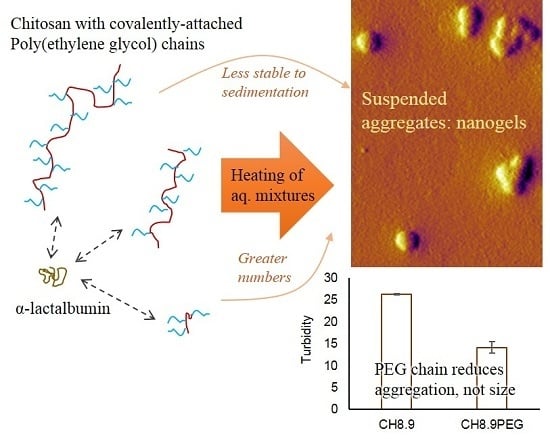
Impact of Chitosan Molecular Weight and Attached Non-Interactive Chains on the Formation of α-Lactalbumin Nanogel Particles
Abstract
:1. Introduction
2. Results and Discussion
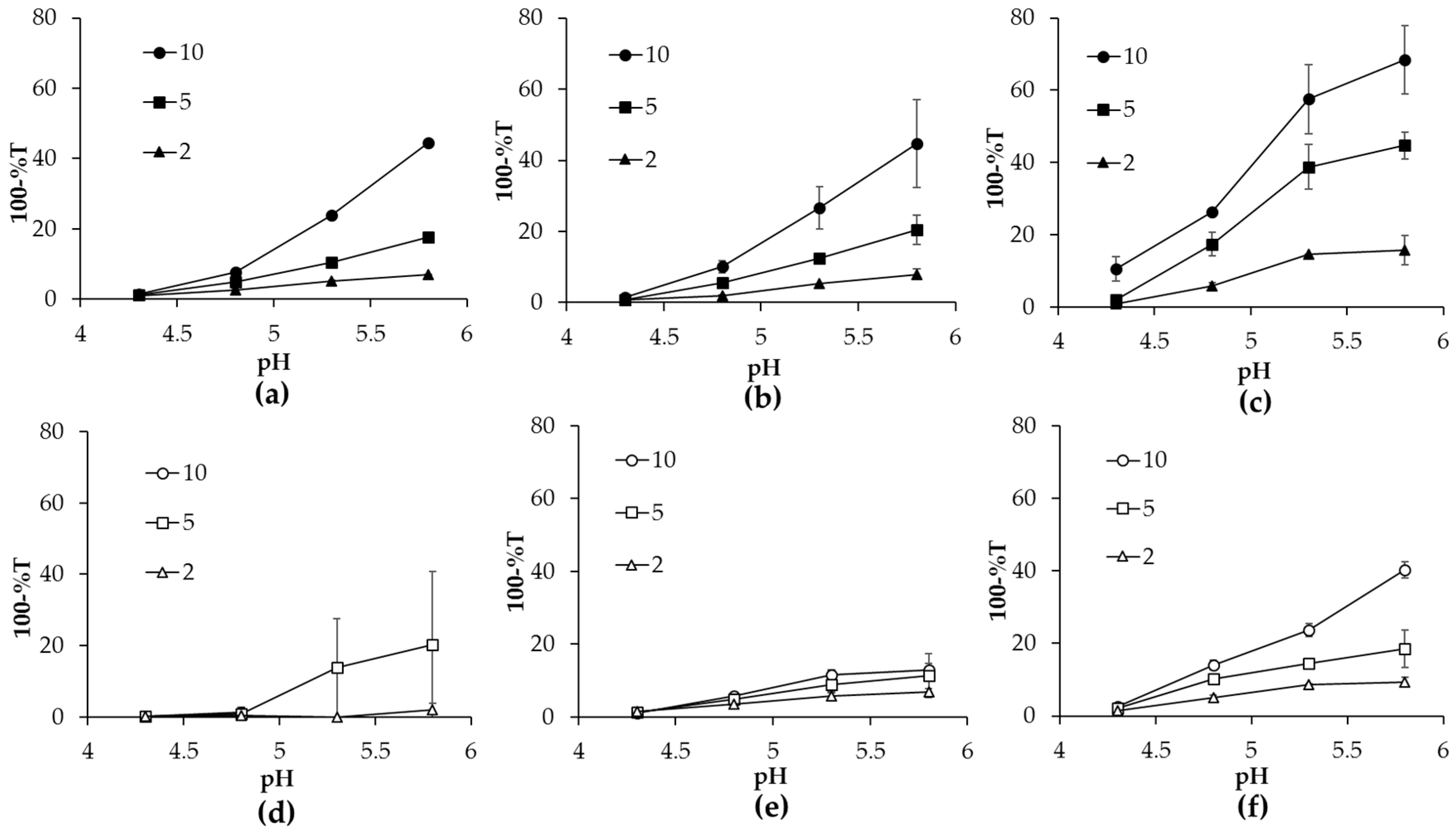
2.1. Turbidity of Heated α-lac/CHXX or CHXXPEG Complexes
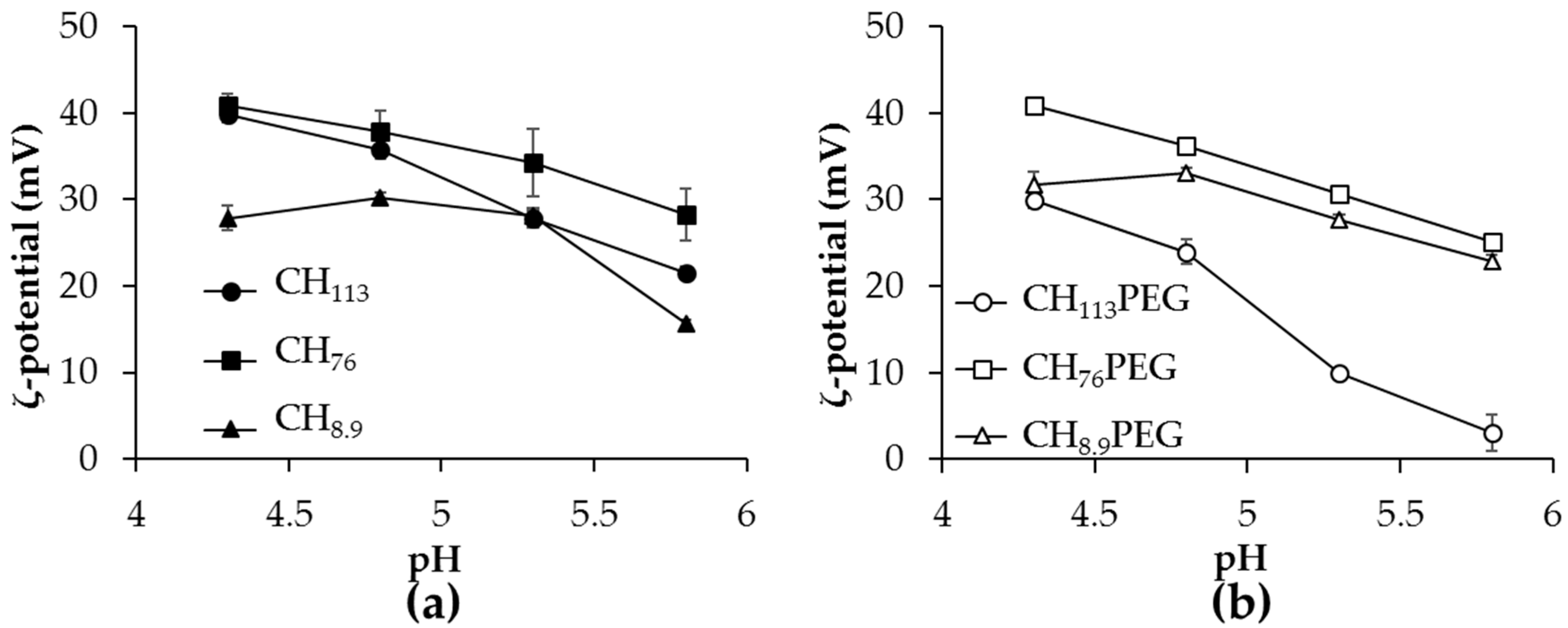
2.2. The pH Dependence of ζ-Potential of Heated α-Lactalbumin/CHXX or CHXXPEG Complexes
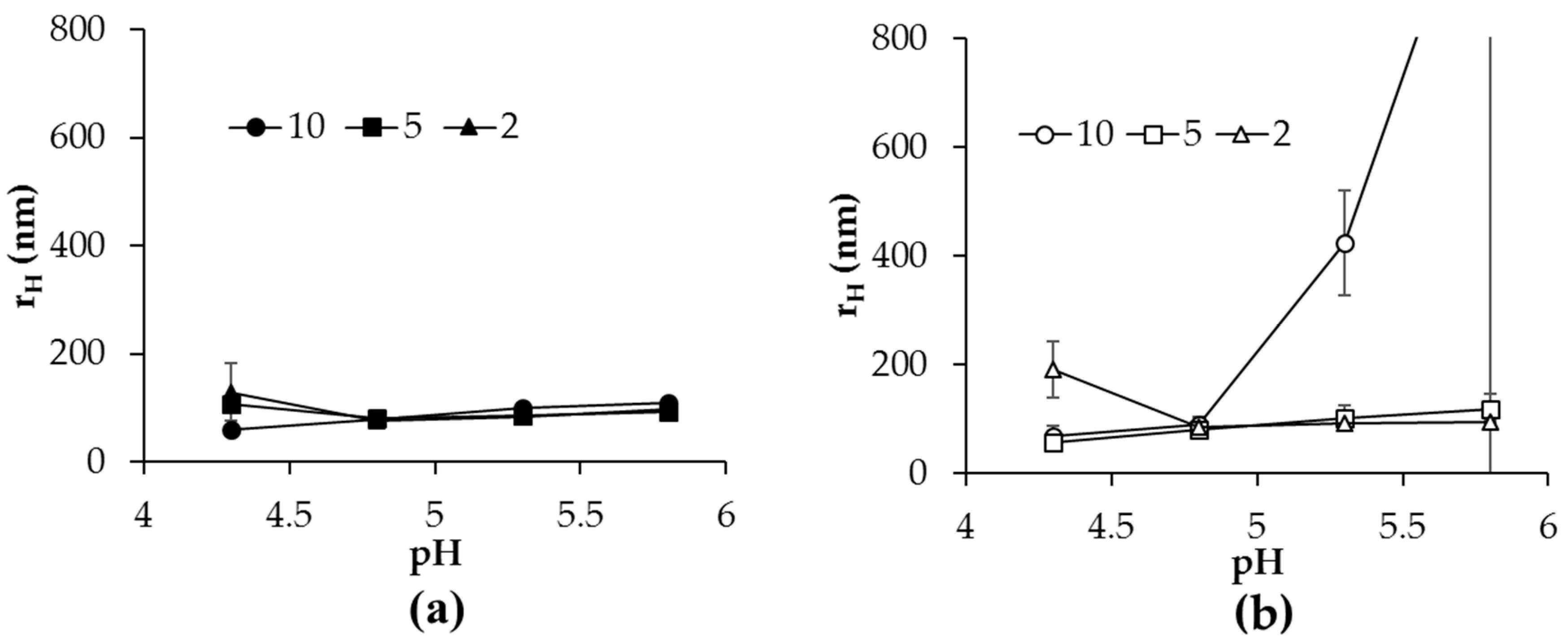
2.3. Hydrodynamic Diameter and Morphology of Aggregates in Heated Mixtures
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Different Molecular Weight of CH Preparation and Characterization
4.3. CHXXPEG Synthesis and Characterization
4.4. Solution Preparation
4.5. Colloidal Sample Characterization
4.6. Statistical Treatment
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, O.G.; McClements, D.J. Functional biopolymer particles: Design, fabrication, and applications. Compr. Rev. Food Sci. Food Saf. 2010, 9, 374–397. [Google Scholar] [CrossRef]
- Barrantes, E.; Tamime, A.; Muir, D.; Sword, A. The effect of substitution of fat by microparticulate whey protein on the quality of set-type, natural yogurt. Int. J. Dairy Technol. 1994, 47, 61–68. [Google Scholar] [CrossRef]
- Phan-Xuan, T.; Durand, D.; Nicolai, T.; Donato, L.; Schmitt, C.; Bovetto, L. On the crucial importance of the ph for the formation and self-stabilization of protein microgels and strands. Langmuir 2011, 27, 15092–15101. [Google Scholar] [CrossRef] [PubMed]
- Sağlam, D.; Venema, P.; de Vries, R.; van Aelst, A.; van der Linden, E. Relation between gelation conditions and the physical properties of whey protein particles. Langmuir 2012, 28, 6551–6560. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, T. Formation and functionality of self-assembled whey protein microgels. Colloids Surf. B 2016, 137, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.G.; McClements, D.J. Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein–polysaccharide complex es. Adv. Colloid Interface Sci. 2011, 167, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.G.; Decker, E.A.; McClements, D.J. Formation of biopolymer particles by thermal treatment of beta-lactoglobulin-pectin complexes. Food Hydrocoll. 2009, 23, 1312–1321. [Google Scholar] [CrossRef]
- Jones, O.G.; Lesmes, U.; Dubin, P.; McClements, D.J. Effect of polysaccharide charge on formation and properties of biopolymer nanoparticles created by heat treatment of beta-lactoglobulin-pectin complexes. Food Hydrocoll. 2010, 24, 374–383. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Biopolymer nanoparticles from heat-treated electrostatic protein–polysaccharide complex es: Factors affecting particle characteristics. J. Food Sci. 2010, 75, N36–N43. [Google Scholar] [CrossRef] [PubMed]
- Hirt, S.; Jones, O.G.; Adijanto, M.; Gilbert, J. Influence of sulphate, chloride, and thiocyanate salts on formation of beta-lactoglobulin-pectin microgels. Food Chem. 2014, 164, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Cho, Y.H.; Farkas, B.; Jones, O.G. Control of thermal fabrication and size of b-lactoglobulin-based microgels and their potential applications. J. Colloid Interface Sci. 2015, 447, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and technofunctional properties of protein–polysaccharide complex es: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 689–753. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Muhoberac, B.B.; Dubin, P.L.; Xia, J.L. Effects of protein charge heterogeneity in protein–polyelectrolyte complexation. Macromolecules 1992, 25, 290–295. [Google Scholar] [CrossRef]
- Mattison, K.W.; Dubin, P.L.; Brittain, I.J. Complex formation between bovine serum albumin and strong polyelectrolytes: Effect of polymer charge density. J. Phys. Chem. B 1998, 102, 3830–3836. [Google Scholar] [CrossRef]
- Voets, I.K.; de Keizer, A.; Cohen Stuart, M.A. Complex coacervate core micelles. Adv. Colloid Interface Sci. 2009, 147–148, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Pergushov, D.V.; Müller, A.H.; Schacher, F.H. Micellar interpolyelectrolyte complexes. Chem. Soc. Rev. 2012, 41, 6888–6901. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Reuhs, B.L.; Jones, O.G. Influence of pegylation on the ability of carboxymethyl-dextran to form complexes with α-lactalbumin. Food Chem. 2016, 196, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-H.; McClements, D.J. Formation of hydrogel particles by thermal treatment of β-lactoglobulin−chitosan complexes. J. Agric. Food Chem. 2007, 55, 5653–5660. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.-C.; Hong, Y.-H. Coacervate formation of α-lactalbumin–chitosan and β-lactoglobulin–chitosan complexes. Food Res. Int. 2009, 42, 733–738. [Google Scholar] [CrossRef]
- Zhang, M.; Desai, T.; Ferrari, M. Proteins and cells on peg immobilized silicon surfaces. Biomaterials 1998, 19, 953–960. [Google Scholar] [CrossRef]
- De Souza, H.K.S.; Bai, G.; Gonçalves, M.D.P.; Bastos, M. Whey protein isolate–chitosan interactions: A calorimetric and spectroscopy study. Thermochim. Acta 2009, 495, 108–114. [Google Scholar] [CrossRef]
- Halabalová, V.; Šimek, L. A study of the interaction between chitosan and poly(ethylene glycol) by viscosity method. Int. J. Polym. Anal. Charact. 2006, 11, 185–195. [Google Scholar] [CrossRef]
- Cooper, C.L.; Goulding, A.; Kayitmazer, A.B.; Ulrich, S.; Stoll, S.; Turksen, S.; Yusa, S.-I.; Kumar, A.; Dubin, P.L. Effects of polyelectrolyte chain stiffness, charge mobility, and charge sequences on binding to proteins and micelles. Biomacromolecules 2006, 7, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Moitzi, C.; Bovay, C.; Rouvet, M.; Bovetto, L.; Donato, L.; Leser, M.E.; Schurtenberger, P.; Stradner, A. Internal structure and colloidal behaviour of covalent whey protein microgels obtained by heat treatment. Soft Matter 2010, 6, 4876–4884. [Google Scholar] [CrossRef]
- Jones, O.G.; Decker, E.A.; McClements, D.J. Comparison of protein-polysaccharide nanoparticle fabrication methods: Impact of biopolymer complexation before or after particle formation. J. Colloid Interface Sci. 2010, 344, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, T. Food characterisation using scattering methods. In Understanding and Controlling the Microstructure of Complex Foods; Mcclements, D.J., Ed.; Woodhead Publishing: Cambridge, UK, 2007; pp. 288–310. [Google Scholar]
- Mao, S.; Shuai, X.; Unger, F.; Simon, M.; Bi, D.; Kissel, T. The depolymerization of chitosan: Effects on physicochemical and biological properties. Int. J. Pharm. 2004, 281, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.M.; Cho, Y.W.; Chung, H.; Kwon, I.C.; Jeong, S.Y.; Ooya, T.; Lee, W.K.; Sasaki, S.; Yui, N. Supramolecular hydrogel formation based on inclusion complexation between poly(ethylene glycol)-modified chitosan and α-cyclodextrin. Macromol. Biosci. 2004, 4, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Kim, D.-G.; Jang, M.-K.; Nah, J.-W. Preparation and spectroscopic characterization of methoxy poly(ethylene glycol)-grafted water-soluble chitosan. Carbohydr. Res. 2008, 343, 282–289. [Google Scholar] [CrossRef] [PubMed]

| Sample | MW (kDa) 1 | DDA (%) 2 | Name Post-Modification | DS (%) 3 |
|---|---|---|---|---|
| CH113 | 113 | 77.46 ± 0.59 | CH113PEG | 6.78 ± 0.94 |
| CH76 | 76 | 83.37 ± 0.44 | CH76PEG | 8.14 ± 1.41 |
| CH8.9 | 8.9 | 77.20 ± 2.54 | CH8.9PEG | 9.94 ± 1.58 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Cho, Y.-H.; Murphy, R.; Jones, O.G. Impact of Chitosan Molecular Weight and Attached Non-Interactive Chains on the Formation of α-Lactalbumin Nanogel Particles. Gels 2017, 3, 14. https://doi.org/10.3390/gels3020014
Du J, Cho Y-H, Murphy R, Jones OG. Impact of Chitosan Molecular Weight and Attached Non-Interactive Chains on the Formation of α-Lactalbumin Nanogel Particles. Gels. 2017; 3(2):14. https://doi.org/10.3390/gels3020014
Chicago/Turabian StyleDu, Juan, Young-Hee Cho, Ryan Murphy, and Owen Griffith Jones. 2017. "Impact of Chitosan Molecular Weight and Attached Non-Interactive Chains on the Formation of α-Lactalbumin Nanogel Particles" Gels 3, no. 2: 14. https://doi.org/10.3390/gels3020014