Carboxymethyl Cellulose-Grafted Mesoporous Silica Hybrid Nanogels for Enhanced Cellular Uptake and Release of Curcumin
Abstract
:1. Introduction
2. Results and Discussion
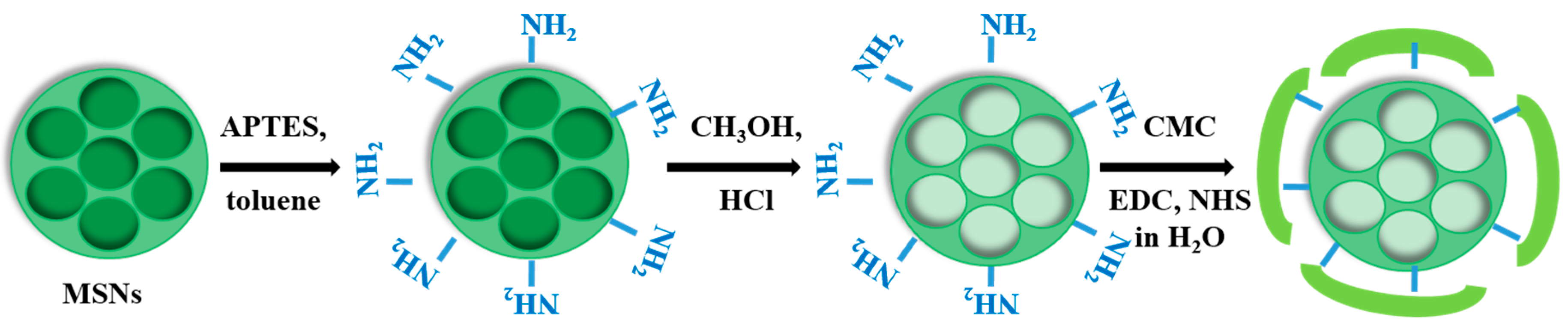
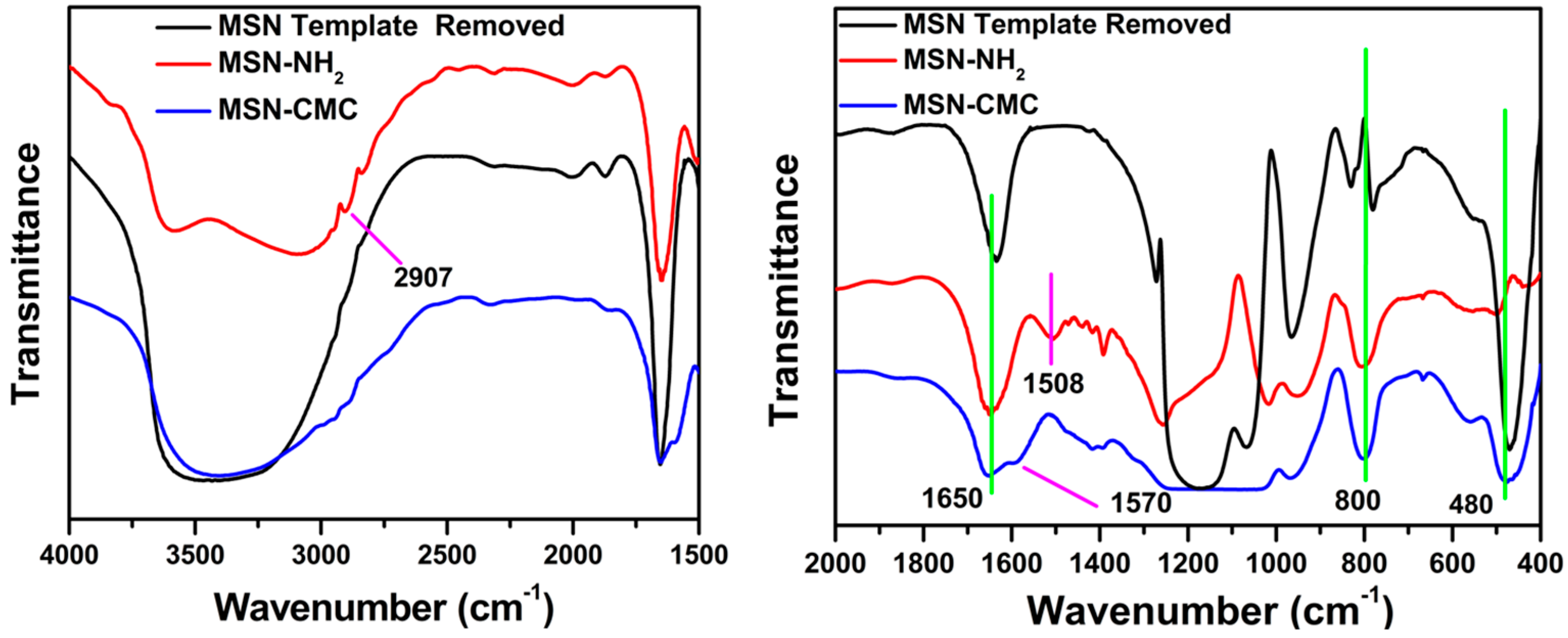
2.1. Functionalization and Characterization of Mesoporous Silica Nanoparticles (MSNs)
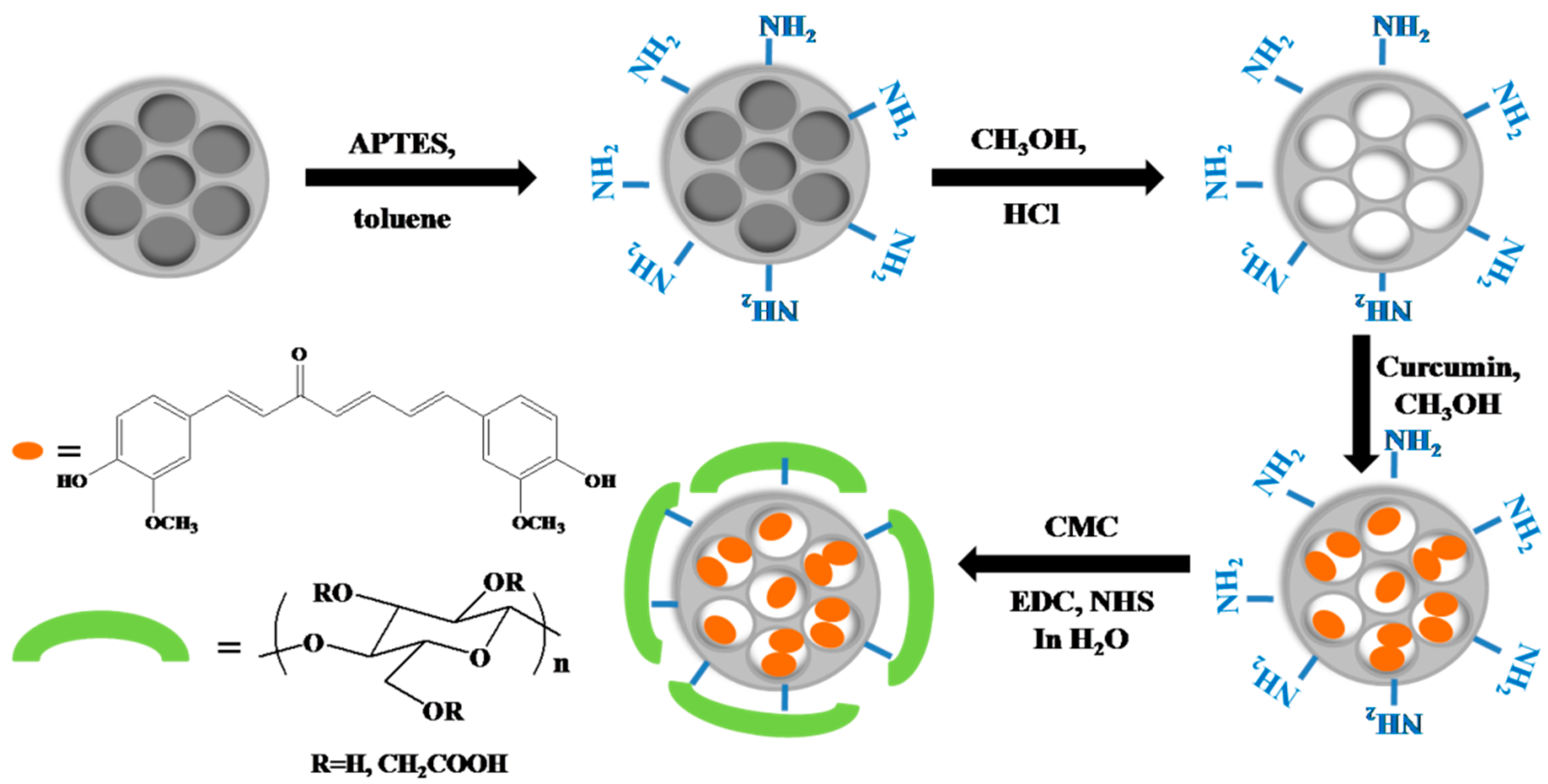
2.2. Synthesis of Curcumin-Loaded MSNs-Carboxymethyl Cellulose (CMC) Nanoparticles
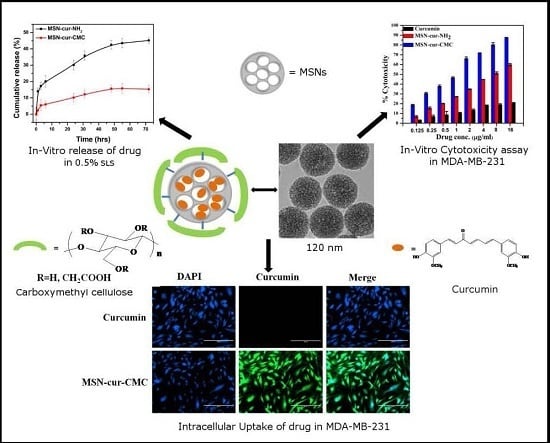
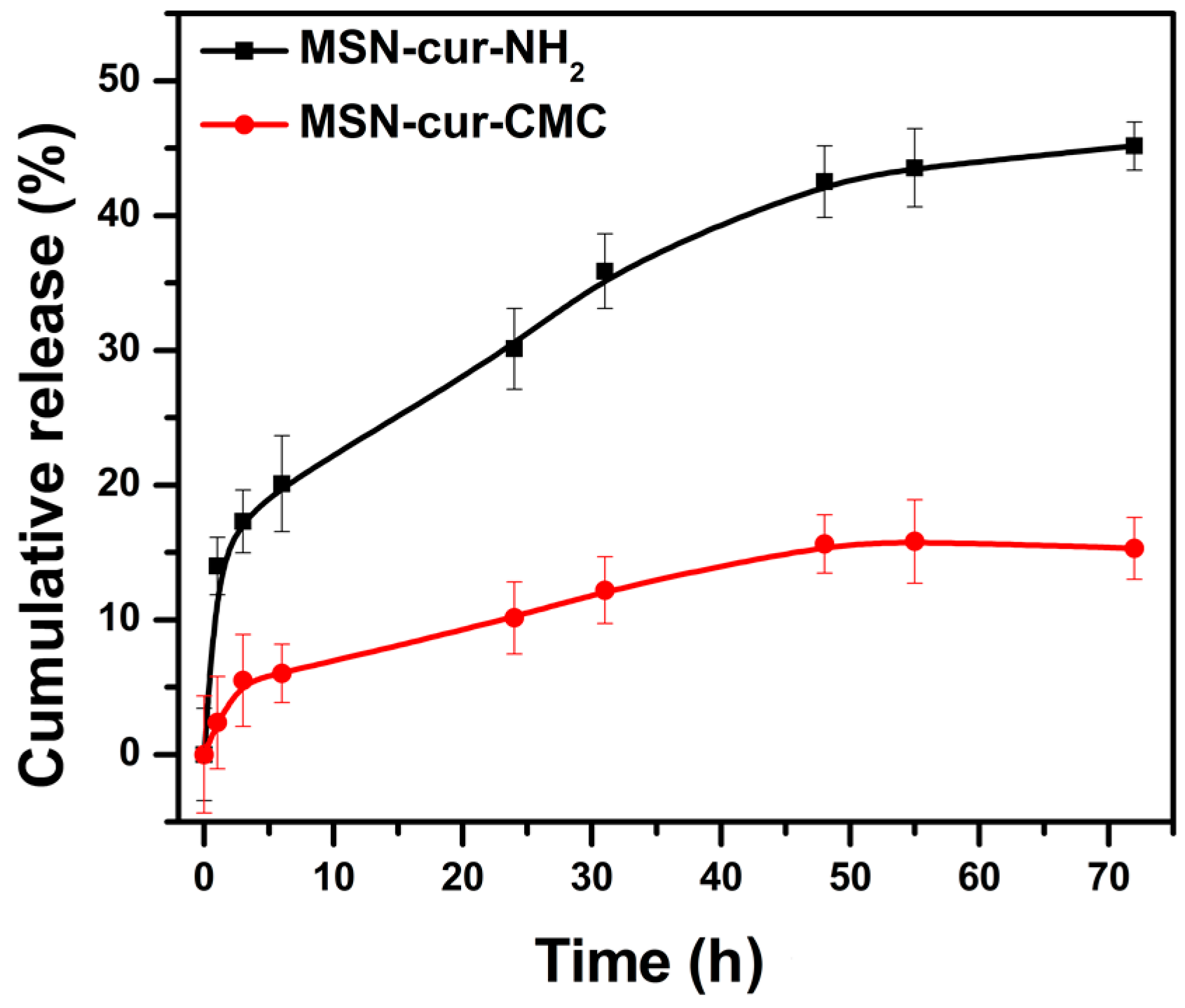
2.3. Release Study of Curcumin from MSN in 0.5% Sodium Lauryl Sulphate
2.4. In Vitro Cytotoxicity Assay
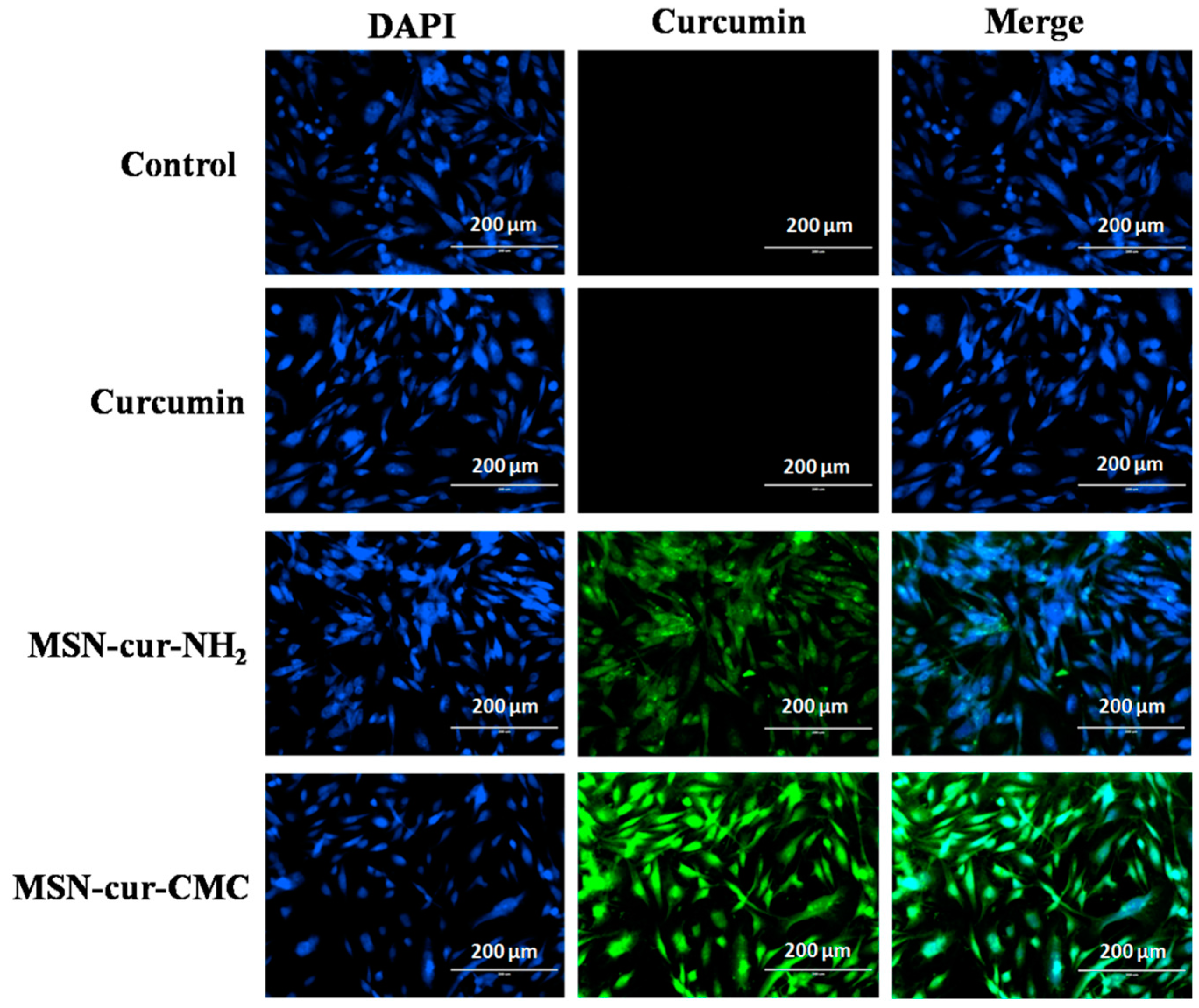
2.5. Intracellular Uptake of MSN Particles
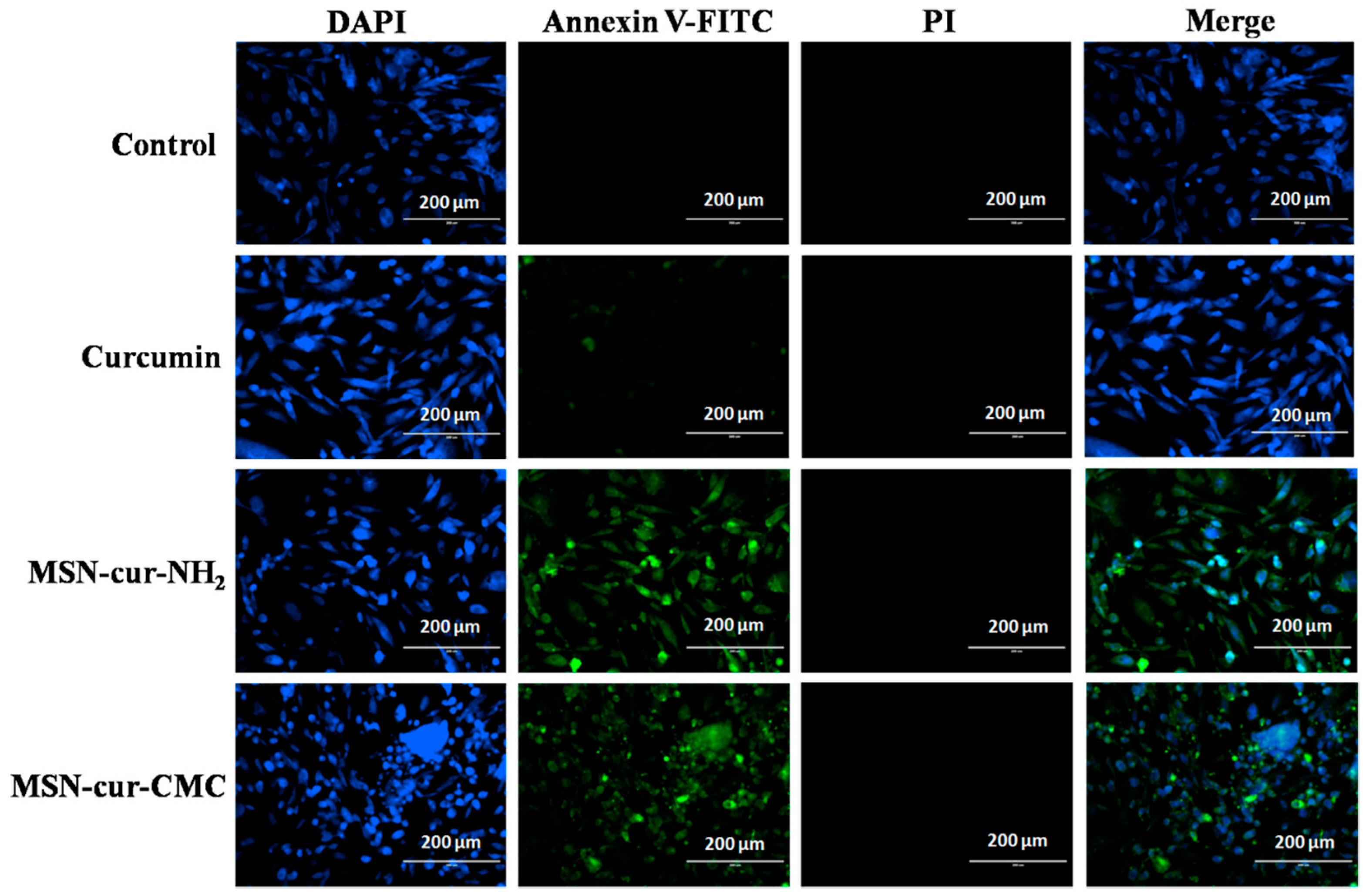
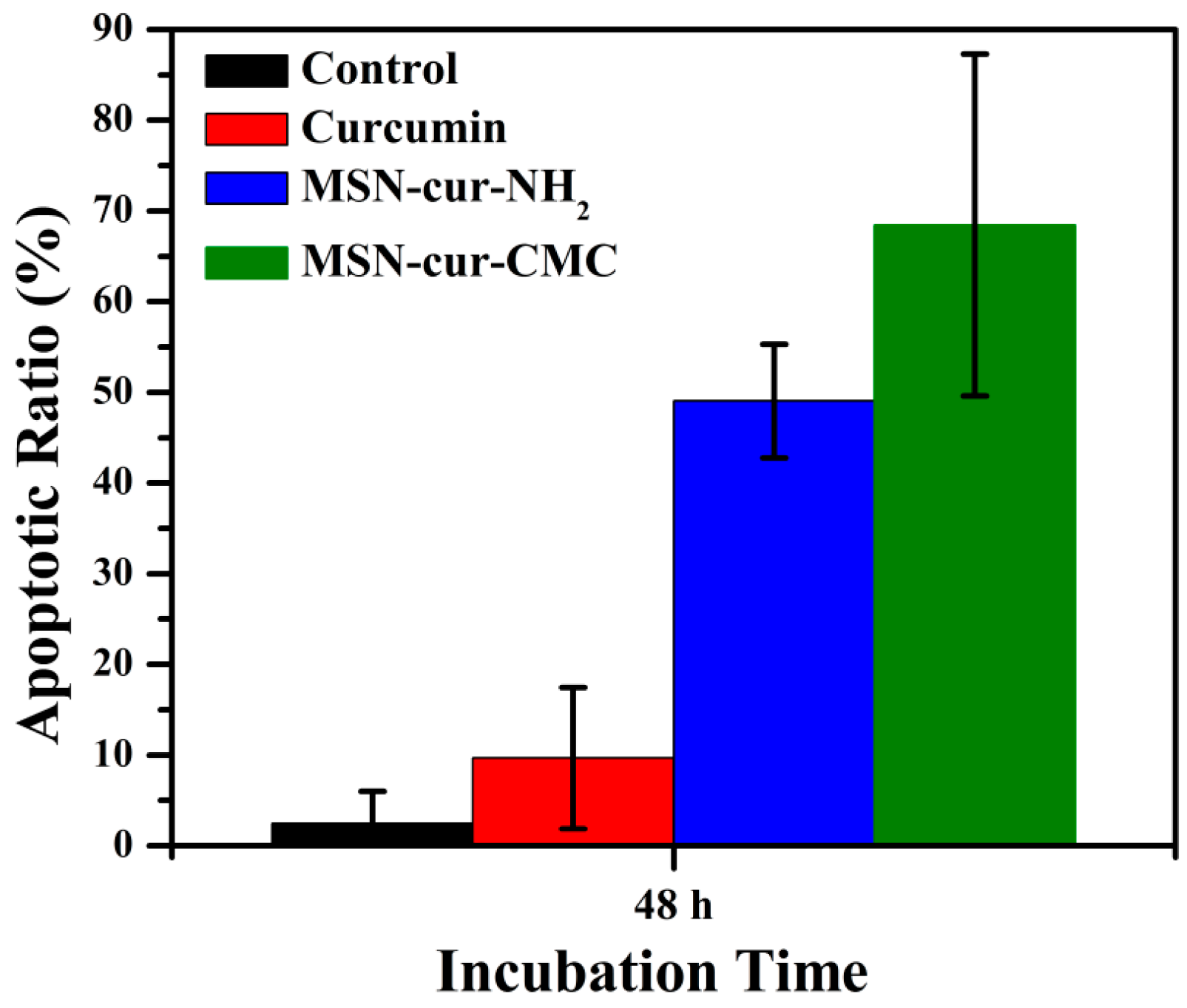
2.6. Apoptosis by Fluorescein Isothiocyanate (FITC)-Labeled Annexin V (Annexin V-FITC)/Propidium Iodide (PI) Staining
3. Conclusions
4. Materials and Synthesis
4.1. Materials
4.2. Synthesis
4.2.1. Synthesis of Mesoporous Silica Nanoparticles
4.2.2. Outer Surface Functionalization of MSNs with Amino Groups
4.2.3. Curcumin Loading in MSN-NH2
4.2.4. Functionalization of MSN-NH2 and MSN-cur-NH2 by Carboxymethyl Cellulose
4.2.5. In Vitro Curcumin Release Studies
4.2.6. In Vitro Cytotoxicity Assay
4.2.7. Intracellular Uptake of MSNs
4.2.8. Apoptosis by Annexin V-FITC/PI Staining
5. Characterizations
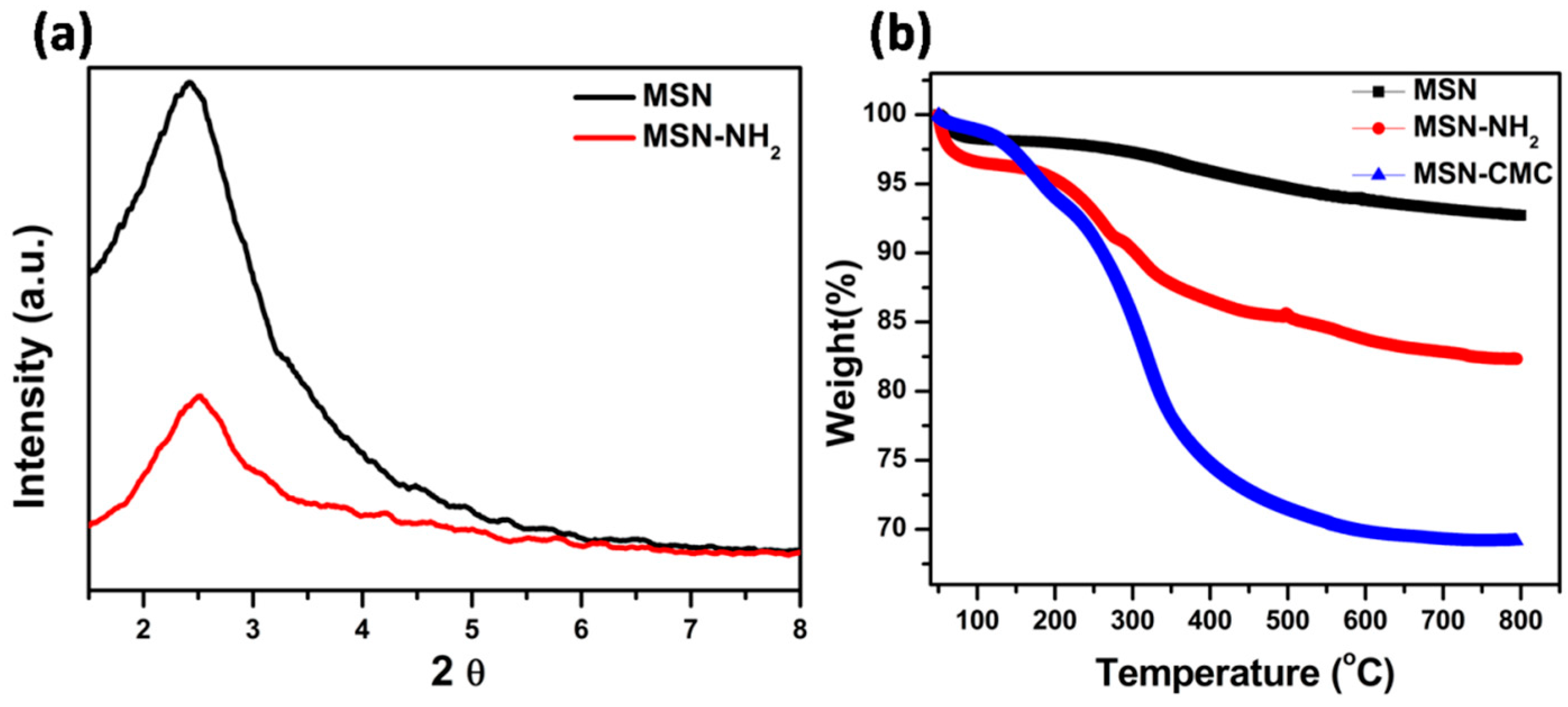
5.1. Fourier Transform Infrared Spectroscopy (FT-IR)
5.2. Thermo Gravimetric Analysis
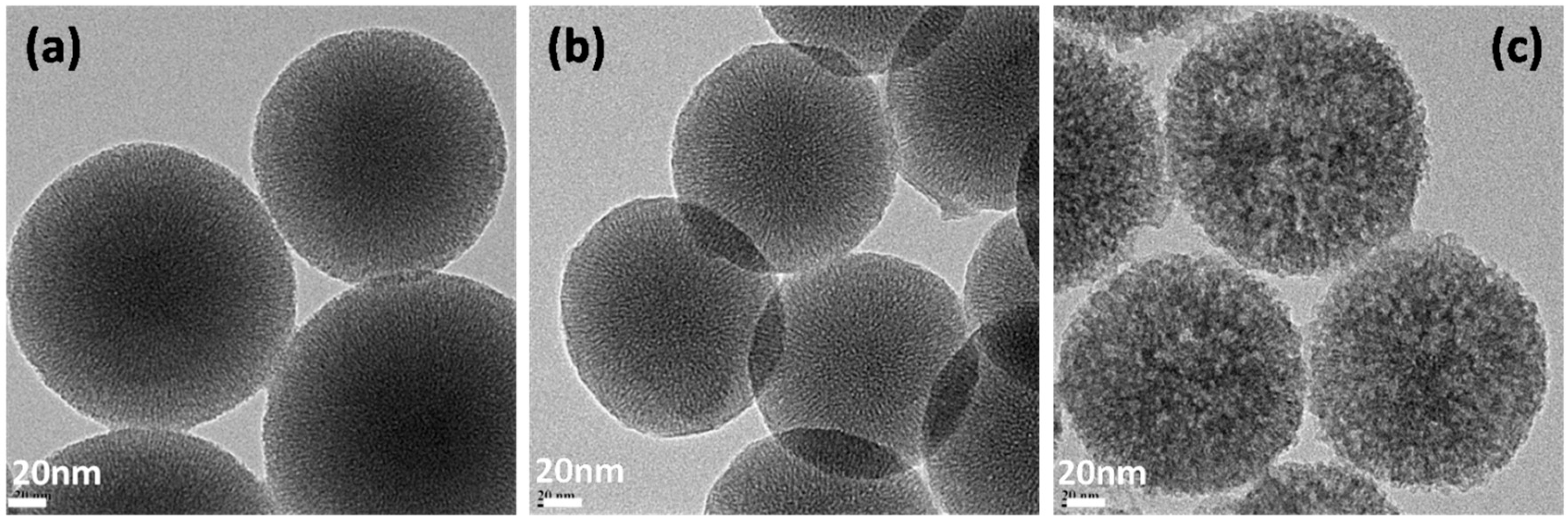
5.3. Transmission Electron Microscopy (TEM)
5.4. Scanning Electron Microscopy (SEM)
5.5. Nitrogen Adsorption/Desorption
5.6. ζ Potential and Size Determination
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, K.N.; Zhang, C.Q.; Wang, W.; Wang, P.C.; Zhou, J.P.; Liang, X.J. pH-responsive mesoporous silica nanoparticles employed in controlled drug delivery systems for cancer treatment. Cancer Biol. Med. 2014, 11, 34–43. [Google Scholar] [PubMed]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of nanoparticles in biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Singh, R.K.; Perez, R.A.; Abou Neel, E.A.; Kim, H.W.; Chrzanowski, W. Silica-based mesoporous nanoparticles for controlled drug delivery. J. Tissue Eng. 2013, 4, 2041731413503357. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Ordered mesoporous materials for drug delivery. Microporous Mesoporous Mater. 2009, 117, 1–9. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ruiz-González, L.; Izquierdo-Barba, I.; González-Calbet, J.M. Revisiting silica based ordered mesoporous materials: Medical applications. J. Mater. Chem. 2006, 16, 26–31. [Google Scholar] [CrossRef]
- Bouchoucha, M.; Côté, M.-F.; C.-Gaudreault, R.; Fortin, M.-A.; Kleitz, F. Size-controlled functionalized mesoporous silica nanoparticles for tunable drug release and enhanced anti-tumoral activity. Chem. Mater. 2016, 28, 4243–4258. [Google Scholar] [CrossRef]
- Harrison, W. Synthesis and characterization of ordered mesoporous silica nanoparticles with tunable physical properties by varying molar composition of reagents. Afr. J. Pharm. Pharmacol. 2011, 5, 2402–2410. [Google Scholar] [CrossRef]
- Morell, J.; Güngerich, M.; Wolter, G.; Jiao, J.; Hunger, M.; Klar, P.J.; Fröba, M. Synthesis and characterization of highly ordered bifunctional aromatic periodic mesoporous organosilicas with different pore sizes. J. Mater. Chem. 2006, 16, 2809–2818. [Google Scholar] [CrossRef]
- Malgras, V.; Ji, Q.; Kamachi, Y.; Mori, T.; Shieh, F.-K.; Wu, K.C.-W.; Ariga, K.; Yamauchi, Y. Templated synthesis for nanoarchitectured porous materials. Bull. Chem. Soc. Jpn. 2015, 88, 1171–1200. [Google Scholar] [CrossRef]
- Yamamoto, E.; Kuroda, K. Colloidal mesoporous silica nanoparticles. Bull. Chem. Soc. Jpn. 2016, 89, 501–539. [Google Scholar] [CrossRef]
- Zhan, G.; Zeng, H.C. Integrated nanocatalystswithmesoporoussilica/silicateand microporous mofmaterials. Coord. Chem. Rev. 2016, 320–321, 181–192. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Mirshekari, H.; Aliakbari, M.; Sahandi-Zangabad, P.; Hamblin, M.R. Smart mesoporous silica nanoparticles for controlled-release drug delivery. Nanotechnol. Rev. 2016, 5, 195–207. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Giri, S.; Slowing, I.I.; Lin, V.S. Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem. Commun. 2007, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Tiwari, N.; Tiwari, M.; Lahiri, M.; Gupta, S.S. Poly-l-arginine grafted silica mesoporous nanoparticles for enhanced cellular uptake and their application in DNA delivery and controlled drug release. Part. Part. Syst. Charact. 2013, 30, 166–179. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Zink, J.I.; Tamanoi, F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small 2007, 3, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Cheng, S.H.; Huang, I.P.; Souris, J.S.; Yang, C.S.; Mou, C.Y.; Lo, L.W. Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew. Chem. 2010, 49, 8214–8219. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, Z.; Gao, Y.; Shi, J.; Li, Y. Intracellular localization and cytotoxicity of spherical mesoporous silica nano- and microparticles. Small 2009, 5, 2722–2729. [Google Scholar] [CrossRef] [PubMed]
- Kecht, J.; Schlossbauer, A.; Bein, T. Selective functionalization of the outer and inner surfaces in msn. Chem. Mater. 2008, 20, 7207–7214. [Google Scholar] [CrossRef]
- Ma’mani, L.; Nikzad, S.; Kheiri-Manjili, H.; Al-Musawi, S.; Saeedi, M.; Askarlou, S.; Foroumadi, A.; Shafiee, A. Curcumin-loaded guanidine functionalized pegylated I3AD mesoporous silica nanoparticles KIT-6: Practical strategy for the breast cancer therapy. Eur. J. Med. Chem. 2014, 83, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Z.; Zink, J.I.; Tamanoi, F. In vivo tumor suppression efficacy of mesoporous silica nanoparticles-based drug-delivery system: Enhanced efficacy by folate modification. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Neoh, K.G.; Xu, L.; Lu, S.; Kang, E.T.; Mahendran, R.; Chiong, E. Functionalized mesoporous silica nanoparticles with mucoadhesive and sustained drug release properties for potential bladder cancer therapy. Langmuir 2014, 30, 6151–6161. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Tang, Q.; Yang, D.; Zhang, J.Z.; Zhang, F.; Hu, J. Preparation of pH-responsive mesoporous silica nanoparticles and their application in controlled drug delivery. J. Phys. Chem. C 2011, 115, 9926–9932. [Google Scholar] [CrossRef]
- Gulfam, M.; Chung, B.G. Development of pH-responsive chitosan-coated mesoporous silica nanoparticl. Macromol. Res. 2014, 22, 412–417. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, Y.; Zhang, L.; Shi, J. Preparation of chitosan/mesoporous silica nanoparticle composite hydrogels for sustained co-delivery of biomacromolecules and small chemical drugs. Sci. Technol. Adv. Mater. 2013, 14, 045005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Geng, H.; Wang, Y.; Gao, Y.; Huang, J.; Wang, Y.; Zhang, J.; Wang, S. Hyaluronic acid oligosaccharide modified redox-responsive mesoporous silica nanoparticles for targeted drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 20290–20299. [Google Scholar] [CrossRef] [PubMed]
- Gary-Bobo, M.; Mir, Y.; Rouxel, C.; Brevet, D.; Basile, I.; Maynadier, M.; Vaillant, O.; Mongin, O.; Blanchard-Desce, M.; Morère, A.; et al. Mannose-functionalized mesoporous silica nanoparticles for efficient two-photon photodynamic therapy of solid tumors. Angew. Chem. Int. Ed. 2011, 50, 11425–11429. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sun, C.; Song, A.; Chang, D.; Zheng, X.; Gao, Y.; Jiang, T.; Wang, S. Alginate encapsulated mesoporous silica nanospheres as a sustained drug delivery system for the poorly water-soluble drug indomethacin. Asian J. Pharm. Sci. 2014, 9, 183–190. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.-T.; Lin, V.S.-Y. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol–gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Froba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Young, N.P.; Townley, H.E. Characterization and comparison of mesoporous silica particles for optimized drug delivery. Nanomater. Nanotechnol. 2014, 4, 2. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Salis, A.; Fanti, M.; Medda, L.; Nairi, V.; Cugia, F.; Piludu, M.; Sogos, V.; Monduzzi, M. Mesoporous silica nanoparticles functionalized with hyaluronic acid and chitosan biopolymers. Effect of functionalization on cell internalization. ACS Biomater. Sci. Eng. 2016, 2, 741–751. [Google Scholar] [CrossRef]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. 2011, 50, 2597. [Google Scholar] [CrossRef] [PubMed]


| Sample Name | Weight Loss (%) | Hydrodynamic Diameter (nm) | Mean ζ Potential (mV) in Water |
|---|---|---|---|
| MSN | 7.3 | 167 ± 5 | −34.2 ± 2 |
| MSN-NH2 | 17.5 | 210 ± 7 | 38.48 ± 3 |
| MSN-CMC | 30.7 | 333 ± 4 | −3.07 ± 2 |
| Sample Name | Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| MSN | 939.6 | 3 | 0.738 |
| MSN-NH2 | 659.1 | 2.6 | 0.642 |
| MSN-cur-CMC | 262.9 | 2 | 0.339 |
| Sample Name | Drug Loading Content (%) | Drug Entrapment Efficiency (%) |
|---|---|---|
| MSN-cur-NH2 | 10.7 | 71.3 |
| MSN-cur-CMC | 8 | 53.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, N.; Nawale, L.; Sarkar, D.; Badiger, M.V. Carboxymethyl Cellulose-Grafted Mesoporous Silica Hybrid Nanogels for Enhanced Cellular Uptake and Release of Curcumin. Gels 2017, 3, 8. https://doi.org/10.3390/gels3010008
Tiwari N, Nawale L, Sarkar D, Badiger MV. Carboxymethyl Cellulose-Grafted Mesoporous Silica Hybrid Nanogels for Enhanced Cellular Uptake and Release of Curcumin. Gels. 2017; 3(1):8. https://doi.org/10.3390/gels3010008
Chicago/Turabian StyleTiwari, Neha, Laxman Nawale, Dhiman Sarkar, and Manohar V. Badiger. 2017. "Carboxymethyl Cellulose-Grafted Mesoporous Silica Hybrid Nanogels for Enhanced Cellular Uptake and Release of Curcumin" Gels 3, no. 1: 8. https://doi.org/10.3390/gels3010008