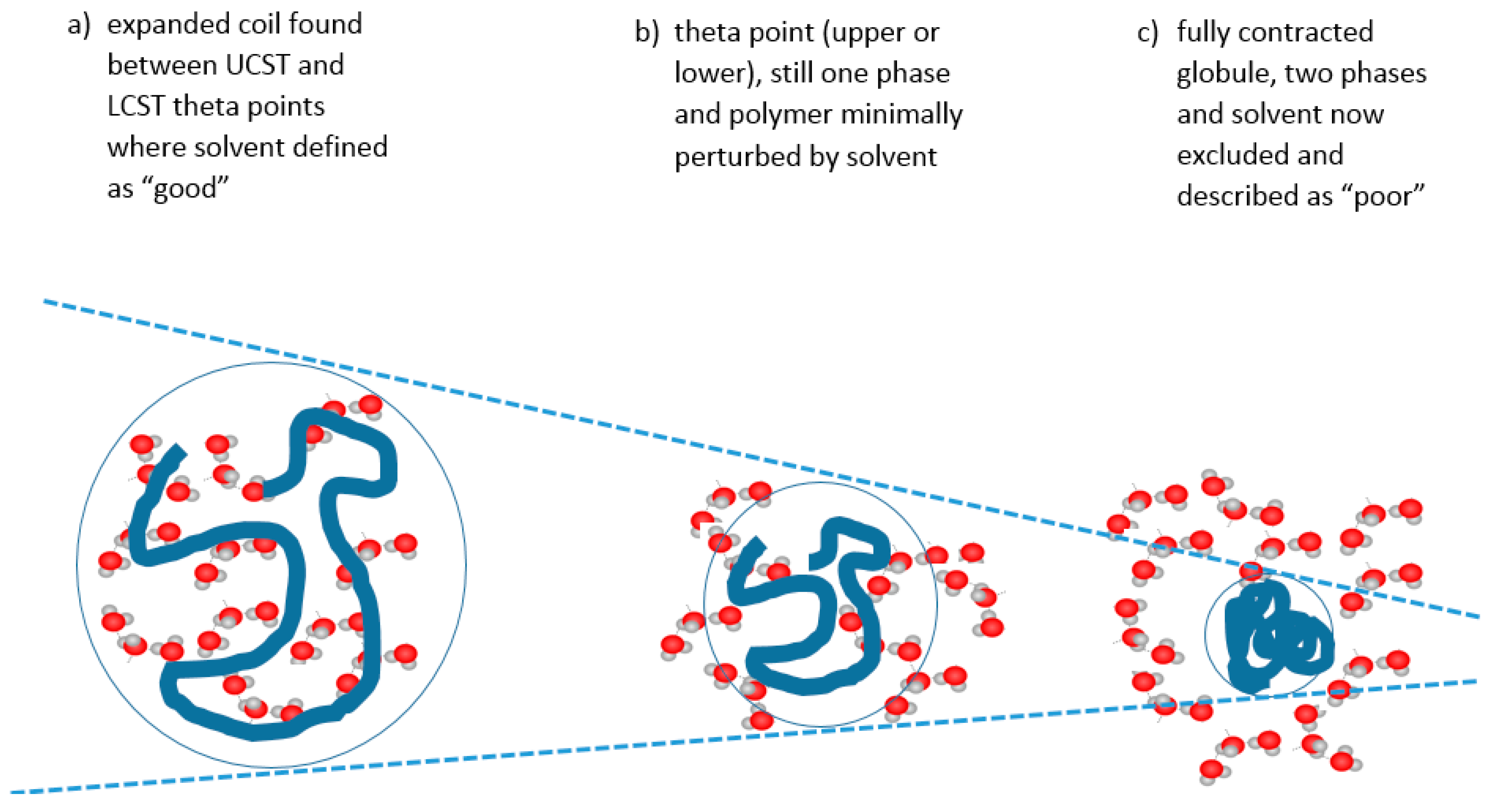
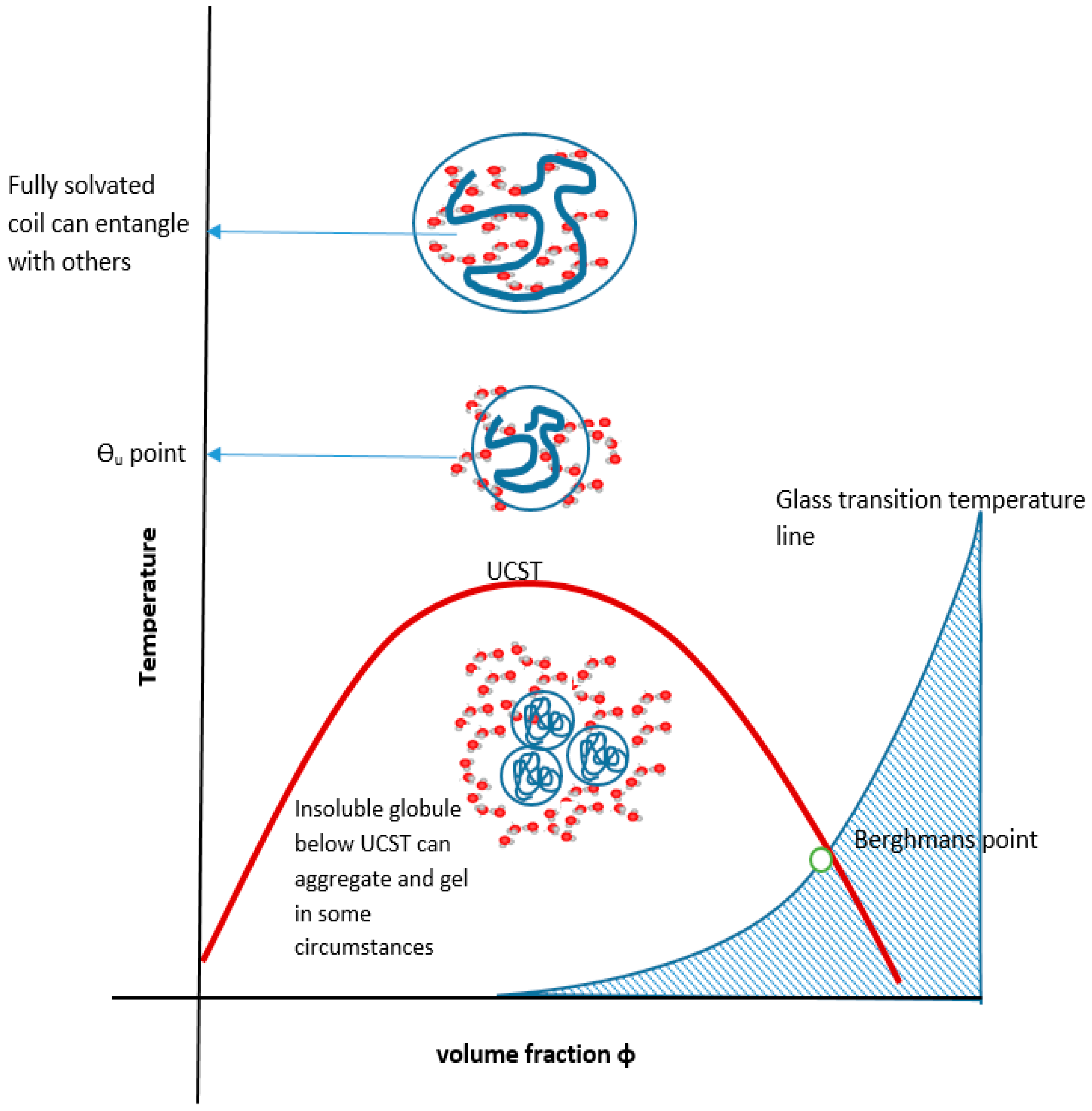
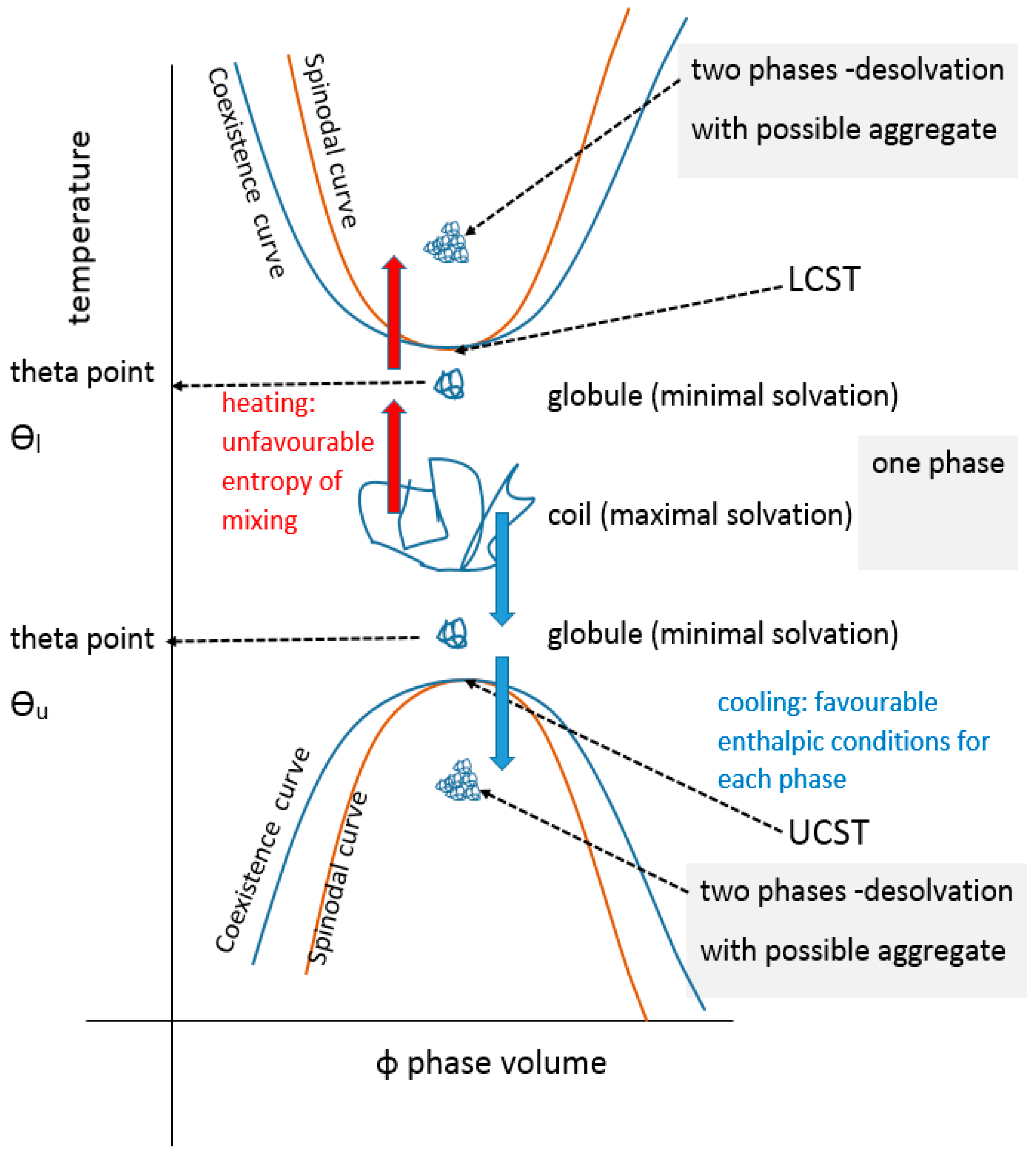
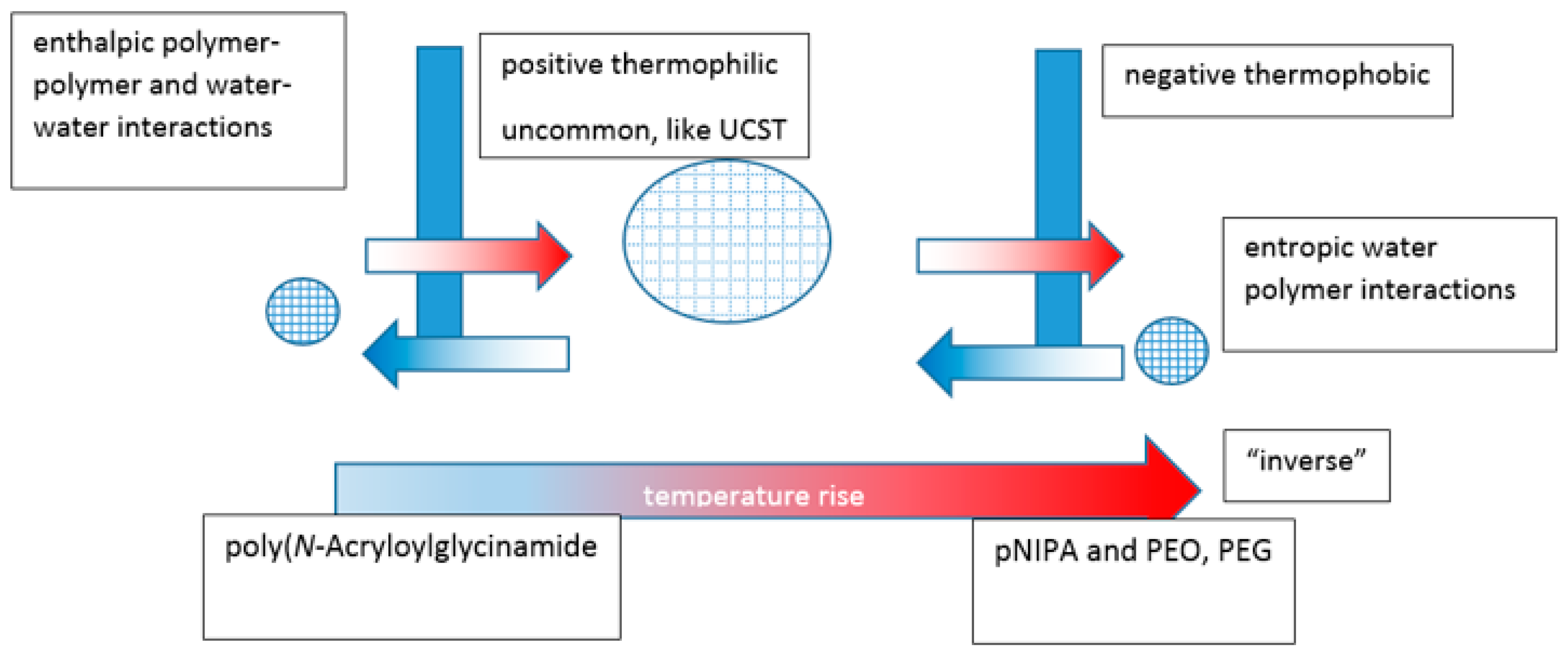
2.1. The Upper Critical Solution Temperature (UCST)
2.2. The Lower Critical Solution Temperature (LCST)
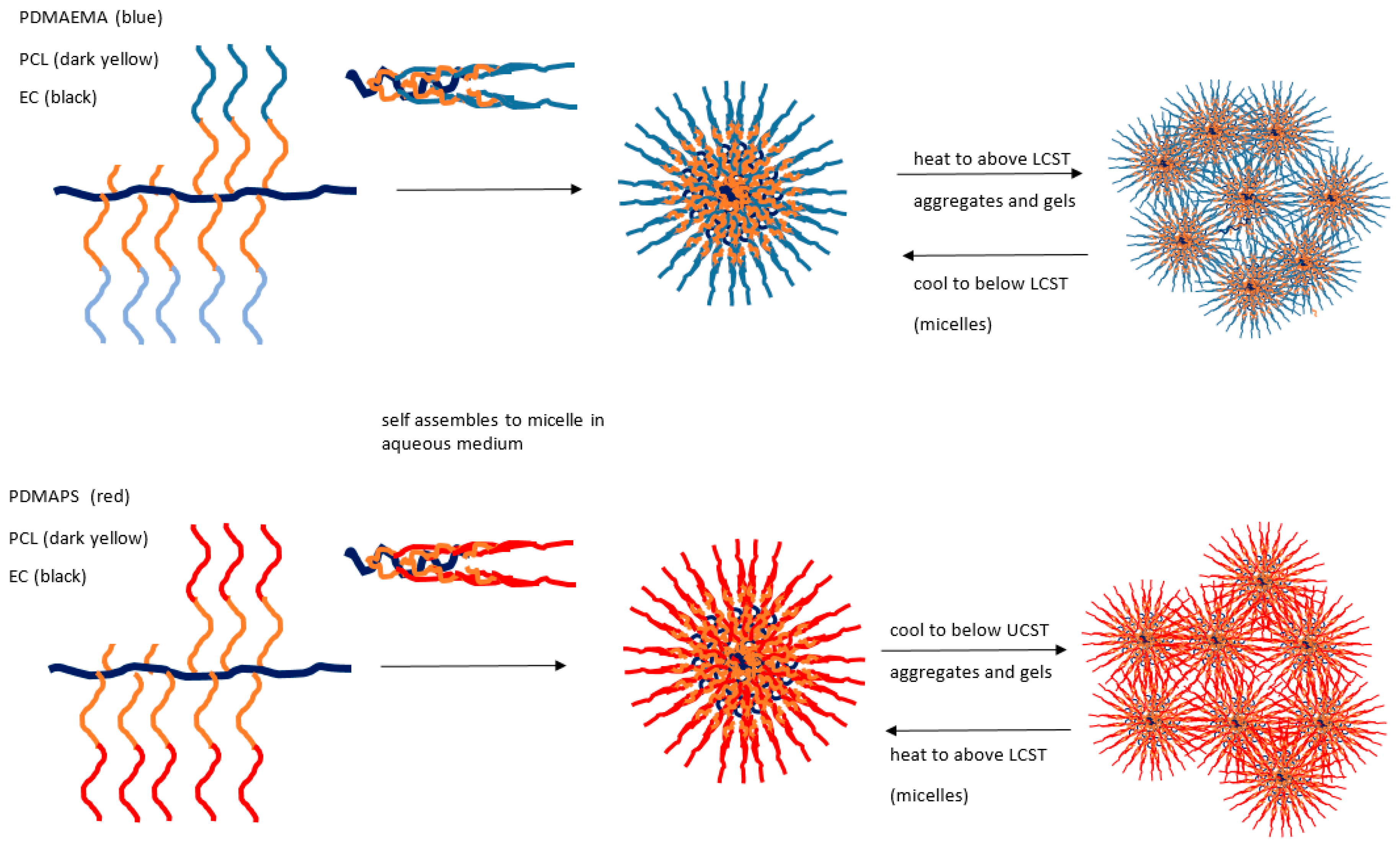
2.3. Micellar Systems with UCST and/or LCST
2.3.1. Micellar UCST Systems
2.3.2. Micellar LCST Systems
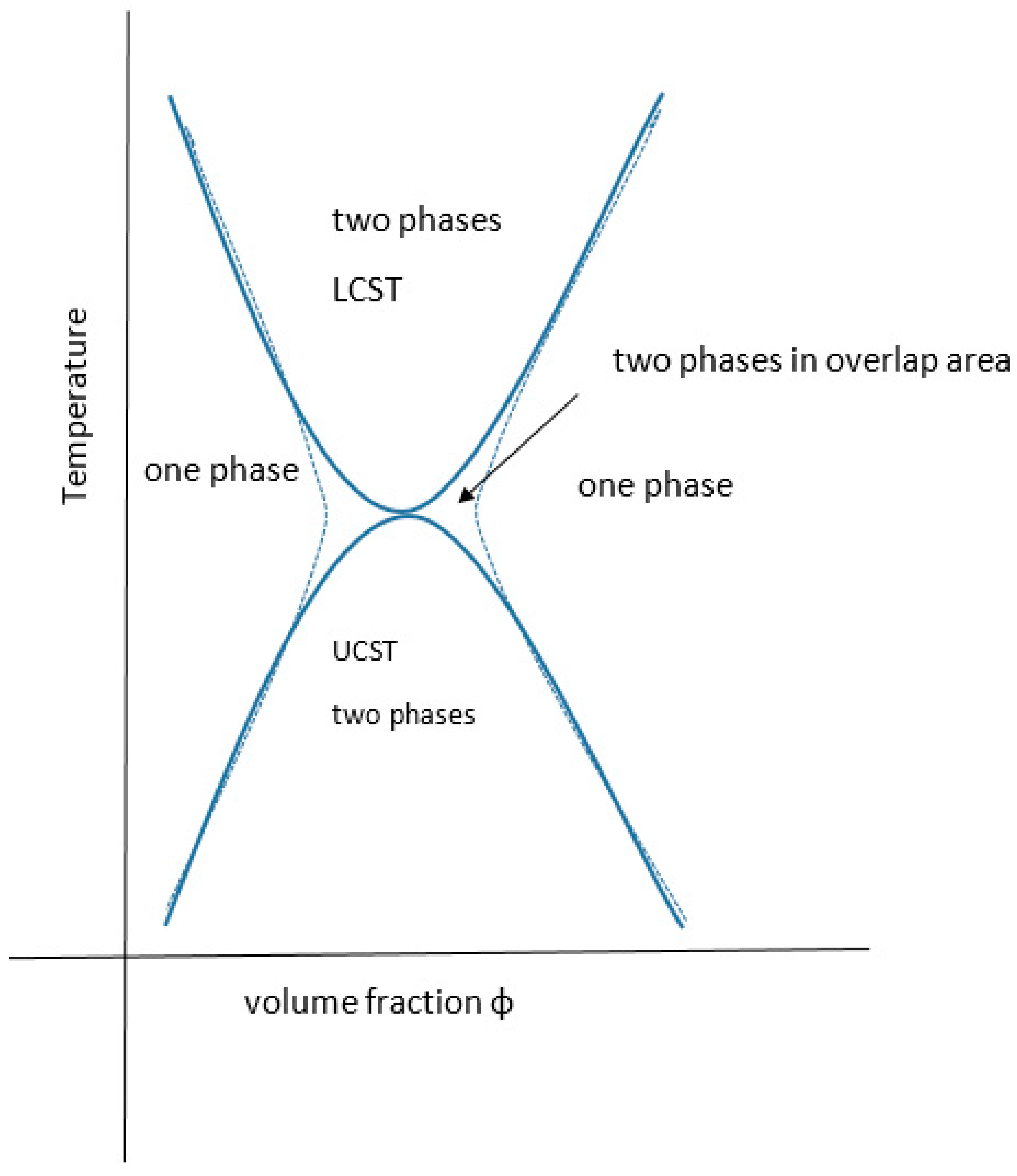
2.4. Materials Displaying Both a UCST and LCST
3. Hydrogels and Crosslinked Organogels Including Particulate Forms
4. Proteins
5. Thermoresponsive Vitrimers and Composites
6. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.R.; San Román, J. 1—Introduction to smart polymers and their applications. In Smart Polymers and Their Applications; Aguilar, M.R., Román, J.S., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 1–11. [Google Scholar]
- Matanović, M.R.; Kristl, J.; Grabnar, P.A. Thermoresponsive polymers: Insights into decisive hydrogel characteristics, mechanisms of gelation, and promising biomedical applications. Int. J. Pharm. 2014, 472, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, L.; Feng, J. Dynamic covalent gels assembled from small molecules: From discrete gelators to dynamic covalent polymers. Chin. Chem. Lett. 2016, in press. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Asghar, A.; Aslam Maan, A. Chapter 13—Food Gels: Gelling Process and New Applications. In Advances in Food Rheology and Its Applications; Ahmed, J., Ptaszek, P., Basu, S., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 335–353. [Google Scholar]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels (Republished 2012, first published 2002). Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Goponenko, A.V.; Dzenis, Y.A. Role of mechanical factors in applications of stimuli-responsive polymer gels—Status and prospects. Polymer 2016, 101, 415–449. [Google Scholar] [CrossRef]
- Sahota, T.S.; Sawicka, K.; Taylor, M.J.; Tanna, S. Effect of varying molecular weight of dextran on acrylic derivatised dextran and concanavalin A glucose-responsive materials for closed-loop insulin delivery. Drug Dev. Ind. Pharm. 2011, 37, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.G.; Pinheiro, M.N.C. Diffusion Coefficients of Timolol Maleate in Polymeric Membranes Based on Methacrylate Hydrogels. J. Chem. Eng. Data 2013, 58, 2280–2289. [Google Scholar] [CrossRef]
- Esposito, E.; Ravani, L.; Mariani, P.; Puglia, C.; Mazzitelli, S.; Huang, N.; Cortesi, R.; Nastruzzi, C. Gelified reverse micellar dispersions as percutaneous formulations. J. Drug Deliv. Sci. Technol. 2016, 32, 270–282. [Google Scholar] [CrossRef]
- Feng, G.; Wang, H.; Yang, Y. Diffusion and Release of the Guest Molecules in Supramolecular Organogel. Acta Chim. Sin. 2008, 66, 576. [Google Scholar]
- Wang, D.; Zhao, J.; Liu, X.; Sun, F.; Zhou, Y.; Teng, L.; Li, Y. Parenteral thermo-sensitive organogel for schizophrenia therapy, in vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2014, 60, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, A.P.; Georgiou, T.K. Tuning the gelation of thermoresponsive gels. Eur. Polym. J. 2016, 78, 366–375. [Google Scholar] [CrossRef]
- Chabert, E.; Vial, J.; Cauchois, J.P.; Mihaluta, M.; Tournilhac, F. Multiple welding of long fiber epoxy vitrimer composites. Soft Matter 2016, 12, 4838–4845. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of aerogels, cryogels and xerogels of cellulose with hierarchical porous structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- Teotia, A.K.; Sami, H.; Kumar, A. 1—Thermo-responsive polymers: Structure and design of smart materials. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Zhang, Z., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 3–43. [Google Scholar]
- Zhu, Y.; Hoshi, R.; Chen, S.; Yi, J.; Duan, C.; Galiano, R.D.; Zhang, H.F.; Ameer, G.A. Sustained release of stromal cell derived factor-1 from an antioxidant thermoresponsive hydrogel enhances dermal wound healing in diabetes. J. Control. Release 2016, 238, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jiang, H.; Ye, S.; Yoshizumi, T.; Wagner, W.R. Tailoring the degradation rates of thermally responsive hydrogels designed for soft tissue injection by varying the autocatalytic potential. Biomaterials 2015, 53, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Van Hove, A.H.; Burke, K.; Antonienko, E.; Brown, E., III; Benoit, D.S.W. Enzymatically-responsive pro-angiogenic peptide-releasing poly(ethylene glycol) hydrogels promote vascularization in vivo. J. Control. Release 2015, 217, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Okano, T. 9—Temperature-responsive polymers for cell culture and tissue engineering applications. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Zhang, Z., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 203–233. [Google Scholar]
- Lin, J.B.; Isenberg, B.C.; Shen, Y.; Schorsch, K.; Sazonova, O.V.; Wong, J.Y. Thermo-responsive poly(N-isopropylacrylamide) grafted onto microtextured poly(dimethylsiloxane) for aligned cell sheet engineering. Colloids Surfaces B Biointerfaces 2012, 99, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Smink, A.M.; de Haan, B.J.; Paredes-Juarez, G.A.; Wolters, A.H.; Kuipers, J.; Giepmans, B.N.; Schwab, L.; Engelse, M.A.; van Apeldoorn, A.A.; de Koning, E.; et al. Selection of polymers for application in scaffolds applicable for human pancreatic islet transplantation. Biomed. Mater. 2016, 11, 035006. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.; Edberg, J.; Hamedi, M.M.; Gabrielsson, R.; Granberg, H.; Wågberg, L.; Engquist, I.; Berggren, M.; Crispin, X. Thermoelectric Polymers and Their Elastic Aerogels. Adv. Mater. 2016, 28, 4556–4562. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Y.A.; Martínez, J.G.; Al Harrasi, A.S.; Kim, S.J.; Otero, T.F. Sensing characteristics of a conducting polymer/hydrogel hybrid microfiber artificial muscle. Sens. Actuators B Chem. 2011, 160, 1180–1190. [Google Scholar] [CrossRef]
- Ge, D.; Qi, R.; Mu, J.; Ru, X.; Hong, S.; Ji, S.; Linkov, V.; Shi, W. A self-powered and thermally-responsive drug delivery system based on conducting polymers. Electrochem. Commun. 2010, 12, 1087–1090. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Priya James, H.; John, R.; Alex, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.S.; Kwon, Y.J. Stimuli-responsive polymers and nanomaterials for gene delivery and imaging applications. Adv. Drug Deliv. Rev. 2012, 64, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Lemanowicz, M. Thermosensitive aggregation under conditions of repeated heating-cooling cycles. Int. J. Miner. Process. 2015, 144, 26–32. [Google Scholar] [CrossRef]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- Casado, N.; Hernández, G.; Sardon, H.; Mecerreyes, D. Current trends in redox polymers for energy and medicine. Prog. Polym. Sci. 2016, 52, 107–135. [Google Scholar] [CrossRef]
- Li, J.; Stachowski, M.; Zhang, Z. 11—Application of responsive polymers in implantable medical devices and biosensors. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Zhang, Z., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 259–298. [Google Scholar]
- Bilanovic, D.; Starosvetsky, J.; Armon, R.H. Cross-linking xanthan and other compounds with glycerol. Food Hydrocoll. 2015, 44, 129–135. [Google Scholar] [CrossRef]
- Bhatia, M.; Ahuja, M.; Mehta, H. Thiol derivatization of Xanthan gum and its evaluation as a mucoadhesive polymer. Carbohydr. Polym. 2015, 131, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hwang, J.; Kang, H.; Choi, J. Chlorhexidine-loaded xanthan gum-based biopolymers for targeted, sustained release of antiseptic agent. J. Ind. Eng. Chem. 2015, 32, 44–48. [Google Scholar] [CrossRef]
- Bassas-Galia, M.; Follonier, S.; Pusnik, M.; Zinn, M. 2—Natural polymers: A source of inspiration. In Bioresorbable Polymers for Biomedical Applications; Perale, G., Hilborn, J., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 31–64. [Google Scholar]
- Zhu, B.; Ma, D.; Wang, J.; Zhang, J.; Zhang, S. Multi-responsive hydrogel based on lotus root starch. Int. J. Biol. Macromol. 2016, 89, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Karolewicz, B. A review of polymers as multifunctional excipients in drug dosage form technology. Saudi Pharm. J. 2015, 24, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Shang, H.; Wei, D.; Jiang, S. A multi-stimuli-responsive organogel based on salicylidene Schiff base. Sens. Actuators B Chem. 2013, 185, 389–397. [Google Scholar] [CrossRef]
- Seeboth, A.; Lotzsch, D.; Ruhmann, R.; Muehling, O. Thermochromic polymers—Function by design. Chem. Rev. 2014, 114, 3037–3068. [Google Scholar] [CrossRef] [PubMed]
- Mojtabavi, M.; Jodhani, G.; Rao, R.; Zhang, J.; Gouma, P. A PANI–Cellulose acetate composite as a selective and sensitive chemomechanical actuator for acetone detection. Adv. Device Mater. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Serpe, J.M. Stimuli-Responsive Assemblies for Sensing Applications. Gels 2016, 2, 8. [Google Scholar] [CrossRef]
- Alzari, V.; Ruiu, A.; Nuvoli, D.; Sanna, R.; Martinez, J.I.; Appelhans, D.; Voit, B.; Zschoche, S.; Mariani, A. Three component terpolymer and IPN hydrogels with response to stimuli. Polymer 2014, 55, 5305–5313. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Multicompartment thermoresponsive gels: Does the length of the hydrophobic side group matter? Polym. Chem. 2013, 4, 1893–1902. [Google Scholar] [CrossRef]
- Flory, P. Selected Works of Paul J Flory; Mandelkern, L., Mark, J., Suter, U., Yoon, D.Y., Eds.; Stanford University Press: Redwood City, CA, USA, 1985; Volume 1. [Google Scholar]
- Kawaguchi, H. Biomedical Applications of Hydrogels Handbook; Ottenbrite, R., Park, K., Okano, T., Eds.; Springer Science and Business Media: New York, NY, USA, 2010. [Google Scholar]
- Aseyev, V.; Tenhu, H.; Winnik, F.M. Non-Ionic Thermoresponsive Polymers in Water. In Self Organized Nanostructures of Amphiphilic Block Copolymers II; Müller, H.E.A., Borisov, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 29–89. [Google Scholar]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef] [PubMed]
- Generalova, A.N.; Oleinikov, V.A.; Sukhanova, A.; Artemyev, M.V.; Zubov, V.P.; Nabiev, I. Quantum dot-containing polymer particles with thermosensitive fluorescence. Biosens. Bioelectron. 2013, 39, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Haas, H.C.; Chiklis, C.K.; Moreau, R.D. Synthetic thermally reversible gel systems. III. J. Polym. Sci. A-1 Polym. Chem. 1970, 8, 1131–1145. [Google Scholar] [CrossRef]
- Boustta, M.; Colombo, P.; Lenglet, S.; Poujol, S.; Vert, M. Versatile UCST-based thermoresponsive hydrogels for loco-regional sustained drug delivery. J. Control. Release 2014, 174, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vert, M. Polymeric biomaterials: Strategies of the past vs. strategies of the future. Prog. Polym. Sci. 2007, 32, 755–761. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Doulabi, M. Multiwalled carbon nanotube-polyelectrolyte gels: Preparation and swelling behavior for organic solvents. Solid State Ion. 2014, 257, 32–37. [Google Scholar] [CrossRef]
- Louro, H.; Pinhão, M.; Santos, J.; Tavares, A.; Vital, N.; Silva, M.J. Evaluation of the cytotoxic and genotoxic effects of benchmark multi-walled carbon nanotubes in relation to their physicochemical properties. Toxicol. Lett. 2016, 262, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Mooney, J.E.; Rolfe, B.E.; Osborne, G.W.; Sester, D.P.; van Rooijen, N.; Campbell, G.R.; Hume, D.A.; Campbell, J.H. Cellular Plasticity of Inflammatory Myeloid Cells in the Peritoneal Foreign Body Response. Am. J. Pathol. 2010, 176, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, E.; Tsianou, M. Solution properties of dextran in water and in formamide. J. Appl. Polym. Sci. 2012, 125, 1681–1692. [Google Scholar] [CrossRef]
- Maffi, C.; Baiesi, M.; Casetti, L.; Piazza, F.; de Los Rios, P. First-order coil-globule transition driven by vibrational entropy. Nat. Commun. 2012, 3, 1065. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Chan Bae, Y. Role of intermolecular interactions for upper and Lower Critical Solution Temperature Behaviors in polymer solutions: Molecular simulations and thermodynamic modeling. Polymer 2012, 53, 3772. [Google Scholar] [CrossRef]
- Deshmukh, P.K.; Ramani, K.P.; Singh, S.S.; Tekade, A.R.; Chatap, V.K.; Patil, G.B.; Bari, S.B. Stimuli-sensitive layer-by-layer (LbL) self-assembly systems: Targeting and biosensory applications. J. Control. Release 2013, 166, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhove, I.; Salamon, A.; Peters, K.; Graulus, G.; Martins, J.C.; Frankel, D.; Kersemans, K.; de Vos, F.; van Vlierberghe, S.; Dubruel, P. Gelatin- and starch-based hydrogels. Part A: Hydrogel development, characterization and coating. Carbohydr. Polym. 2016, 152, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Z.; Hao, Y.; Zhao, Y.; Qiu, Y.; Jiang, J.; Yu, T.; Ji, P.; Liu, Y.; Wu, C. Preparation of a Novel Form of Gelatin With a Three-Dimensional Ordered Macroporous Structure to Regulate the Release of Poorly Water-Soluble Drugs. J. Pharm. Sci. 2016, 105, 2940–2948. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Sahoo, R.K.; Biswas, N.; Guha, A.; Kuotsu, K. Recent advancement of gelatin nanoparticles in drug and vaccine delivery. Int. J. Biol. Macromol. 2015, 81, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, G.; Seijo, B.; Sanchez, A. Handbook of Polymers for Pharmaceutical Technologies, Structure and Chemistry; Thakur, V.K., Thakur, M.K., Eds.; Wiley: Hoboken, NJ, USA, 2015; Volume 1. [Google Scholar]
- Duconseille, A.; Andueza, D.; Picard, F.; Santé-Lhoutellier, V.; Astruc, T. Molecular changes in gelatin aging observed by NIR and fluorescence spectroscopy. Food Hydrocoll. 2016, 61, 496–503. [Google Scholar] [CrossRef]
- Badii, F.; Martinet, C.; Mitchell, J.R.; Farhat, I.A. Enthalpy and mechanical relaxation of glassy gelatin films. Food Hydrocoll. 2006, 20, 879–884. [Google Scholar] [CrossRef]
- Badii, F.; MacNaughtan, W.; Farhat, I.A. Enthalpy relaxation of gelatin in the glassy state. Int. J. Biol. Macromol. 2005, 36, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G. Chapter 5—Temperature-Sensitive Pharmaceutical Nanocarriers in Smart Pharmaceutical Nanocarriers. Torchilin, V., Ed.; Imperial College Press: London, UK, 2016; pp. 143–177. [Google Scholar]
- Shikanov, A.; Domb, A.J. Chapter 23—Polymer-based drug delivery systems in Focal Controlled drug delivery. Springer Science & Business Media: New York, NY, USA, 2014; pp. 511–556. [Google Scholar]
- Gupta, A.; Mohanty, B.; Bohidar, H.B. Flory temperature and upper critical solution temperature of gelatin solutions. Biomacromolecules 2005, 6, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Seuring, J.; Agarwal, S. Polymers with upper critical solution temperature in aqueous solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef] [PubMed]
- Chejara, D.R.; Mabrouk, M.; Badhe, R.V.; Mulla, J.A.S.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. A bio-injectable algin-aminocaproic acid thixogel with tri-stimuli responsiveness. Carbohydr. Polym. 2016, 135, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Maji, T.; Banerjee, S.; Biswas, Y.; Mandal, T.K. Dual-Stimuli-Responsive l-Serine-Based Zwitterionic UCST-Type Polymer with Tunable Thermosensitivity. Macromolecules 2015, 48, 4957–4966. [Google Scholar] [CrossRef]
- Ji, Y.; Zhu, M.; Gong, Y.; Tang, H.; Li, J.; Cao, Y. Thermoresponsive Polymers with Lower Critical Solution Temperature- or Upper Critical Solution Temperature-Type Phase Behaviour Do Not Induce Toxicity to Human Endothelial Cells. Basic Clin. Pharmacol. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liang, Y.; Peng, X.; Hu, Y.; Zhang, L.; Wu, H.; He, B. In situ injection of phenylboronic acid based low molecular weight gels for efficient chemotherapy. Biomaterials 2016, 105, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; You, X.; Lin, Q.; Li, J.; Guo, Y.; Wei, T.; Zhang, Y. Multi-stimuli responsive metal-organic gel of benzimidazol-based ligands with lead nitrate and their use in removal of dyes from waste-water. Chin. Chem. Lett. 2013, 24, 703–706. [Google Scholar] [CrossRef]
- Covitch, M.J.; Trickett, K.J. How Polymers Behave as Viscosity Index Improvers in Lubricating Oils. Adv. Chem. Eng. Sci. 2015, 5, 134–151. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Singh, V.K.; Kulanthaivel, S.; Banerjee, I.; Basak, P.; Battachrya, M.K.; Pal, K. Stearate organogel-gelatin hydrogel based bigels: Physicochemical, thermal, mechanical characterizations and in vitro drug delivery applications. J. Mech. Behav. Biomed. Mater. 2015, 43, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Hafez, S.A.; Mahdy, M.M. Organogels, hydrogels and bigels as transdermal delivery systems for diltiazem hydrochloride. Asian J. Pharm. Sci. 2013, 8, 48–57. [Google Scholar] [CrossRef]
- Zhu, M.; Xu, Y.; Ge, C.; Ling, Y.; Tang, H. Synthesis and UCST-type phase behavior of OEGylated poly(γ-benzyl-l-glutamate) in organic media. J. Polym. Sci. A Polym. Chem. 2016, 54, 1348–1356. [Google Scholar] [CrossRef]
- Hanabusa, K.; Suzuki, M. Development of low-molecular-weight gelators and polymer-based gelators. Polym. J. 2014, 46, 776–782. [Google Scholar] [CrossRef]
- Kumar, P.; Kadam, M.M.; Gaikar, V.G. Low Molecular Weight Organogels and Their Application in the Synthesis of CdS Nanoparticles. Ind. Eng. Chem. Res. 2012, 51, 15374–15385. [Google Scholar] [CrossRef]
- Guo, H.; Jiao, T.; Shen, X.; Zhang, Q.; Li, A.; Zhou, J.; Gao, F. Binary organogels based on glutamic acid derivatives and different acids: Solvent effect and molecular skeletons on self-assembly and nanostructures. Colloids Surface Physicochem. Eng. Asp. 2014, 447, 88–96. [Google Scholar] [CrossRef]
- Mateescu, M.A. 1—The concept of self-assembling and the interactions involved. In Controlled Drug Delivery; Mateescu, M.A., Ispas-Szabo, P., Assaad, E., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 1–20. [Google Scholar]
- Ilbasmis-Tamer, S.; Unsal, H.; Tugcu-Demiroz, F.; Kalaycioglu, G.D.; Degim, I.T.; Aydogan, N. Stimuli-responsive lipid nanotubes in gel formulations for the delivery of doxorubicin. Colloids Surfaces B Biointerfaces 2016, 143, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Peng, S.; Hughes, T.C. Multistimuli responsive organogels based on a reactive azobenzene gelator. Soft Matter 2014, 10, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Yang, Y. Stimuli-responsive fluorescent supramolecular polymers based on pillarenes for controlled drug release. J. Control. Release 2015, 213, e137. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Katsumoto, Y.; Nakano, S.; Kita, R. LCST phase separation and thermoreversible gelation in aqueous solutions of stereo-controlled poly(N-isopropylacrylamide)s. React. Funct. Polym. 2013, 73, 894–897. [Google Scholar] [CrossRef]
- Chu, B. Scattering Techniques Applied to Supramolecular and Nonequilibrium Systems; Chen, S.H., Chu, B., Nossal, R., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Clark, E.A.; Lipson, J.E.G. LCST and UCST behavior in polymer solutions and blends. Polymer 2012, 53, 536–545. [Google Scholar] [CrossRef]
- Masuelli, M.A. Dextrans in Aqueous Solution. Experimental Review on Intrinsic Viscosity Measurements and Temperature Effect. J. Polym. Biopolym. Phys. Chem. 2013, 1, 13–21. [Google Scholar]
- Güner, A.; Kibarer, G. The important role of thermodynamic interaction parameter in the determination of theta temperature, dextran/water system. Eur. Polym. J. 2001, 37, 619–622. [Google Scholar] [CrossRef]
- Rao, M.K.; Rao, S.K.; Ha, C. Stimuli Responsive Poly(Vinyl Caprolactam) Gels for Biomedical Applications. Gels 2016, 2, 6. [Google Scholar] [CrossRef]
- Zhang, Q.; Hoogenboom, R. Polymers with upper critical solution temperature behavior in alcohol/water solvent mixtures. Prog. Polym. Sci. 2015, 48, 122–142. [Google Scholar] [CrossRef]
- Lima, L.H.; Morales, Y.; Cabral, T. Ocular Biocompatibility of Poly-N-Isopropylacrylamide (pNIPAM). J. Ophthalmol. 2016, 2016, 5356371. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Cyclodextrin-based self-assembled supramolecular hydrogels and cationic polyrotaxanes for drug and gene delivery applications. J. Drug Deliv. Sci. Technol. 2010, 20, 399–405. [Google Scholar] [CrossRef]
- Graziano, G. On the temperature-induced coil to globule transition of poly-N-isopropylacrylamide in dilute aqueous solutions. Int. J. Biol. Macromol. 2000, 27, 89–97. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, X. Collapse kinetics for individual poly(N-isopropylmethacrylamide) chains. Polymer 2010, 51, 897–901. [Google Scholar] [CrossRef]
- Costa, M.C.M.; Silva, S.M.C.; Antunes, F.E. Adjusting the low critical solution temperature of poly(N-isopropyl acrylamide) solutions by salts, ionic surfactants and solvents: A rheological study. J. Mol. Liq. 2015, 210, 113–118. [Google Scholar] [CrossRef]
- Wang, M. A single polymer folding and thickening from different dilute solution. Phys. Lett. A 2015, 379, 2761–2765. [Google Scholar] [CrossRef]
- Milewska, A.; Szydlowski, J.; Rebelo, L.P.N. Viscosity and ultrasonic studies of poly(N-isopropylacrylamide)—Water solutions. J. Polym. Sci. B Polym. Phys. 2003, 41, 1219–1233. [Google Scholar] [CrossRef]
- Shoji, K.; Nakayama, M.; Koseki, T.; Nakabayashi, K.; Mori, H. Threonine-based chiral homopolymers with multi-stimuli-responsive property by RAFT polymerization. Polymer 2016, 97, 20–30. [Google Scholar] [CrossRef]
- Silva, S.M.C.; Pinto, F.V.; Antunes, F.E.; Miguel, M.G.; Sousa, J.J.S.; Pais, A.A.C.C. Aggregation and gelation in hydroxypropylmethyl cellulose aqueous solutions. J. Colloid Interface Sci. 2008, 327, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, J.; Zhao, J.; Akiyama, H.; Yoshida, M. Photo-controllable coil-to-globule transition of single polymer molecules. Polymer 2016, 97, 309–313. [Google Scholar] [CrossRef]
- Boutris, C.; Chatzi, E.G.; Kiparissides, C. Characterization of the LCST behaviour of aqueous poly(N-isopropylacrylamide) solutions by thermal and cloud point techniques. Polymer 1997, 38, 2567–2570. [Google Scholar] [CrossRef]
- Zhou, K.; Lu, Y.; Li, J.; Shen, L.; Zhang, G.; Xie, Z.; Wu, C. The Coil-to-Globule-to-Coil Transition of Linear Polymer Chains in Dilute Aqueous Solutions: Effect of Intrachain Hydrogen Bonding. Macromolecules 2008, 41, 8927–8931. [Google Scholar] [CrossRef]
- Zhang, L.P.; Noda, I.; Wu, Y. Quantitative comparison of reversibility in thermal-induced hydration of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide) in aqueous solutions by “concatenated” 2D correlation analysis. Vib. Spectrosc. 2012, 60, 200–205. [Google Scholar] [CrossRef]
- Jeong, B.; Gutowska, A. Lessons from nature: Stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2002, 20, 305–311. [Google Scholar] [CrossRef]
- Jones, S.T.; Walsh-Korb, Z.; Barrow, S.J.; Henderson, S.L.; del Barrio, J.; Scherman, O.A. The Importance of Excess Poly(N-isopropylacrylamide) for the Aggregation of Poly(N-isopropylacrylamide)-Coated Gold Nanoparticles. ACS Nano 2016, 10, 3158–3165. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, J.; Zhou, X.; Cao, S. Hierarchically organization of biomineralized alginate beads for dual stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2015, 73, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Geng, S.; Li, H.; Wan, J.; Peng, X.; Liu, W.; Zhao, Y.; Yang, X.; Xu, H. The stimuli-responsive multiphase behavior of core-shell nanogels with opposite charges and their potential application in in situ gelling system. Colloids Surfaces B Biointerfaces 2015, 136, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Masuda, Y.; Kadota, J.; Nishiyama, T.; Horibe, H. Dual stimuli-responsive homopolymers: Thermo- and photo-responsive properties of coumarin-containing polymers in organic solvents. Eur. Polym. J. 2015, 69, 605–615. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Singh, V.K.; Banerjee, I.; Pramanik, K.; Basak, P.; Pal, K. Core-shell-type organogel-alginate hybrid microparticles: A controlled delivery vehicle. Chem. Eng. J. 2015, 264, 134–145. [Google Scholar] [CrossRef]
- Strandman, S.; Zhu, X.X. Thermo-responsive block copolymers with multiple phase transition temperatures in aqueous solutions. Prog. Polym. Sci. 2015, 42, 154–176. [Google Scholar] [CrossRef]
- Wagner, H.J.; Sprenger, A.; Rebmann, B.; Weber, W. Upgrading biomaterials with synthetic biological modules for advanced medical applications. Adv. Drug Deliv. Rev. 2016, 105, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Gu, L.; Ren, W.; Liu, Y. Stimuli-responsive polymers for anti-cancer drug delivery. Mater. Sci. Eng. C 2014, 45, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Ma, C.; Shan, G.; Bao, Y.; Pan, P. Poly(lactic acid)/poly(ethylene glycol) supramolecular diblock copolymers based on three-fold complementary hydrogen bonds: Synthesis, micellization, and stimuli responsivity. Polymer 2016, 90, 122–131. [Google Scholar] [CrossRef]
- Jain, A.; Kunduru, K.R.; Basu, A.; Mizrahi, B.; Domb, A.J.; Khan, W. Injectable formulations of poly(lactic acid) and its copolymers in clinical use. Adv. Drug Deliv. Rev. 2016, 107, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, A.; Shimada, N.; Maruyama, A.; Ishihara, K.; Nakai, K.; Yusa, S. Preparation of upper critical solution temperature (UCST) responsive diblock copolymers bearing pendant ureido groups and their micelle formation behavior in water. Soft Matter 2015, 11, 5204–5213. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Rawat, K.; Bohidar, H.B. Interface versus bulk gelation and UCST in hydrophobically assembled TX-100 molecular gels. Colloids Surface Physicochem. Eng. Asp. 2016, 499, 113–122. [Google Scholar] [CrossRef]
- Huang, G.; Li, H.; Feng, S.; Li, X.; Tong, G.; Liu, J.; Quan, C.; Jiang, Q.; Zhang, C.; Li, Z. Self-assembled UCST-Type Micelles as Potential Drug Carriers for Cancer Therapeutics. Macromol. Chem. Phys. 2015, 216, 1014–1023. [Google Scholar] [CrossRef]
- Yuan, H.; Chi, H.; Yuan, W. Ethyl cellulose amphiphilic graft copolymers with LCST–UCST transition: Opposite self-assembly behavior, hydrophilic-hydrophobic surface and tunable crystalline morphologies. Carbohydr. Polym. 2016, 147, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, R.; Rogers, S.; Can, A.; Becer, C.R.; Guerrero-Sanchez, C.; Wouters, D.; Hoeppener, S.; Schubert, U.S. Self-assembly of double hydrophobic block copolymers in water-ethanol mixtures: From micelles to thermoresponsive micellar gels. Chem. Commun. (Camb) 2009, 5582–5584. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hu, S.; Liu, D.; Chen, S.; Chen, I. Biomedical nanoparticle carriers with combined thermal and magnetic responses. Nano Today 2009, 4, 52–65. [Google Scholar] [CrossRef]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.I.; O’Reilly, R.K. To aggregate, or not to aggregate? considerations in the design and application of polymeric thermally-responsive nanoparticles. Chem. Soc. Rev. 2013, 42, 7204–7213. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Fatouros, D.G. Smart materials: In situ gel-forming systems for nasal delivery. Drug Discov. Today 2016, 21, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariépy, E.; Chenite, A.; Chaput, C.; Guirguis, S.; Leroux, J. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int. J. Pharm. 2000, 203, 89–98. [Google Scholar] [CrossRef]
- Shi, K.; Wang, Y.L.; Qu, Y.; Liao, J.F.; Chu, B.Y.; Zhang, H.P.; Luo, F.; Qian, Z.Y. Synthesis, characterization, and application of reversible PDLLA-PEG-PDLLA copolymer thermogels in vitro and in vivo. Sci. Rep. 2016, 6, 19077. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Kunduru, K.R.; Doppalapudi, S.; Domb, A.J.; Khan, W. Poly(lactic acid) based hydrogels. Adv. Drug Deliv. Rev. 2016, 107, 92–205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Sanoj Rejinold, N.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly(N-isopropylacrylamide) nanocarrier for curcumin drug delivery. Int. J. Biol. Macromol. 2011, 49, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, Z.; Li, Y.; Jiang, Z.; Hu, Q.; Liu, M.; Zhao, Q. Dual stimuli-responsive N-phthaloylchitosan-graft-(poly(N-isopropylacrylamide)-block-poly(acrylic acid)) copolymer prepared via RAFT polymerization. Carbohydr. Polym. 2013, 92, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Guerry, A.; Cottaz, S.; Fleury, E.; Bernard, J.; Halila, S. Redox-stimuli responsive micelles from DOX-encapsulating polycaprolactone-g-chitosan oligosaccharide. Carbohydr. Polym. 2014, 112, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Hu, S. “Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications. Materials 2013, 6, 738–781. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Hung, C. In vitro dual-modality chemo-photodynamic therapy via stimuli-triggered polymeric micelles. React. Funct. Polym. 2016, 98, 56–64. [Google Scholar] [CrossRef]
- He, L.; Shang, J.; Theato, P. Preparation of dual stimuli-responsive block copolymers based on different activated esters with distinct reactivities. Eur. Polym. J. 2015, 69, 523–531. [Google Scholar] [CrossRef]
- Kim, S.; Joseph, V.S.; Hong, J. Dual stimuli-responsive copolymers comprising poly(N-isopropylacrylamide) and poly(cyano malachite green). Colloids Surface Physicochem. Eng. Asp. 2015, 476, 8–16. [Google Scholar] [CrossRef]
- Weiss, J.; Laschewsky, A. Temperature-induced self-assembly of triple-responsive triblock copolymers in aqueous solutions. Langmuir 2011, 27, 4465–4473. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luo, S.; Shi, W.; Liu, S. Two-stage collapse of unimolecular micelles with double thermoresponsive coronas. Langmuir 2006, 22, 989–997. [Google Scholar] [CrossRef] [PubMed]
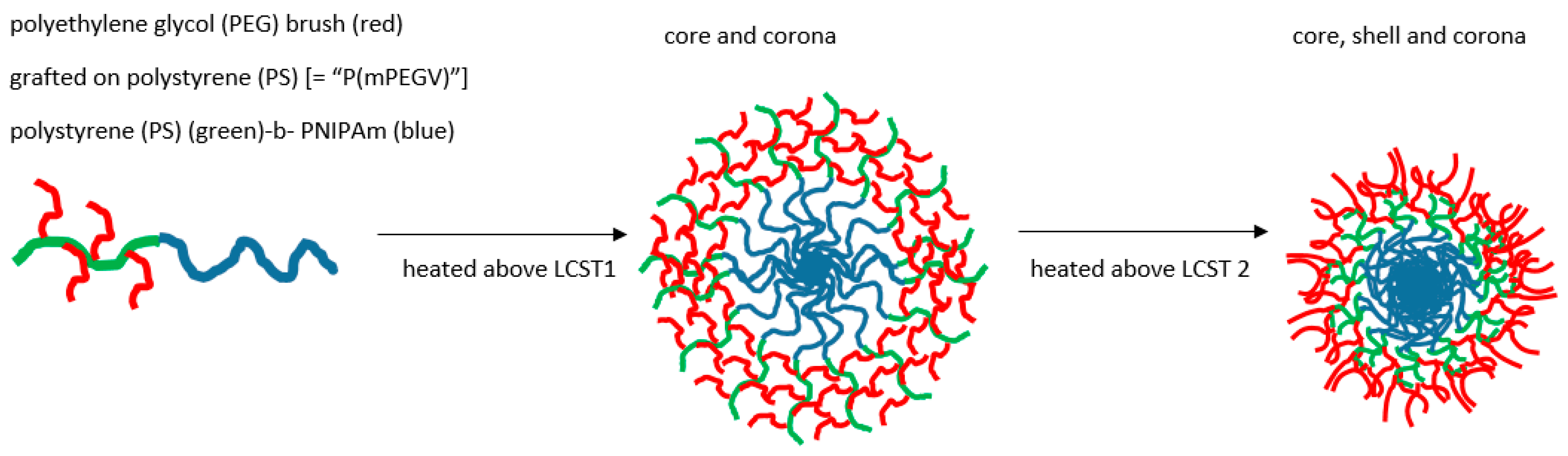
- Su, Y.; Li, Q.; Li, S.; Dan, M.; Huo, F.; Zhang, W. Doubly thermo-responsive brush-linear diblock copolymers and formation of core-shell-corona micelles. Polymer 2014, 55, 1955–1963. [Google Scholar] [CrossRef]
- Koynova, R.; Tenchov, B. Transitions between lamellar and non-lamellar phases in membrane lipids and their physiological roles. Open Access Biochem. 2013, 1, 1–9. [Google Scholar]
- Sundarajan, P.R. Physical Properties of Polymers Handbook, 2nd ed.; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Siow, K.S.; Delmas, G.; Patterson, D. Cloud-Point Curves in Polymer Solutions with Adjacent Upper and Lower Critical Solution Temperatures. Macromolecules 1972, 5, 29–34. [Google Scholar] [CrossRef]
- Zhu, Y.; Batchelor, R.; Lowe, A.B.; Roth, P.J. Design of Thermoresponsive Polymers with Aqueous LCST, UCST, or Both: Modification of a Reactive Poly(2-vinyl-4,4-dimethylazlactone) Scaffold. Macromolecules 2016, 49, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Caneba, G. Free-Radical Retrograde-Precipitation Polymerization (FRRPP): Novel Concept, Processes, Materials, and Energy Aspects; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Balu, R.; Dutta, N.K.; Choudhury, N.R.; Elvin, C.M.; Lyons, R.E.; Knott, R.; Hill, A.J. An16-resilin: An advanced multi-stimuli-responsive resilin-mimetic protein polymer. Acta Biomater. 2014, 10, 4768–4777. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Zhang, Q.; Rudolph, T.; Schacher, F.H.; Gohy, J.; Schubert, U.S.; Hoogenboom, R. Schizophrenic thermoresponsive block copolymer micelles based on LCST and UCST behavior in ethanol–water mixtures. Eur. Polym. J. 2015, 69, 460–471. [Google Scholar] [CrossRef]
- Zhang, Q.; Hong, J.; Hoogenboom, R. A triple thermoresponsive schizophrenic diblock copolymer. Polym. Chem. 2013, 4, 4322–4325. [Google Scholar] [CrossRef]
- Borgogna, M.; Marsich, E.; Donati, I.; Paoletti, S.; Travan, A. Polysaccharide Hydrogels: Characterization and Biomedical Applications; Matricardi, P., Alhaique, F., Coviello, T., Eds.; Pan Stanford Publishing: Singapore, 2016. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, M.T.; Hussain, M.A.; Yuk, S.H.; Bashir, S.; Nauman, M. Polysaccharides based superabsorbent hydrogel from Linseed: Dynamic swelling, stimuli responsive on–off switching and drug release. Carbohydr. Polym. 2016, 136, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.G.; Kumar, A.; De, P. Amino acid containing cross-linked co-polymer gels: PH, thermo and salt responsiveness. Polymer 2016, 85, 1–9. [Google Scholar] [CrossRef]
- Engberg, K.; Waters, D.J.; Kelmanovich, S.; Parke-Houben, R.; Hartmann, L.; Toney, M.F.; Frank, C.W. Self-assembly of cholesterol tethered within hydrogel networks. Polymer 2016, 84, 371–382. [Google Scholar] [CrossRef]
- Vakili, M.R.; Rahneshin, N. Synthesis and characterization of novel stimuli-responsive hydrogels based on starch and l-aspartic acid. Carbohydr. Polym. 2013, 98, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.C. Sol-Gel Behavior of Hydroxypropyl Methylcellulose (HPMC) in Ionic Media Including Drug Release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef]
- Mah, E.; Ghosh, R. Thermo-Responsive Hydrogels for Stimuli-Responsive Membranes. Processes 2013, 1, 238–262. [Google Scholar] [CrossRef]
- Drozdov, A.D.; Sanporean, C.-G.; Christiansen, J.D. Modeling the effects of temperature and pH on swelling of stimuli-responsive gels. Eur. Polym. J. 2015, 73, 278–296. [Google Scholar] [CrossRef]
- Cong, H.; Zheng, S. Poly(N-isopropylacrylamide)-block-poly(acrylic acid) hydrogels: Synthesis and rapid thermoresponsive properties. Colloid Polym. Sci. 2014, 292, 2633–2645. [Google Scholar] [CrossRef]
- Deen, G.R.; Mah, C.H. Influence of external stimuli on the network properties of cationic poly(N-acryloyl-N′-propyl piperazine) hydrogels. Polymer 2016, 89, 55–68. [Google Scholar] [CrossRef]
- Casolaro, M.; Casolaro, I.; Bottari, S.; Del Bello, B.; Maellaro, E.; Demadis, K.D. Long-term doxorubicin release from multiple stimuli-responsive hydrogels based on α-amino-acid residues. Eur. J. Pharm. Biopharm. 2014, 88, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Heidarinasab, A.; Ahmad Panahi, H.; Faramarzi, M.; Farjadian, F. Synthesis of thermosensitive magnetic nanocarrier for controlled sorafenib delivery. Mater. Sci. Eng. C 2016, 67, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, S. Thermosensitive/superparamagnetic iron oxide nanoparticle-loaded nanocapsule hydrogels for multiple cancer hyperthermia. Biomaterials 2016, 106, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Apopei Loghin, D.F.; Cocarta, A.; Doroftei, M. Multi-stimuli-responsive semi-IPN cryogels with native and anionic potato starch entrapped in poly(N,N-dimethylaminoethyl methacrylate) matrix and their potential in drug delivery. React. Funct. Polym. 2016, 105, 66–77. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Wang, Z.; Liu, H.; Li, C. Preparation and characterization of a positive thermoresponsive hydrogel for drug loading and release. J. Appl. Polym. Sci. 2009, 111, 1417–1425. [Google Scholar] [CrossRef]
- Wei, X.; Lv, X.; Zhao, Q.; Qiu, L. Thermosensitive β-cyclodextrin modified poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) micelles prolong the anti-inflammatory effect of indomethacin following local injection. Acta Biomater. 2013, 9, 6953–6963. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.V.K.J.; Hetzer, M.; Ritter, H.; Barner-Kowollik, C. Complex macromolecular architecture design via cyclodextrin host/guest complexes. Prog. Polym. Sci. 2014, 39, 235–249. [Google Scholar] [CrossRef]
- Feng, Q.; Wei, K.; Lin, S.; Xu, Z.; Sun, Y.; Shi, P.; Li, G.; Bian, L. Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials 2016, 101, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wan, S.; Chen, B.; Gao, W.; Chen, J.; Liu, M.; He, B.; Wu, H. Dual pH and temperature responsive hydrogels based on β-cyclodextrin derivatives for atorvastatin delivery. Carbohydr. Polym. 2016, 136, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, M.; Gibson, H.W. Recent developments in polypseudorotaxanes and polyrotaxanes. Prog. Polym. Sci. 2014, 39, 1043–1073. [Google Scholar] [CrossRef]
- Gregory, A.; Stenzel, M.H. Complex polymer architectures via RAFT polymerization: From fundamental process to extending the scope using click chemistry and nature's building blocks. Prog. Polym. Sci. 2012, 37, 38–105. [Google Scholar] [CrossRef]
- Ahmed, M.; Narain, R. Progress of RAFT based polymers in gene delivery. Prog. Polym. Sci. 2013, 38, 767–790. [Google Scholar] [CrossRef]
- Lynam, D.; Peterson, C.; Maloney, R.; Shahriari, D.; Garrison, A.; Saleh, S.; Mehrotra, S.; Chan, C.; Sakamoto, J. Augmenting protein release from layer-by-layer functionalized agarose hydrogels. Carbohydr. Polym. 2014, 103, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, Z.; He, C.; Zhu, J.; Ma, G.; Wang, G.; Zhang, H.; Xiao, J.; Chen, S. Protein diffusion characteristics in the hydrogels of poly(ethylene glycol) and zwitterionic poly(sulfobetaine methacrylate) (pSBMA). Acta Biomater. 2016, 40, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Yom-Tov, O.; Neufeld, L.; Seliktar, D.; Bianco-Peled, H. A novel design of injectable porous hydrogels with in situ pore formation. Acta Biomater. 2014, 10, 4236–4246. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Samanta, P.; Dhara, D. Temperature, pH and redox responsive cellulose based hydrogels for protein delivery. Int. J. Biol. Macromol. 2016, 87, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Helgeson, M.E.; Moran, S.E.; An, H.Z.; Doyle, P.S. Mesoporous organohydrogels from thermogelling photocrosslinkable nanoemulsions. Nat. Mater. 2012, 11, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.G.; De, P. Swelling properties of amino acid containing cross-linked polymeric organogels and their respective polyelectrolytic hydrogels with pH and salt responsive property. Polymer 2014, 55, 5425–5434. [Google Scholar] [CrossRef]
- Singh, V.K.; Banerjee, I.; Agarwal, T.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Guar gum and sesame oil based novel bigels for controlled drug delivery. Colloids Surfaces B Biointerfaces 2014, 123, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Pal, K.; Banerjee, I.; Pramanik, K.; Anis, A.; Al-Zahrani, S.M. Novel organogel based lyotropic liquid crystal physical gels for controlled delivery applications. Eur. Polym. J. 2015, 68, 326–337. [Google Scholar] [CrossRef]
- Amin, S.; Barnett, G.V.; Pathak, J.A.; Roberts, C.J.; Sarangapani, P.S. Protein aggregation, particle formation, characterization & rheology. Curr. Opin. Colloid Interface Sci. 2014, 19, 438–449. [Google Scholar]
- Liu, L.; Qi, W.; Schwartz, D.K.; Randolph, T.W.; Carpenter, J.F. The Effects of Excipients on Protein Aggregation During Agitation: An Interfacial Shear Rheology Study. J. Pharm. Sci. 2013, 102, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhong, Q. Aggregation and gelation properties of preheated whey protein and pectin mixtures at pH 1.0–4.0. Food Hydrocoll. 2016, 60, 11–20. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Hnatuszko-Konka, K.; Gerszberg, A.; Kononowicz, A.K. Elastin-like polypeptides as a promising family of genetically-engineered protein based polymers. World J. Microbiol. Biotechnol. 2014, 30, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xu, Q.; Hu, X.; Qin, G.; Kaplan, D.L. Tunable Self-Assembly of Genetically Engineered Silk–Elastin-like Protein Polymers. Biomacromolecules 2011, 12, 3844–3850. [Google Scholar] [CrossRef] [PubMed]
- Glassman, M.J.; Olsen, B.D. Arrested Phase Separation of Elastin-like Polypeptide Solutions Yields Stiff, Thermoresponsive Gels. Biomacromolecules 2015, 16, 3762–3773. [Google Scholar] [CrossRef] [PubMed]
- Glassman, M.J.; Avery, R.K.; Khademhosseini, A.; Olsen, B.D. Toughening of Thermoresponsive Arrested Networks of Elastin-Like Polypeptides to Engineer Cytocompatible Tissue Scaffolds. Biomacromolecules 2016, 17, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.M.S.; Mano, J.F. Extremely strong and tough hydrogels as prospective candidates for tissue repair—A review. Eur. Polym. J. 2015, 72, 344–364. [Google Scholar] [CrossRef]
- Pritchard, R.H.; Redmann, A.; Pei, Z.; Ji, Y.; Terentjev, E.M. Vitrification and plastic flow in transient elastomer networks. Polymer 2016, 95, 45–51. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef]
- Snijkers, F.; Pasquino, R.; Maffezzoli, A. Curing and viscoelasticity of vitrimers. Soft Matter 2016. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Q.; Wang, T. Dual-Triggered and Thermally Reconfigurable Shape Memory Graphene-Vitrimer Composites. ACS Appl. Mater. Interfaces 2016, 8, 21691–21699. [Google Scholar] [CrossRef] [PubMed]
- Smallenburg, F.; Leibler, L.; Sciortino, F. Patchy particle model for vitrimers. Phys. Rev. Lett. 2013, 111, 188002. [Google Scholar] [CrossRef] [PubMed]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Montarnal, D.; Drockenmuller, E. Reprocessing and Recycling of Highly Cross-Linked Ion-Conducting Networks through Transalkylation Exchanges of C–N Bonds. J. Am. Chem. Soc. 2015, 137, 6078–6083. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Yang, Y.; Chen, Q.; Wei, Y.; Ji, Y. Regional Shape Control of Strategically Assembled Multishape Memory Vitrimers. Adv. Mater. 2016, 28, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Fortman, D.J.; Brutman, J.P.; Cramer, C.J.; Hillmyer, M.A.; Dichtel, W.R. Mechanically activated, catalyst-free polyhydroxyurethane vitrimers. J. Am. Chem. Soc. 2015, 137, 14019–14022. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.; Sciortino, F. Switching bonds in a DNA gel: An all-DNA vitrimer. Phys. Rev. Lett. 2015, 114, 078104. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Yang, Y.; Chen, Q.; Terentjev, E.M.; Wei, Y.; Ji, Y. Mouldable liquid-crystalline elastomer actuators with exchangeable covalent bonds. Nat. Mater. 2014, 13, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Pilate, F.; Toncheva, A.; Dubois, P.; Raquez, J. Shape-memory polymers for multiple applications in the materials world. Eur. Polym. J. 2016, 80, 268–294. [Google Scholar] [CrossRef]
- Gan, S.; Wu, Z.L.; Xu, H.; Song, Y.; Zheng, Q. Viscoelastic Behaviors of Carbon Black Gel Extracted from Highly Filled Natural Rubber Compounds: Insights into the Payne Effect. Macromolecules 2016, 49, 1454–1463. [Google Scholar] [CrossRef]
- Aabloo, A.; de Luca, V.; di Pasquale, G.; Graziani, S.; Gugliuzzo, C.; Johanson, U.; Marino, C.; Pollicino, A.; Puglisi, R. A new class of ionic electroactive polymers based on green synthesis. Sens. Actuators A Phys. 2016, 249, 32–44. [Google Scholar] [CrossRef]
- Arunkumar, P.; Raju, B.; Vasantharaja, R.; Vijayaraghavan, S.; Preetham Kumar, B.; Jeganathan, K.; Premkumar, K. Near infra-red laser mediated photothermal and antitumor efficacy of doxorubicin conjugated gold nanorods with reduced cardiotoxicity in swiss albino mice. Nanomedicine 2015, 11, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Liu, Z.; Shi, P.; Dong, K.; Ju, E.; Ren, J.; Qu, X. A multi-stimuli responsive gold nanocage-hyaluronic platform for targeted photothermal and chemotherapy. Biomaterials 2014, 35, 9678–9688. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shen, Y.; Zhang, W.; Li, M.; Wang, Y.; Zhou, D.; Guo, S. Efficient, dual-stimuli responsive cytosolic gene delivery using a RGD modified disulfide-linked polyethylenimine functionalized gold nanorod. J. Control. Release 2014, 196, 37–51. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, M.J.; Tomlins, P.; Sahota, T.S. Thermoresponsive Gels. Gels 2017, 3, 4. https://doi.org/10.3390/gels3010004
Taylor MJ, Tomlins P, Sahota TS. Thermoresponsive Gels. Gels. 2017; 3(1):4. https://doi.org/10.3390/gels3010004
Chicago/Turabian StyleTaylor, M. Joan, Paul Tomlins, and Tarsem S. Sahota. 2017. "Thermoresponsive Gels" Gels 3, no. 1: 4. https://doi.org/10.3390/gels3010004





