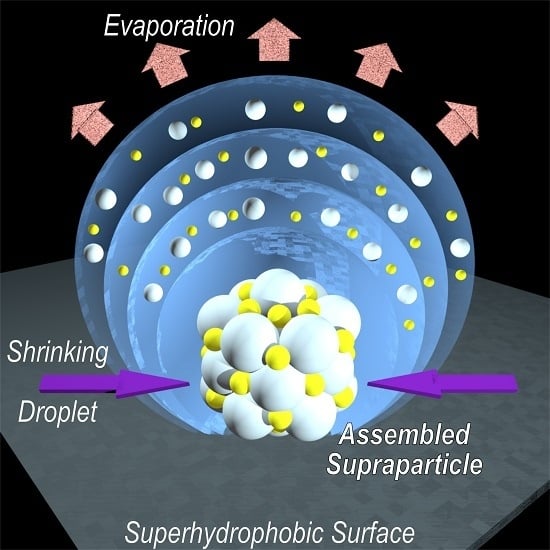
Droplets, Evaporation and a Superhydrophobic Surface: Simple Tools for Guiding Colloidal Particles into Complex Materials
Abstract
:1. Introduction
2. Evaporation-Induced Self-Assembly and the Droplet Templating Method
3. Superhydrophobic Surfaces
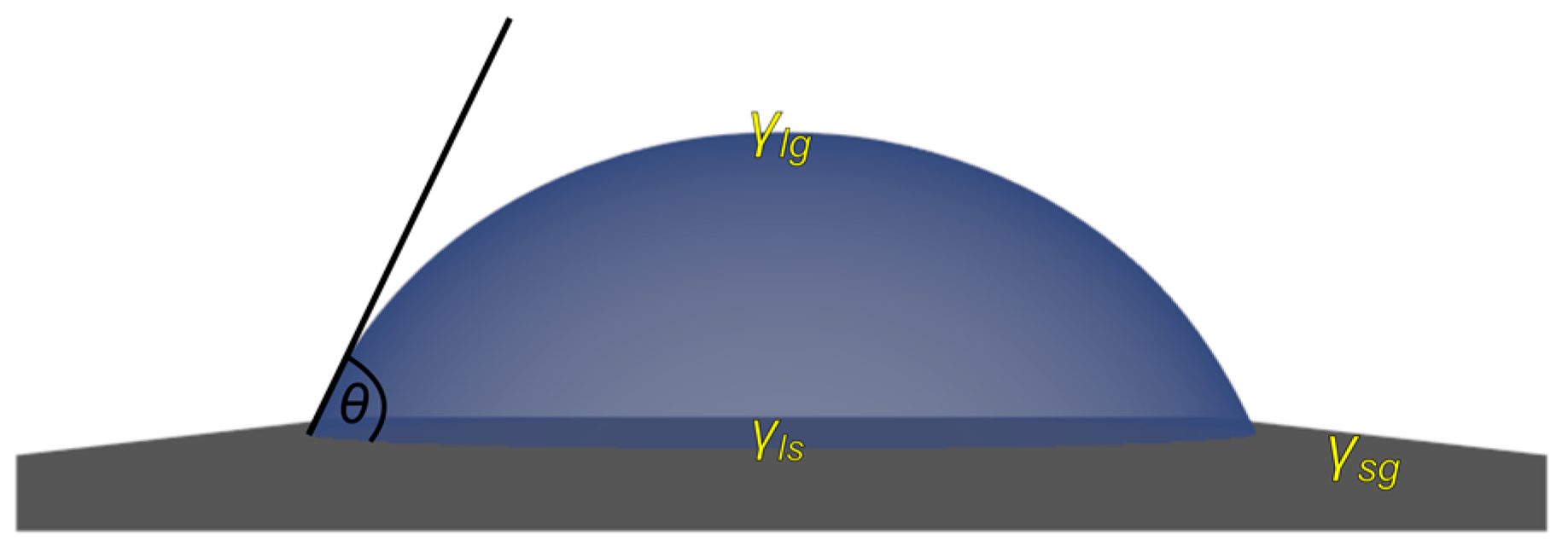
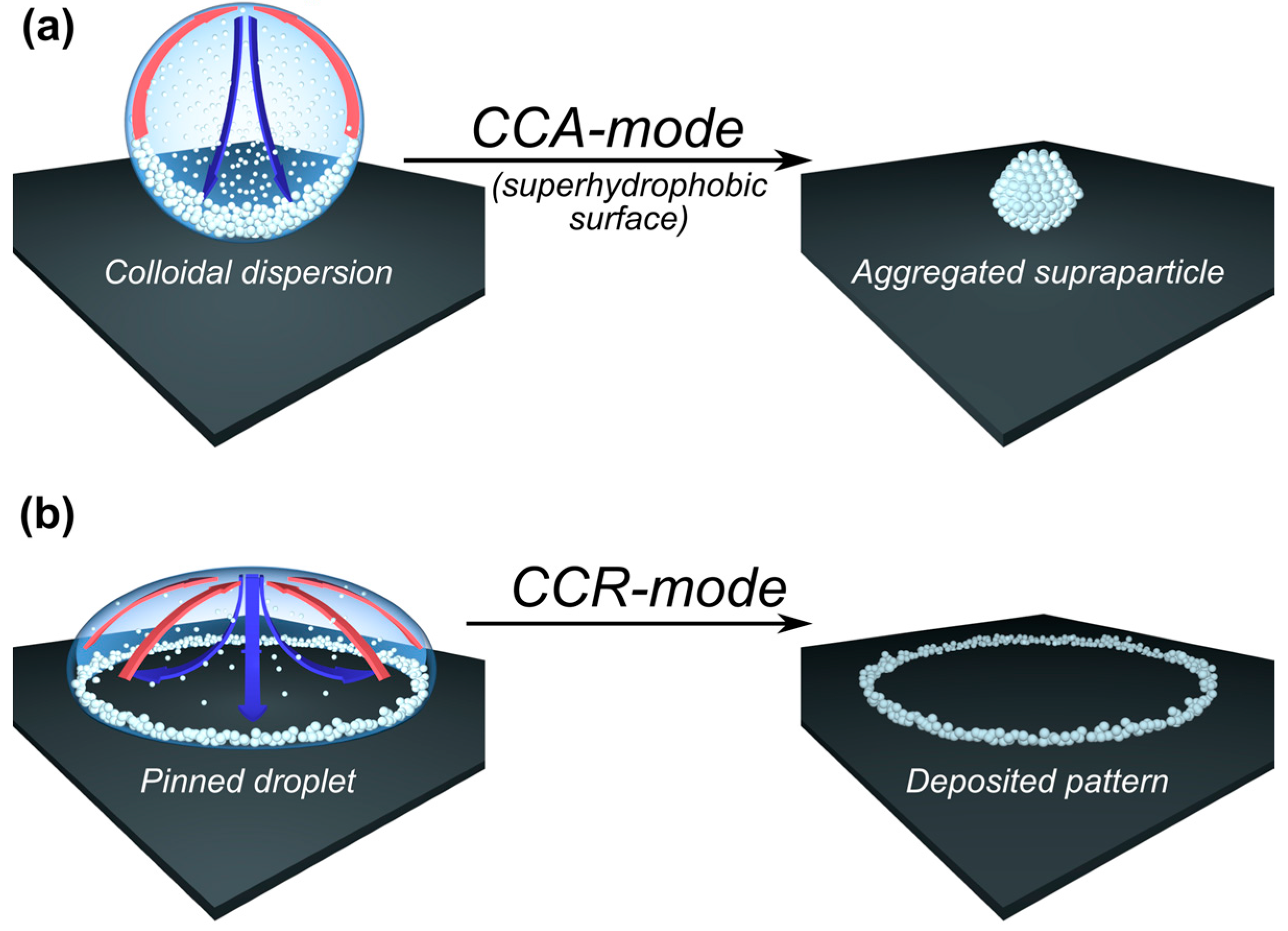
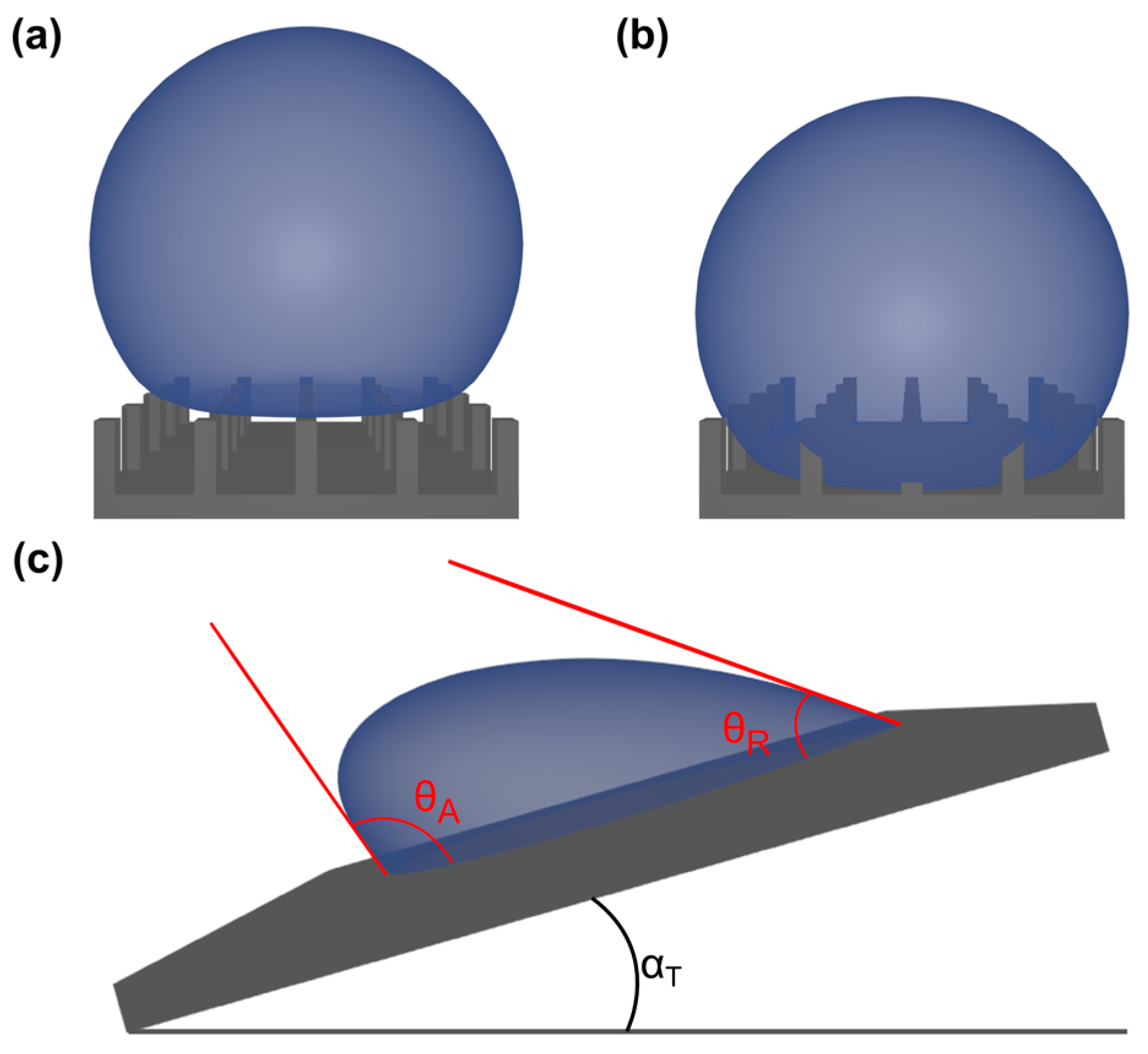
3.1. Wetting Modes
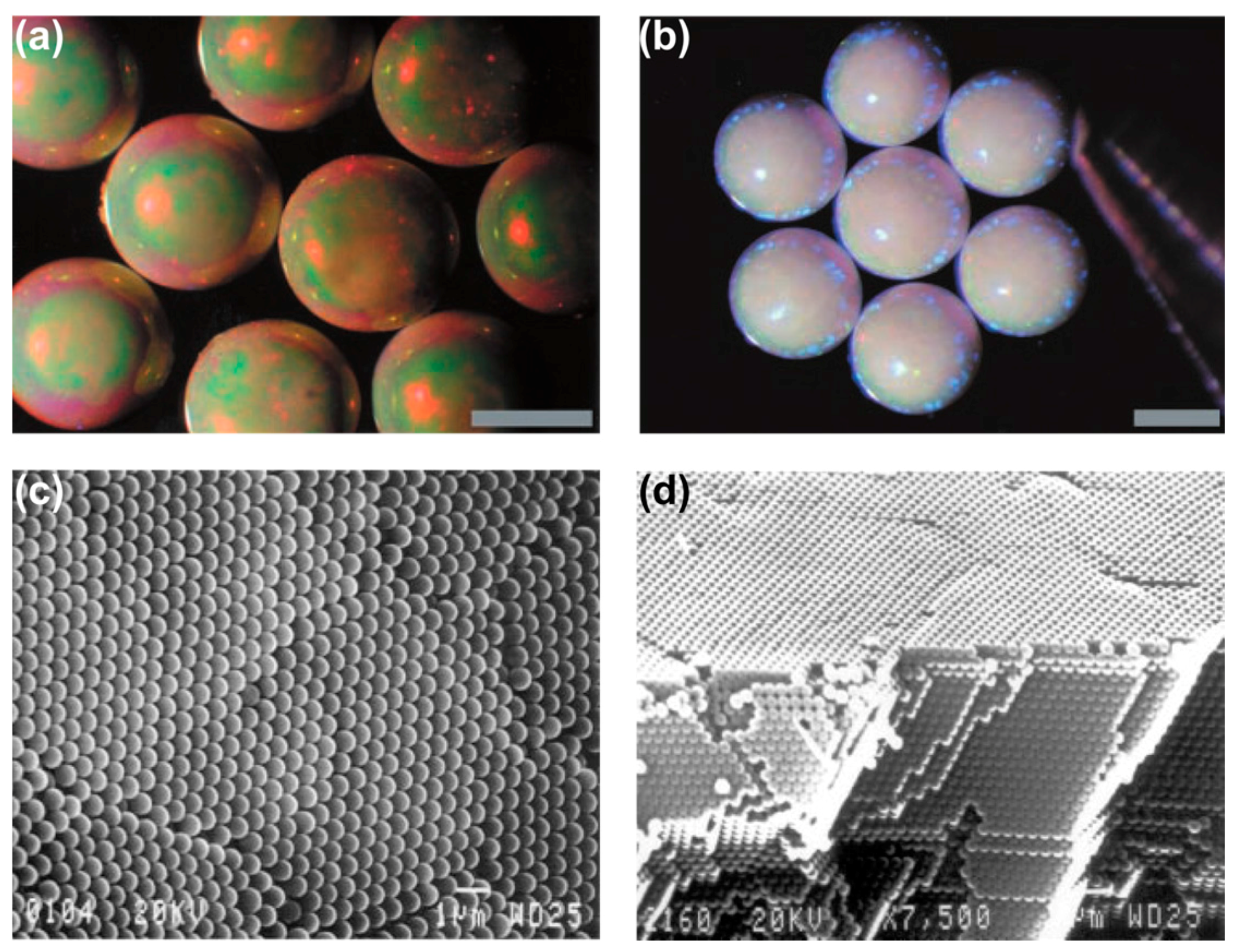
3.2. Production of Superhydrophobic Surfaces
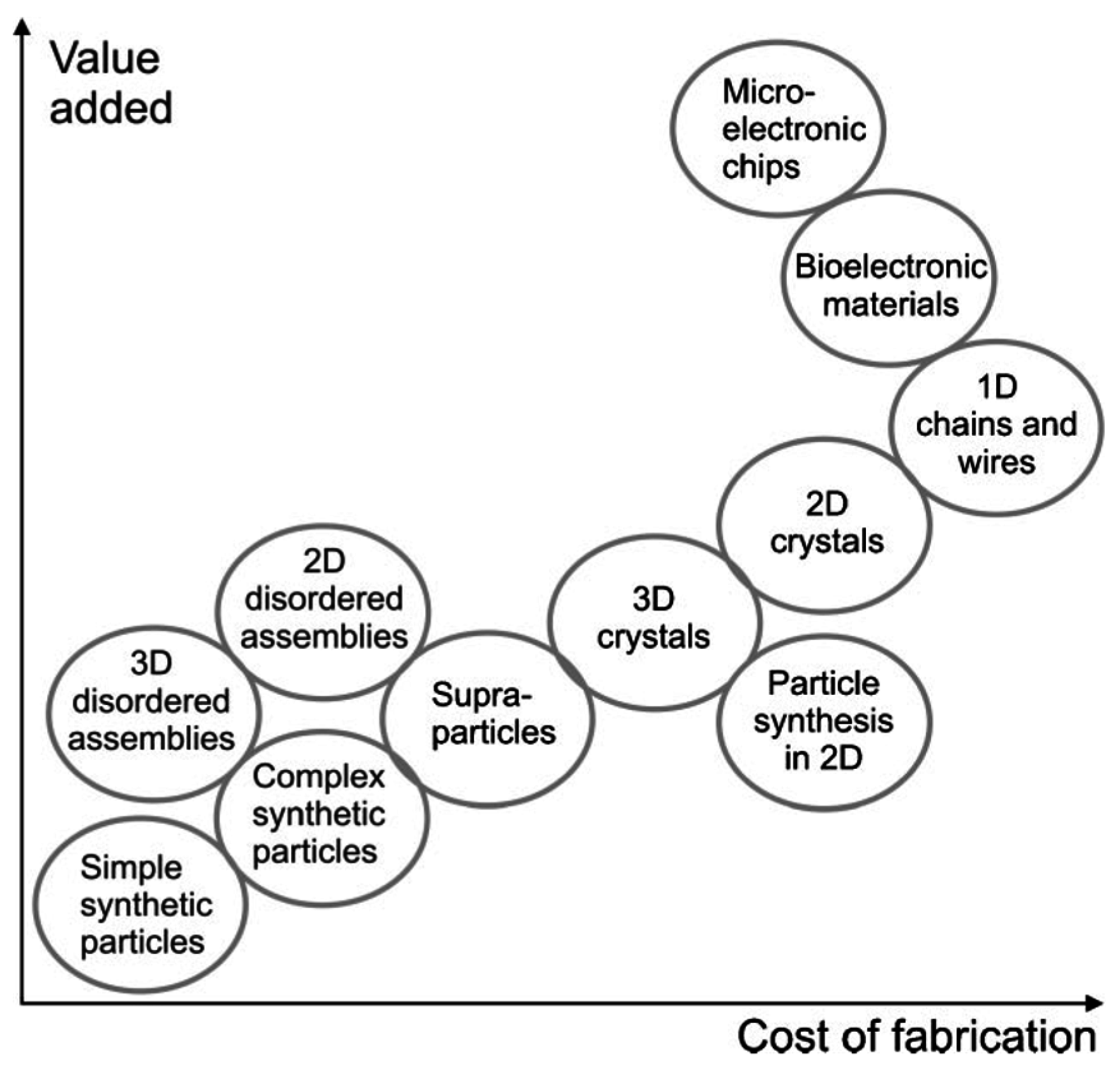
4. The Concept of Supraparticle Formation
5. Supraparticles by EISA on Superhydrophobic Surfaces
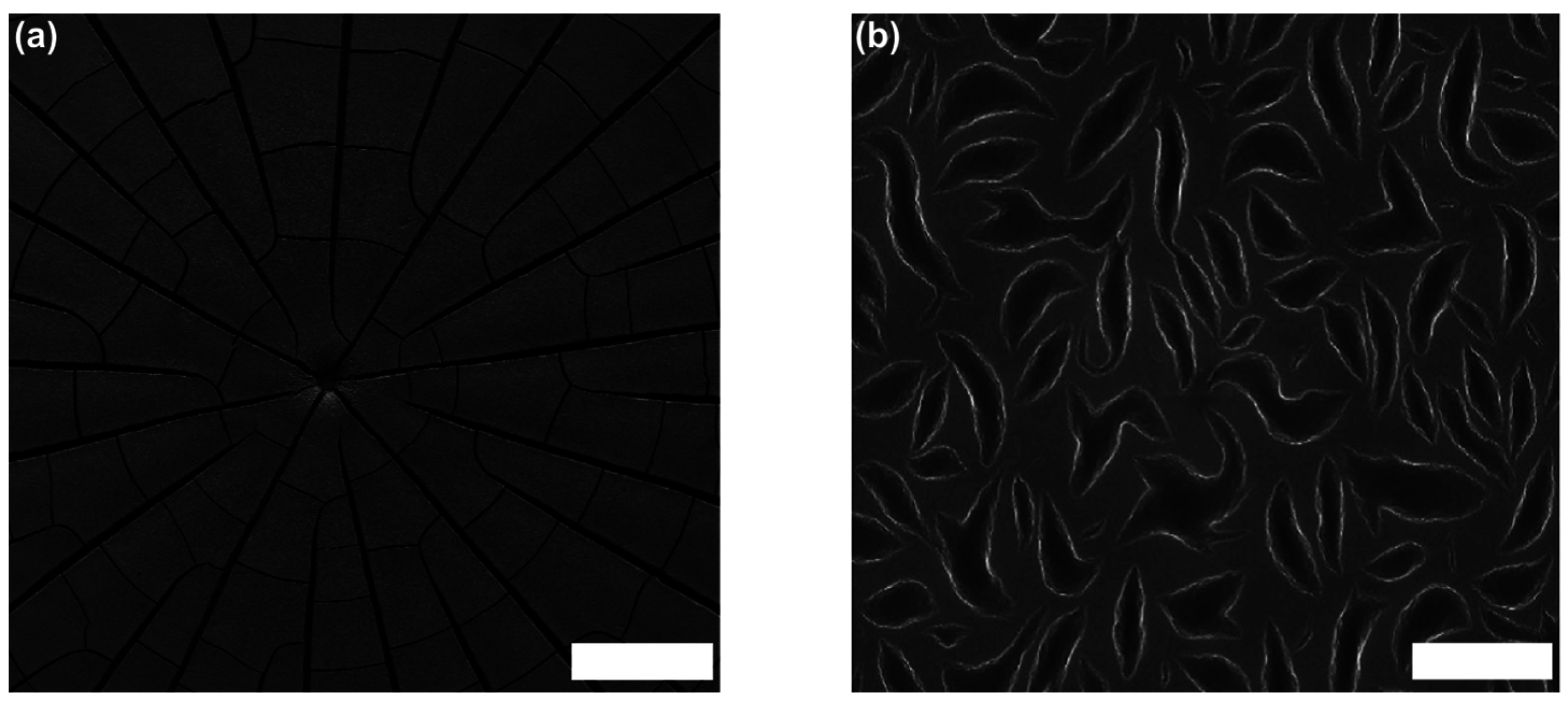
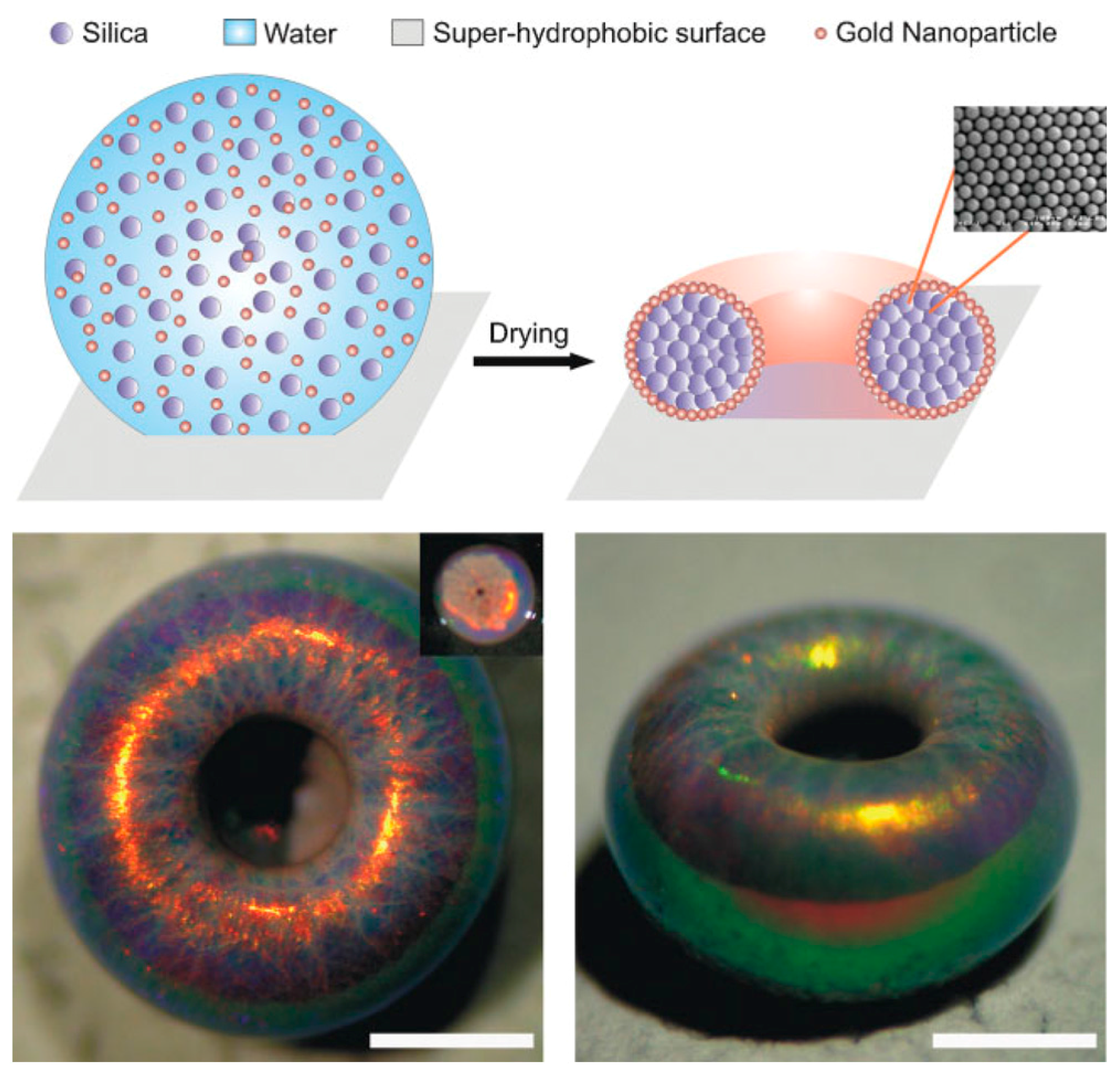
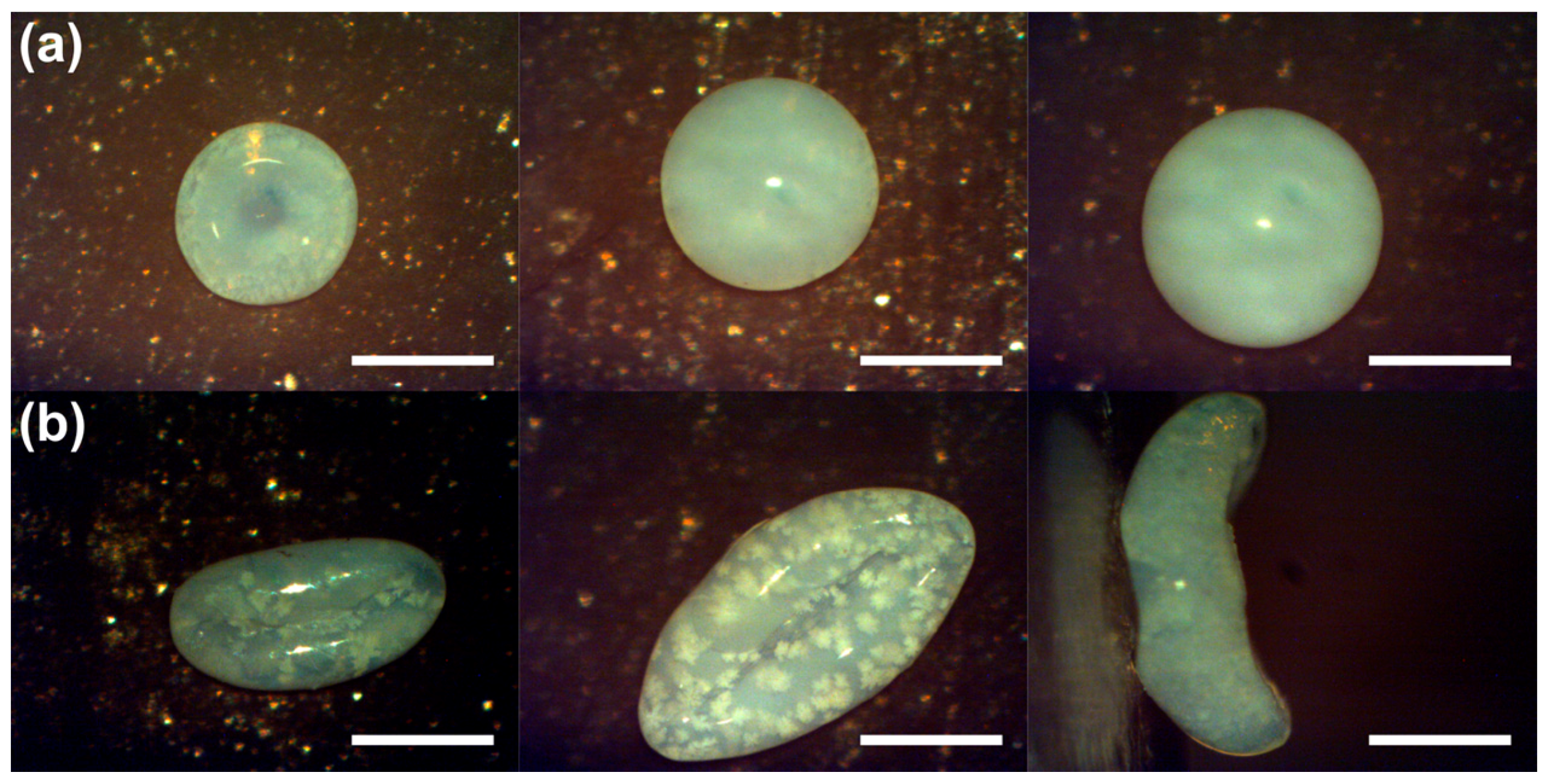
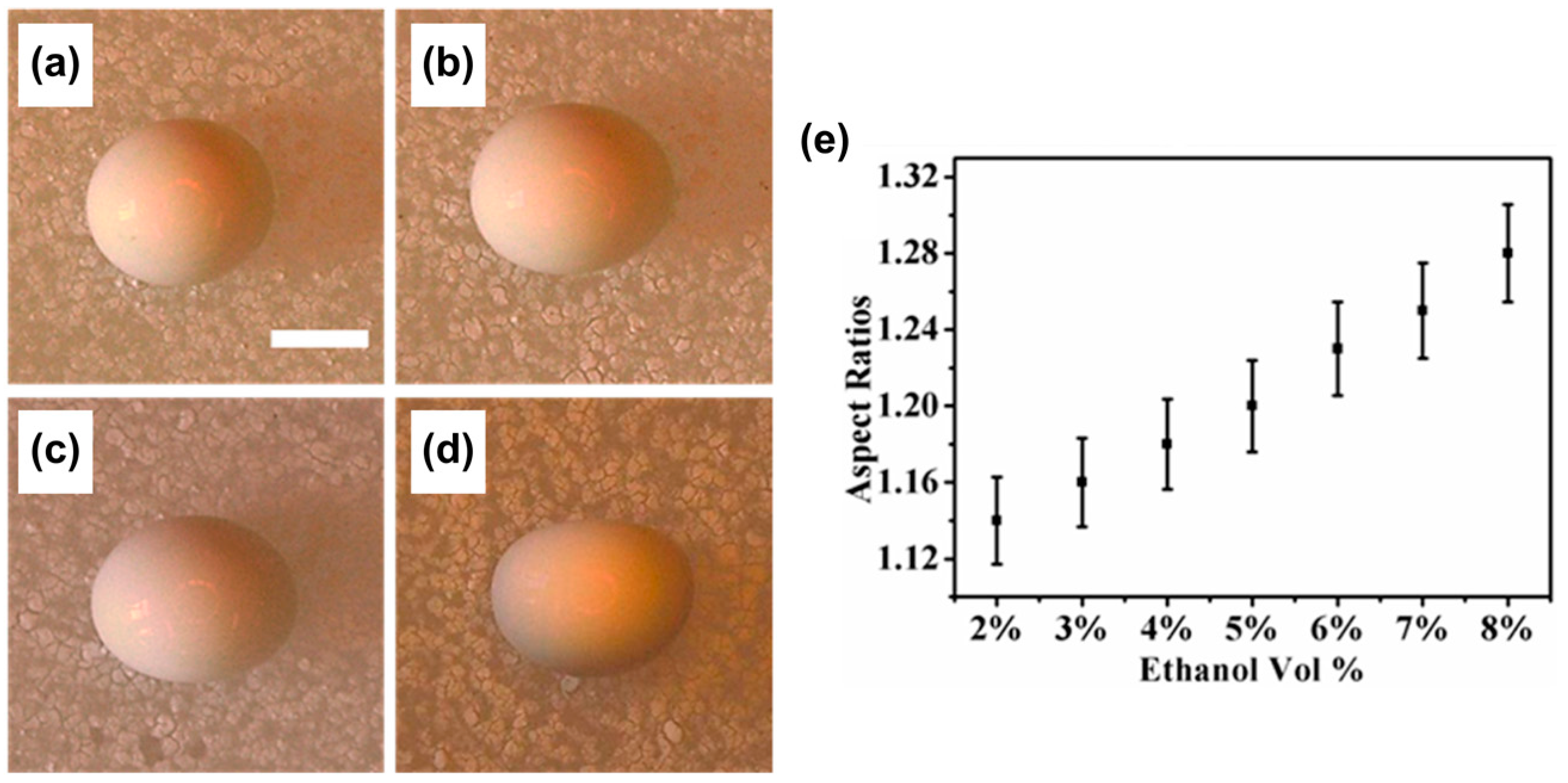
5.1. Shaping of Supraparticles
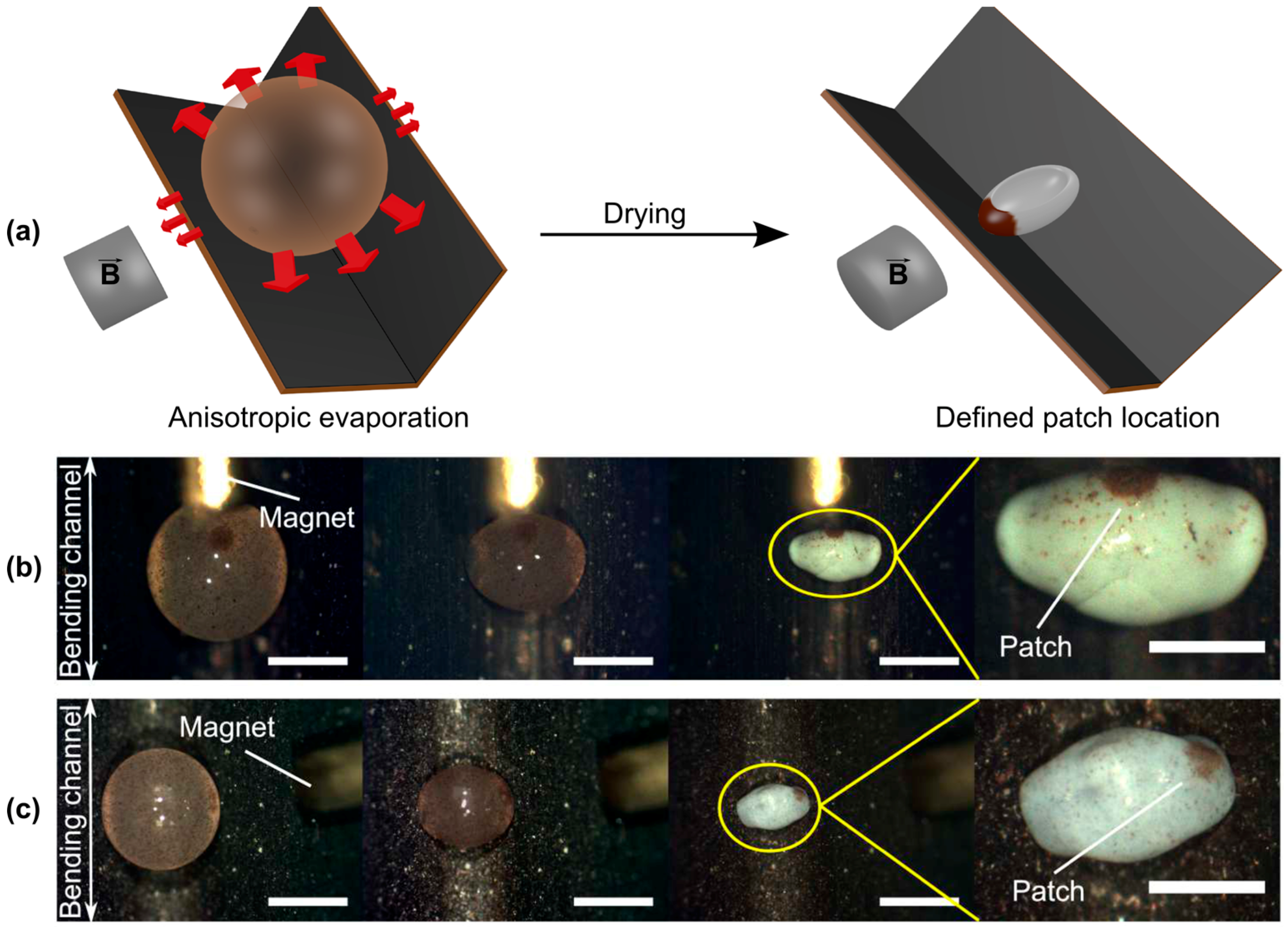
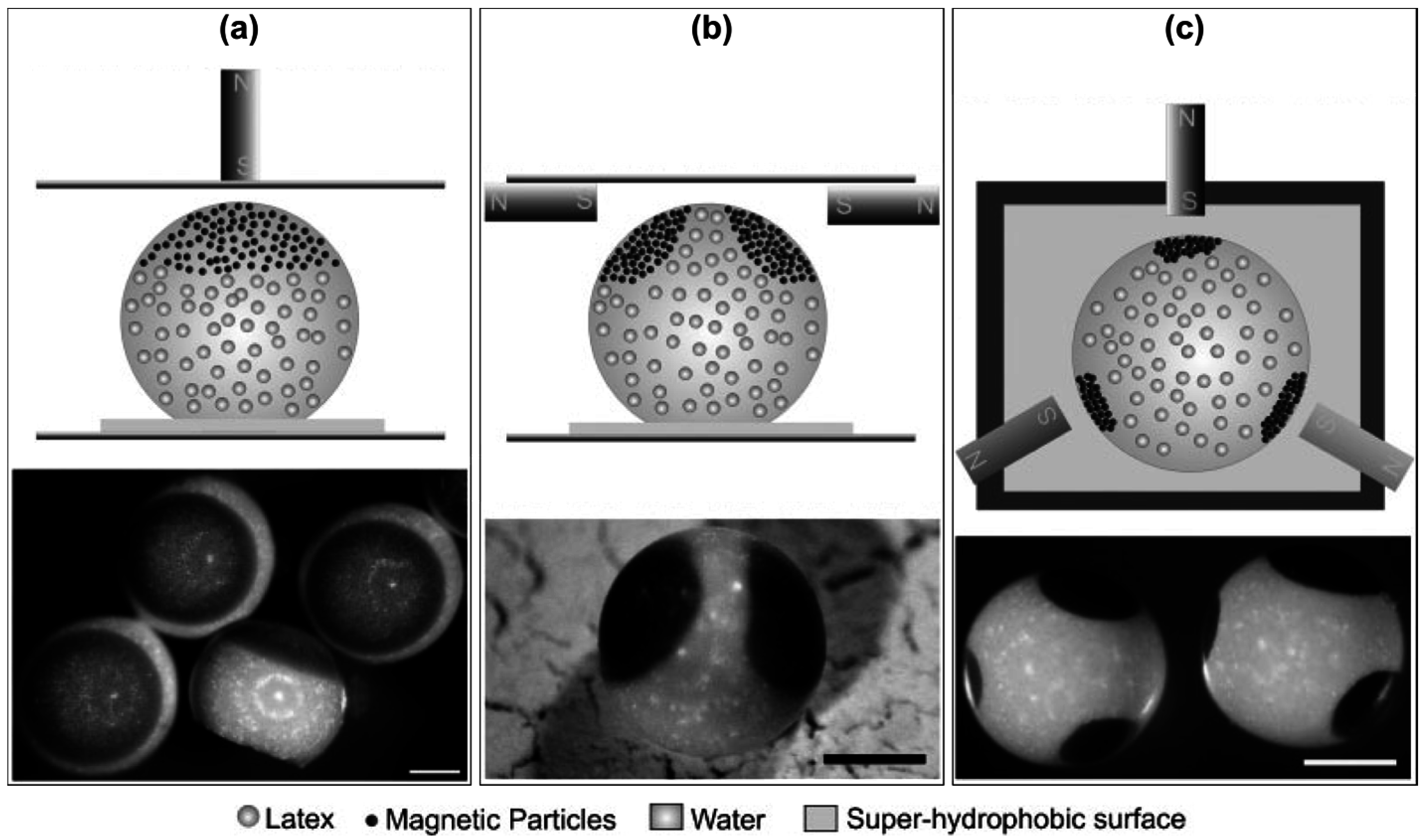
5.2. Substructuring of Supraparticles
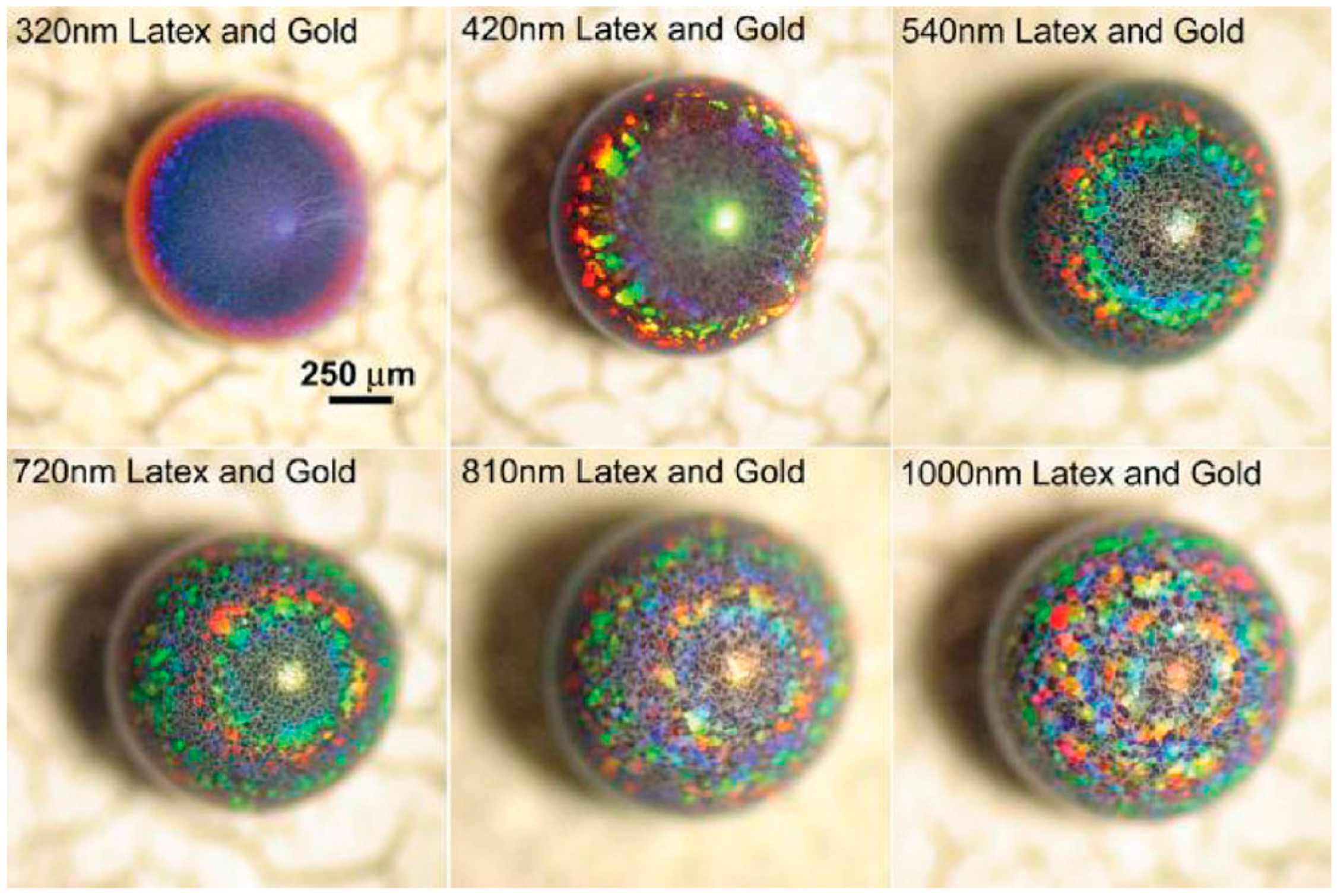
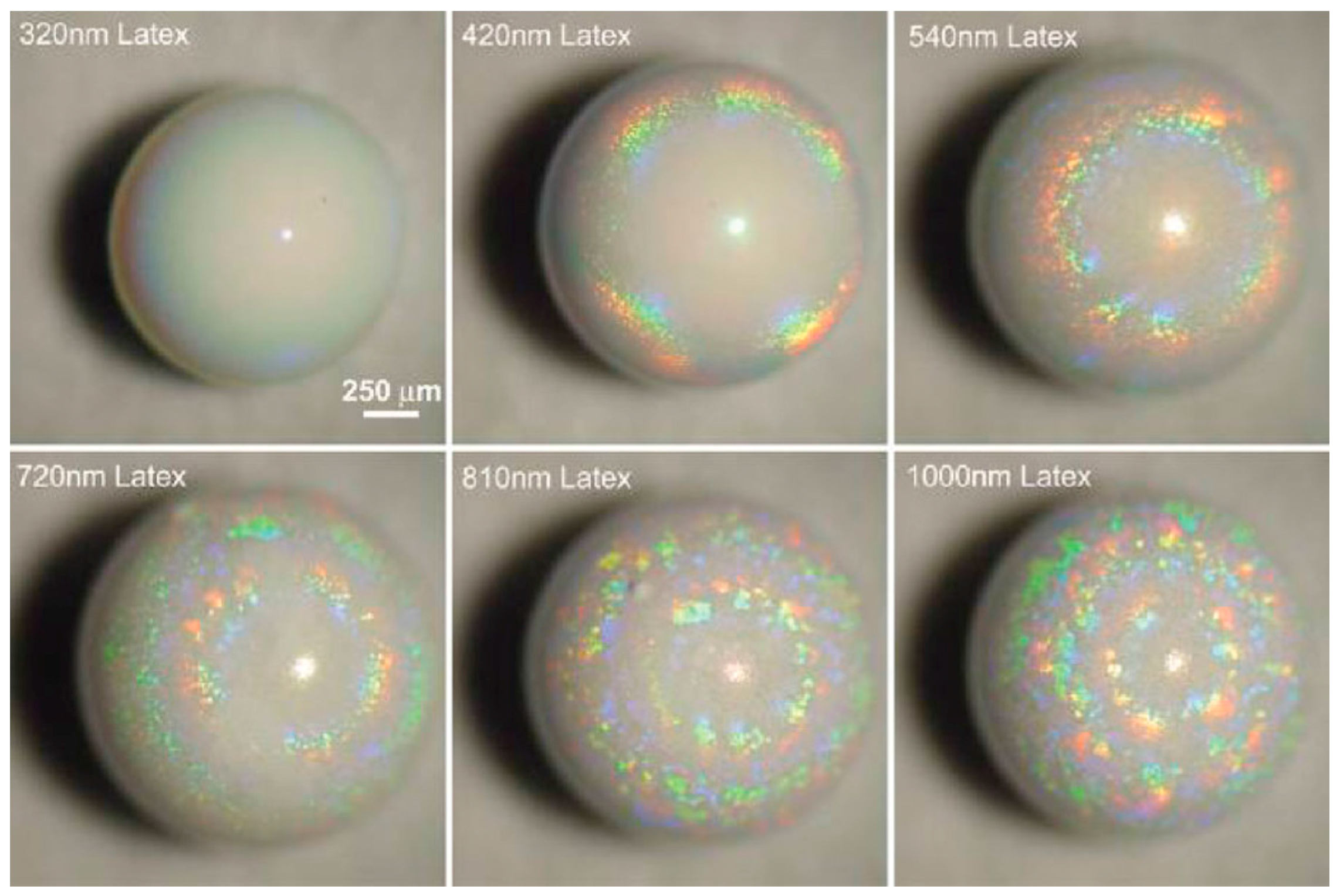
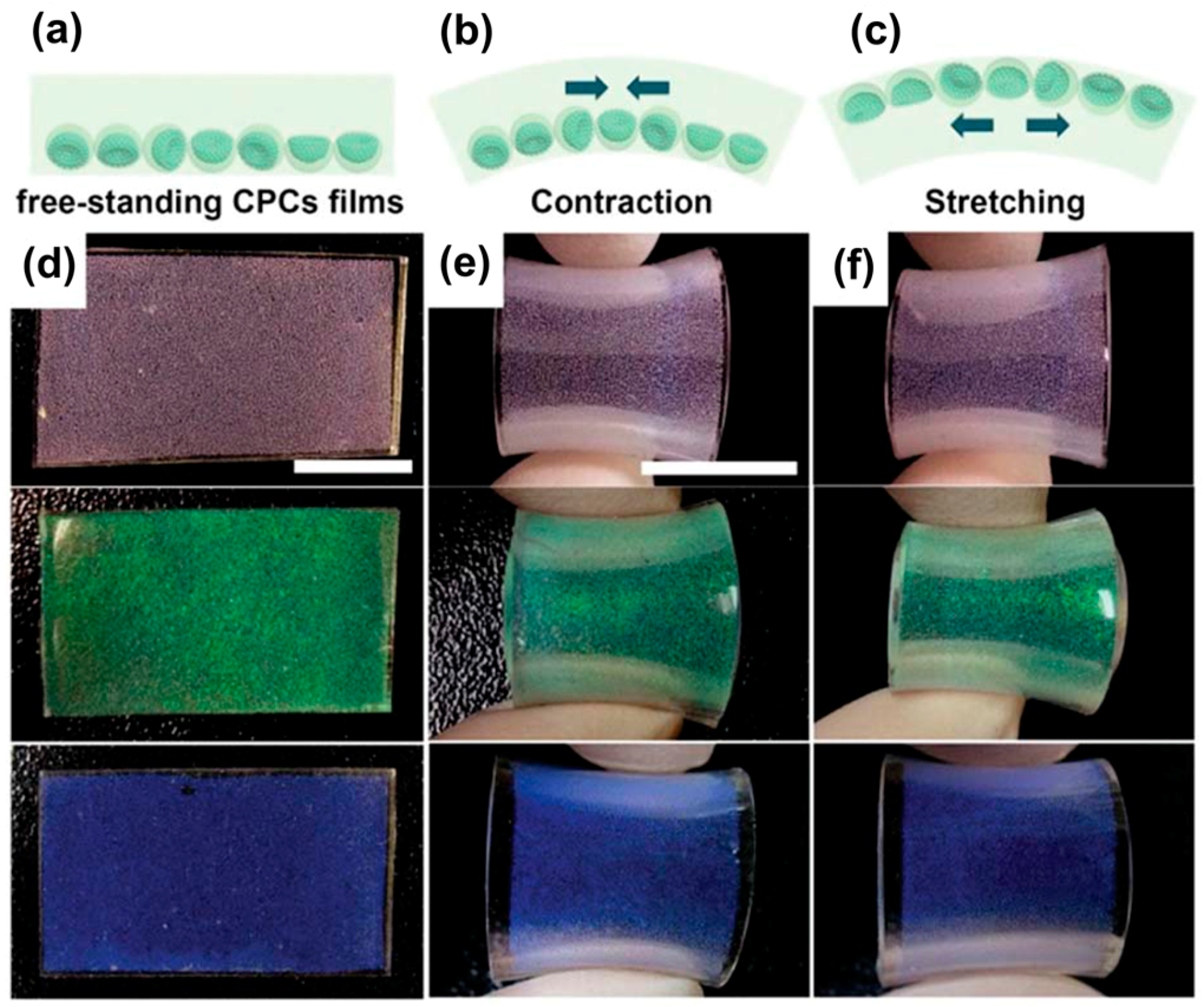
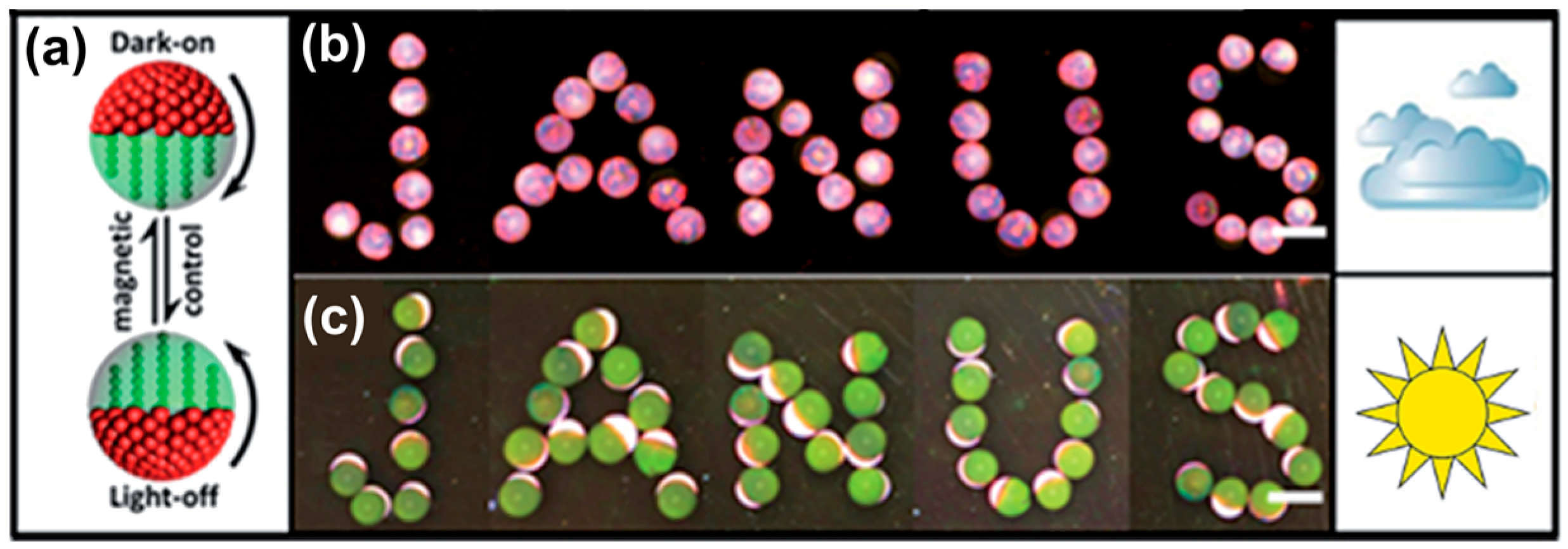
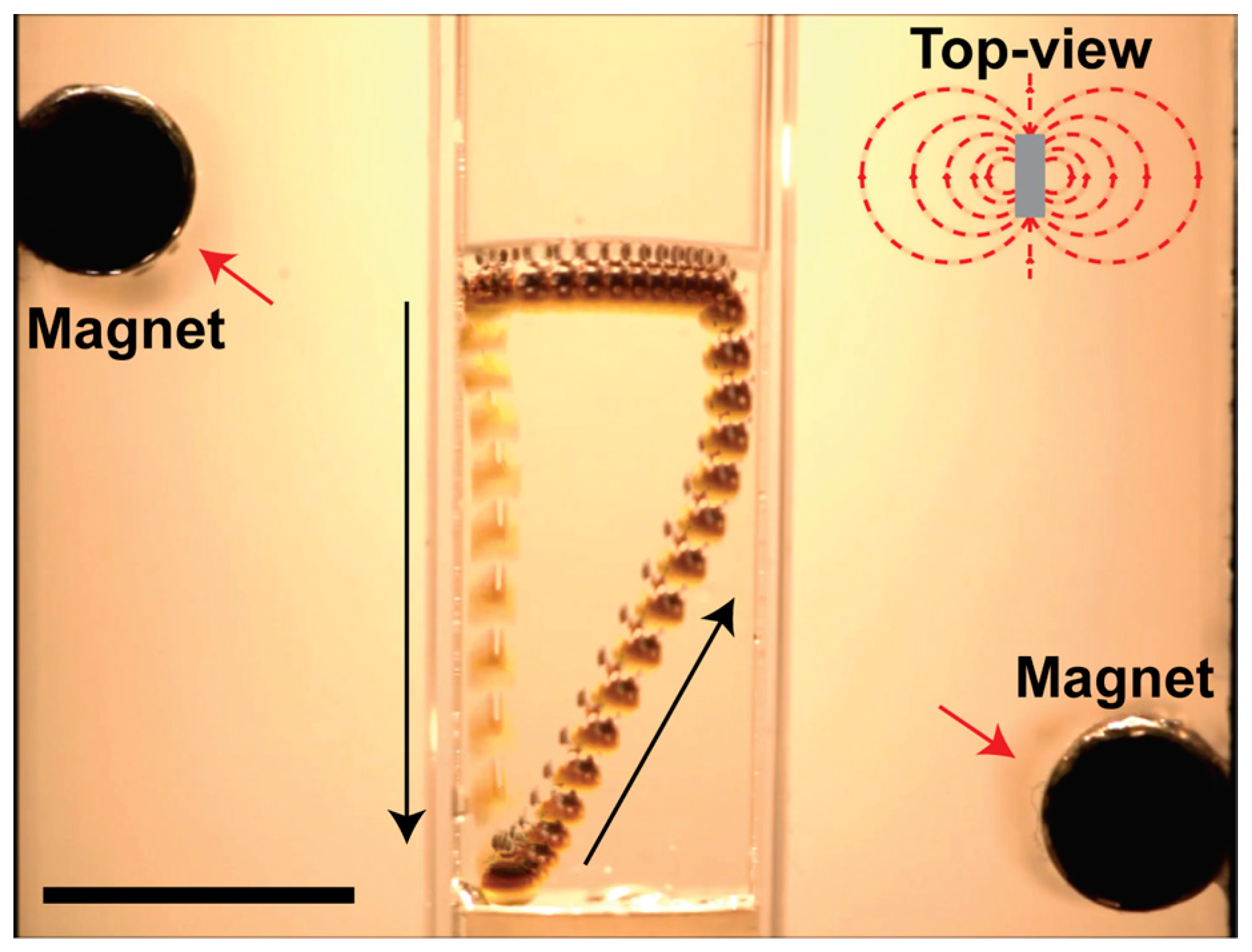
6. Applications of Supraparticles
7. Conclusions
Conflicts of Interest
Abbreviations
| BNDF | brain-derived neurotrophic factor |
| CA | contact angle |
| CCA | constant contact angle |
| CCR | constant contact radius |
| cmc | critical micelle concentration |
| CVD | chemical vapor deposition |
| DEX | dexamethasone |
| ECD | electrochemical deposition |
| EISA | evaporation-induced self-assembly |
| FS | fumed silica |
| PEO | polyethylene oxide |
| PMMA | poly methyl methacrylate |
| PS | polystyrene |
| SEM | scanning electron microscopy |
| TMPTA | trimethylolpropane triacrylate |
| TNF-α | tumor necrosis factor-alpha |
| TPCL | three-phase contact line |
| UV | ultra-violet |
| WCA | water contact angle |
References
- Lu, Z.D.; Yin, Y.D. Colloidal nanoparticle clusters: Functional materials by design. Chem. Soc. Rev. 2012, 41, 6874–6887. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.N.; Yin, Y.D.; Lu, Y.; McLellan, J. Template-assisted self-assembly of spherical colloids into complex and controllable structures. Adv. Funct. Mater. 2003, 13, 907–918. [Google Scholar] [CrossRef]
- Stein, A.; Schroden, R.C. Colloidal crystal templating of three-dimensionally ordered macroporous solids: Materials for photonics and beyond. Curr. Opin. Solid State Mater. Sci. 2001, 5, 553–564. [Google Scholar] [CrossRef]
- Velev, O.D.; Gupta, S. Materials Fabricated by Micro- and Nanoparticle Assembly—The Challenging Path from Science to Engineering. Adv. Mater. 2009, 21, 1897–1905. [Google Scholar] [CrossRef]
- Li, F.; Josephson, D.P.; Stein, A. Colloidal Assembly: The Road from Particles to Colloidal Molecules and Crystals. Angew. Chem. Int. Ed. 2011, 50, 360–388. [Google Scholar] [CrossRef] [PubMed]
- Grzelczak, M.; Vermant, J.; Furst, E.M.; Liz-Marzan, L.M. Directed Self-Assembly of Nanoparticles. ACS Nano 2010, 4, 3591–3605. [Google Scholar] [CrossRef] [PubMed]
- Galisteo-Lopez, J.F.; Ibisate, M.; Sapienza, R.; Froufe-Perez, L.S.; Blanco, A.; Lopez, C. Self-Assembled Photonic Structures. Adv. Mater. 2011, 23, 30–69. [Google Scholar] [CrossRef] [PubMed]
- Van Blaaderen, A.; Ruel, R.; Wiltzius, P. Template-directed colloidal crystallization. Nature 1997, 385, 321–324. [Google Scholar] [CrossRef]
- Bragg, W.L. Diffraction of X-rays by Two-Dimensional Crystal Lattice. Nature 1929, 124, 125. [Google Scholar] [CrossRef]
- Donaldson, J.G.; Kantorovich, S.S. Directional self-assembly of permanently magnetised nanocubes in quasi two dimensional layers. Nanoscale 2015, 7, 3217–3228. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Bian, K.; Wang, Y.; Xu, H.; Hollingsworth, J.A.; Hanrath, T.; Fang, J.; Wang, Z. An Obtuse Rhombohedral Superlattice Assembled by Pt Nanocubes. Nano Lett. 2015, 15, 6254–6260. [Google Scholar] [CrossRef] [PubMed]
- Van der Stam, W.; Gantapara, A.P.; Akkerman, Q.A.; Soligno, G.; Meeldijk, J.D.; van Roij, R.; Dijkstra, M.; de Mello Donega, C. Self-Assembly of Colloidal Hexagonal Bipyramid- and Bifrustum-Shaped ZnS Nanocrystals into Two-Dimensional Superstructures. Nano Lett. 2014, 14, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.; Bian, K.; Baumgardner, W.J.; Smilgies, D.-M.; Hanrath, T. Interface-Induced Nucleation, Orientational Alignment and Symmetry Transformations in Nanocube Superlattices. Nano Lett. 2012, 12, 4791–4798. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Jankowski, E.; Glotzer, S.C. Self-Assembly and Reconfigurability of Shape-Shifting Particles. ACS Nano 2011, 5, 8892–8903. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Yoon, J.; Madhukar, A.; Hsia, K.J.; Braun, P.V. Colloidal Particles that Rapidly Change Shape via Elastic Instabilities. Small 2015, 11, 6051–6057. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fan, H.; Stump, A.; Ward, T.L.; Rieker, T.; Brinker, C.J. Aerosol-assisted self-assembly of mesostructured spherical nanoparticles. Nature 1999, 398, 223–226. [Google Scholar]
- Brezesinski, T.; Groenewolt, M.; Gibaud, A.; Pinna, N.; Antonietti, M.; Smarsly, B. Evaporation-Induced Self-Assembly (EISA) at Its Limit: Ultrathin, Crystalline Patterns by Templating of Micellar Monolayers. Adv. Mater. 2006, 18, 2260–2263. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired Surfaces with Special Wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Genzer, J.; Efimenko, K. Recent developments in superhydrophobic surfaces and their relevance to marine fouling: A review. Biofouling 2006, 22, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-Induced Self-Assembly: Nanostructures Made Easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- Grosso, D.; Cagnol, F.; Soler, G.J.D.A.; Crepaldi, E.L.; Amenitsch, H.; Brunet-Bruneau, A.; Bourgeois, A.; Sanchez, C. Fundamentals of Mesostructuring Through Evaporation-Induced Self-Assembly. Adv. Funct. Mater. 2004, 14, 309–322. [Google Scholar] [CrossRef]
- Velev, O.D.; Lenhoff, A.M.; Kaler, E.W. A class of microstructured particles through colloidal crystallization. Science 2000, 287, 2240–2243. [Google Scholar] [CrossRef] [PubMed]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Contact line deposits in an evaporating drop. Phys. Rev. E 2000, 62, 756–765. [Google Scholar] [CrossRef]
- Kuncicky, D.M.; Velev, O.D. Surface-guided templating of particle assemblies inside drying sessile droplets. Langmuir 2008, 24, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Pauchard, L.; Parisse, F.; Allain, C. Influence of salt content on crack patterns formed through colloidal suspension desiccation. Phys. Rev. E 1999, 59, 3737–3740. [Google Scholar] [CrossRef]
- Brutin, D.; Sobac, B.; Loquet, B.; Sampol, J. Pattern formation in drying drops of blood. J. Fluid Mech. 2011, 667, 85–95. [Google Scholar] [CrossRef]
- Sobac, B.; Brutin, D. Structural and evaporative evolutions in desiccating sessile drops of blood. Phys. Rev. E 2011, 84, 011603. [Google Scholar] [CrossRef] [PubMed]
- Joksimovic, R.; Watanabe, S.; Riemer, S.; Gradzielski, M.; Yoshikawa, K. Self-organized patterning through the dynamic segregation of DNA and silica nanoparticles. Sci. Rep. 2014, 4, 3660. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Velev, O.D. Evaporation-induced particle microseparations inside droplets floating on a chip. Langmuir 2006, 22, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.; von Arnim, V.; McKinley, G.H.; Hosoi, A.E. Marangoni convection in droplets on superhydrophobic surfaces. J. Fluid Mech. 2009, 624, 101–123. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Marangoni Effect Reverses Coffee-Ring Depositions. J. Phys. Chem. B 2006, 110, 7090–7094. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Larson, R.G. Analysis of the Microfluid Flow in an Evaporating Sessile Droplet. Langmuir 2005, 21, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Larson, R.G. Analysis of the effects of Marangoni stresses on the microflow in an evaporating sessile droplet. Langmuir 2005, 21, 3972–3980. [Google Scholar] [CrossRef] [PubMed]
- Eral, H.B.; Augustine, D.M.; Duits, M.H.G.; Mugele, F. Suppressing the coffee stain effect: How to control colloidal self-assembly in evaporating drops using electrowetting. Soft Matter 2011, 7, 4954–4958. [Google Scholar] [CrossRef]
- Krupenkin, T.N.; Taylor, J.A.; Schneider, T.M.; Yang, S. From rolling ball to complete wetting: The dynamic tuning of liquids on nanostructured surfaces. Langmuir 2004, 20, 3824–3827. [Google Scholar] [CrossRef] [PubMed]
- McHale, G.; Brown, C.V.; Newton, M.I.; Wells, G.G.; Sampara, N. Dielectrowetting Driven Spreading of Droplets. Phys. Rev. Lett. 2011, 107, 186101. [Google Scholar] [CrossRef] [PubMed]
- Picknett, R.G.; Bexon, R. Evaporation of Sessile or Pendant Drops in Still Air. J. Colloid Interface Sci. 1977, 61, 336–350. [Google Scholar] [CrossRef]
- Maxwell, J.C. Diffusion. In Collected Scientific Papers: Diffusion; Encyclopedia Britannica: Cambridge, UK, 1877. [Google Scholar]
- Snow, C. Potential Problems and Capacitance for a Conductor Bounded by Two Intersecting Spheres. J. Res. Nat. Bur. Stand. 1949, 43, 377–407. [Google Scholar] [CrossRef]
- Soulie, V.; Karpitschka, S.; Lequien, F.; Prene, P.; Zemb, T.; Moehwald, H.; Riegler, H. The evaporation behavior of sessile droplets from aqueous saline solutions. Phys. Chem. Chem. Phys. 2015, 17, 22296–22303. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.H.; Nguyen, A.V. Increased Evaporation Kinetics of Sessile Droplets by Using Nanoparticles. Langmuir 2012, 28, 16725–16728. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.S.; Wang, Z.Q.; Zhao, Y.P. Experimental and theoretical investigations of evaporation of sessile water droplet on hydrophobic surfaces. J. Colloid Interface Sci. 2012, 365, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W. Self-cleaning surfaces of objects and process for producing same. U.S. Patent 6,660,363, 9 December 2003. [Google Scholar]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Jung, Y.C.; Barthlott, W. Fabrication of artificial Lotus leaves and significance of hierarchical structure for superhydrophobicity and low adhesion. Soft Matter 2009, 5, 1386–1393. [Google Scholar] [CrossRef]
- Feng, X.J.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L.; Butt, H.J.; Vollmer, D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Seeger, S. Superamphiphobic surfaces. Chem. Soc. Rev. 2014, 43, 2784–2798. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Genzer, J.; Marmur, A. Biological and synthetic self-cleaning surfaces. Mrs Bull. 2008, 33, 742–746. [Google Scholar] [CrossRef]
- Papadopoulos, P.; Mammen, L.; Deng, X.; Vollmer, D.; Butt, H.-J. How superhydrophobicity breaks down. Proc. Natl. Acad. Sci. USA 2013, 110, 3254–3258. [Google Scholar] [CrossRef] [PubMed]
- Celia, E.; Darmanin, T.; de Givenchy, E.T.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Y.; Gao, N.; Barthlott, W. Mimicking natural superhydrophobic surfaces and grasping the wetting process: A review on recent progress in preparing superhydrophobic surfaces. Adv. Colloid Interface Sci. 2011, 169, 80–105. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.D.; Ren, H.; Tu, J.P.; Zhang, T.Y. Micro/Nanobinary Structure of Silver Films on Copper Alloys with Stable Water-Repellent Property under Dynamic Conditions. Langmuir 2009, 25, 12299–12307. [Google Scholar] [CrossRef] [PubMed]
- Sperling, M.; Papadopoulos, P.; Gradzielski, M. Understanding the Formation of Anisometric Supraparticles: A Mechanistic Look Inside Droplets Drying on a Superhydrophobic Surface. Langmuir 2016, 32, 6902–6908. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, A.; Choi, W.; Ma, M.L.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.R.; England, G.T.; Sunny, S.; Shirman, E.; Shirman, T.; Vogel, N.; Aizenberg, J. A colloidoscope of colloid-based porous materials and their uses. Chem. Soc. Rev. 2016, 45, 281–322. [Google Scholar] [CrossRef] [PubMed]
- Il Park, J.; Nguyen, T.D.; Silveira, G.D.; Bahng, J.H.; Srivastava, S.; Zhao, G.P.; Sun, K.; Zhang, P.J.; Glotzer, S.C.; Kotov, N.A. Terminal supraparticle assemblies from similarly charged protein molecules and nanoparticles. Nat. Commun. 2014, 5, 3593. [Google Scholar]
- Piccinini, E.; Pallarola, D.; Battaglini, F.; Azzaroni, O. Recognition-driven assembly of self-limiting supramolecular protein nanoparticles displaying enzymatic activity. Chem. Commun. 2015, 51, 14754–14757. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tang, Z. Monodisperse Hollow Supraparticles via Selective Oxidation. Adv. Funct. Mater. 2012, 22, 2585–2593. [Google Scholar] [CrossRef]
- Yang, G.; Zhong, H.; Liu, R.; Li, Y.; Zou, B. In Situ Aggregation of ZnSe Nanoparticles into Supraparticles: Shape Control and Doping Effects. Langmuir 2013, 29, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wan, J.; Chen, K. A facile synthesis of superparamagnetic Fe3O4 supraparticles@MIL-100(Fe) core-shell nanostructures: Preparation, characterization and biocompatibility. J. Colloid Interface Sci. 2016, 461, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, W.; Wang, C. Magnetic Colloidal Supraparticles: Design, Fabrication and Biomedical Applications. Adv. Mater. 2013, 25, 5196–5214. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.W.; Wang, D.; Möhwald, H. Hierarchical Organization of Colloidal Particles: From Colloidal Crystallization to Supraparticle Chemistry. Macromol. Chem. Phys. 2007, 208, 439–445. [Google Scholar] [CrossRef]
- Xia, Y.S.; Nguyen, T.D.; Yang, M.; Lee, B.; Santos, A.; Podsiadlo, P.; Tang, Z.Y.; Glotzer, S.C.; Kotov, N.A. Self-assembly of self-limiting monodisperse supraparticles from polydisperse nanoparticles. Nat. Nanotechnol. 2011, 6, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Yi, G.R.; Kim, S.H.; Elsesser, M.T.; Breed, D.R.; Yang, S.M. Homogeneous and heterogeneous binary colloidal clusters formed by evaporation-induced self-assembly inside droplets. J. Colloid Interface Sci. 2008, 318, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Velev, O.D.; Furusawa, K.; Nagayama, K. Assembly of latex particles by using emulsion droplets as templates. 2. Ball-like and composite aggregates. Langmuir 1996, 12, 2385–2391. [Google Scholar] [CrossRef]
- Velev, O.D.; Furusawa, K.; Nagayama, K. Assembly of latex particles by using emulsion droplets as templates. 1. Microstructured hollow spheres. Langmuir 1996, 12, 2374–2384. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Kim, S.-H.; Yi, G.-R.; Yang, S.-M. Self-organization of colloidal nanospheres inside emulsion droplets: Higher-order clusters, supraparticles, and supraballs. Colloids Surf. A 2009, 345, 237–245. [Google Scholar] [CrossRef]
- Maeda, K.; Onoe, H.; Takinoue, M.; Takeuchi, S. Controlled Synthesis of 3D Multi-Compartmental Particles with Centrifuge-Based Microdroplet Formation from a Multi-Barrelled Capillary. Adv. Mater. 2012, 24, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Y.; Wang, C.F.; Ling, L.T.; Chen, L.; Chen, S. Triphase Microfluidic-Directed Self-Assembly: Anisotropic Colloidal Photonic Crystal Supraparticles and Multicolor Patterns Made Easy. Angew. Chem. Int. Ed. 2012, 51, 2375–2378. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Wang, J.; Han, J.J. Fabrication of Advanced Particles and Particle-Based Materials Assisted by Droplet-Based Microfluidics. Small 2011, 7, 1728–1754. [Google Scholar] [CrossRef] [PubMed]
- Brugarolas, T.; Tu, F.; Lee, D. Directed assembly of particles using microfluidic droplets and bubbles. Soft Matter 2013, 9, 9046–9058. [Google Scholar] [CrossRef]
- Sowade, E.; Blaudeck, T.; Baumann, R.R. Self-Assembly of Spherical Colloidal Photonic Crystals inside Inkjet-Printed Droplets. Cryst. Growth Des. 2016, 16, 1017–1026. [Google Scholar] [CrossRef]
- Sowade, E.; Hammerschmidt, J.; Blaudeck, T.; Baumann, R.R. In-Flight Inkjet Self-Assembly of Spherical Nanoparticle Aggregates. Adv. Eng. Mater. 2012, 14, 98–100. [Google Scholar] [CrossRef]
- Chen, F.C.; Lu, J.P.; Huang, W.K. Using Ink-Jet Printing and Coffee Ring Effect to Fabricate Refractive Microlens Arrays. IEEE Photonics Technol. Lett. 2009, 21, 648–650. [Google Scholar] [CrossRef]
- Wang, D.; Park, M.; Park, J.; Moon, J. Optical properties of single droplet of photonic crystal assembled by ink-jet printing. Appl. Phys. Lett. 2005, 86, 241114. [Google Scholar] [CrossRef]
- Millman, J.R.; Bhatt, K.H.; Prevo, B.G.; Velev, O.D. Anisotropic particle synthesis in dielectrophoretically controlled microdroplet reactors. Nat. Mater. 2005, 4, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Denkov, N.D.; Velev, O.D.; Kralchevsky, P.A.; Ivanov, I.B.; Yoshimura, H.; Nagayama, K. 2-Dimensional Crystallization. Nature 1993, 361, 26. [Google Scholar] [CrossRef]
- Denkov, N.D.; Velev, O.D.; Kralchevsky, P.A.; Ivanov, I.B.; Yoshimura, H.; Nagayama, K. Mechanism of Formation of 2-Dimensional Crystals from Latex-Particles on Substrates. Langmuir 1992, 8, 3183–3190. [Google Scholar] [CrossRef]
- Lee, D.-W.; Jin, M.-H.; Lee, C.-B.; Oh, D.; Ryi, S.-K.; Park, J.-S.; Bae, J.-S.; Lee, Y.-J.; Park, S.-J.; Choi, Y.-C. Facile synthesis of mesoporous silica and titania supraparticles by a meniscus templating route on a superhydrophobic surface and their application to adsorbents. Nanoscale 2014, 6, 3483–3487. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Velikov, K.P.; Velev, O.D. Microfluidic characterization of sustained solute release from porous supraparticles. Phys. Chem. Chem. Phys. 2010, 12, 11975–11983. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, V.N. Colloidal spheres confined by liquid droplets: Geometry, physics, and physical chemistry. Solid State Commun. 2006, 139, 557–561. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, Y.; Zhai, J.; Jiang, L. Bioinspired Super-antiwetting Interfaces with Special Liquid-Solid Adhesion. Acc. Chem. Res. 2010, 43, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Her, E.K. Fabrication of Superhydrophobic Surfaces with High and Low Adhesion Inspired from Rose Petal. Langmuir 2010, 26, 8207–8217. [Google Scholar] [CrossRef] [PubMed]
- Wooh, S.; Huesmann, H.; Tahir, M.N.; Paven, M.; Wichmann, K.; Vollmer, D.; Tremel, W.; Papadopoulos, P.; Butt, H.-J. Synthesis of Mesoporous Supraparticles on Superamphiphobic Surfaces. Adv. Mater. 2015, 27, 7338–7343. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Garcia, A.A.; Marquez, M.; Velev, O.D. Anisotropic Particle Synthesis Inside Droplet Templates on Superhydrophobic Surfaces. Macromol. Rapid Commun. 2010, 31, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Sperling, M.; Velev, O.D.; Gradzielski, M. Controlling the Shape of Evaporating Droplets by Ionic Strength: Formation of Highly Anisometric Silica Supraparticles. Angew. Chem. Int. Ed. 2014, 53, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Sperling, M.; Kim, H.J.; Velev, O.D.; Gradzielski, M. Active Steerable Catalytic Supraparticles Shuttling on Preprogrammed Vertical Trajectories. Adv. Mater. Interf. 2016, 3, 160095. [Google Scholar] [CrossRef]
- Sperling, M.; Velev, O.D.; Gradzielski, M. Formation of Anisometric Fumed Silica Supraparticles—Mechanism and Application Potential. Z. Phys. Chem. 2015, 229, 1055–1074. [Google Scholar] [CrossRef]
- Sperling, M.; Velev, O.D.; Gradzielski, M. Kontrolle der Form verdunstender Tropfen über die Ionenstärke: Bildung anisometrischer SiO2-Suprapartikel. Angew. Chem. 2014, 126, 597–601. [Google Scholar] [CrossRef]
- Rastogi, V.; Melle, S.; Calderon, O.G.; Garcia, A.A.; Marquez, M.; Velev, O.D. Synthesis of Light-Diffracting Assemblies from Microspheres and Nanoparticles in Droplets on a Superhydrophobic Surface. Adv. Mater. 2008, 20, 4263–4268. [Google Scholar] [CrossRef]
- Princen, H.M. Surface and Colloid Science; Matijevic, E., Ed.; Wiley: New York, NY, USA, 1969; p. 1. [Google Scholar]
- Head, D.A. Modeling the elastic deformation of polymer crusts formed by sessile droplet evaporation. Phys. Rev. E 2006, 74, 021601. [Google Scholar] [CrossRef] [PubMed]
- Tsapis, N.; Dufresne, E.R.; Sinha, S.S.; Riera, C.S.; Hutchinson, J.W.; Mahadevan, L.; Weitz, D.A. Onset of Buckling in Drying Droplets of Colloidal Suspensions. Phys. Rev. Lett. 2005, 94, 018302. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, T.; Nishitani, E.; Yamaue, T.; Doi, M. Piling-to-buckling transition in the drying process of polymer solution drop on substrate having a large contact angle. Phys. Rev. E 2006, 73, 011601. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-Z.; Yu, Y.-H.; Zhang, H.; Chen, H.; Lu, Z.; Fujishima, A.; Sato, O. Self-assembly of monodisperse spheres on substrates with different wettability. Appl. Phys. A 2005, 81, 47–49. [Google Scholar] [CrossRef]
- Kuncicky, D.M.; Bose, K.; Costa, K.D.; Velev, O.D. Sessile Droplet Templating of Miniature Porous Hemispheres from Colloid Crystals. Chem. Mater. 2007, 19, 141–143. [Google Scholar] [CrossRef]
- Sen, D.; Bahadur, J.; Mazumder, S.; Santoro, G.; Yu, S.; Roth, S.V. Probing evaporation induced assembly across a drying colloidal droplet using in situ small-angle X-ray scattering at the synchrotron source. Soft Matter 2014, 10, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Kätzel, U.; Vorbau, M.; Stintz, M.; Gottschalk-Gaudig, T.; Barthel, H. Dynamic Light Scattering for the Characterization of Polydisperse Fractal Systems: II. Relation between Structure and DLS Results. Part. Part. Syst. Charact. 2008, 25, 19–30. [Google Scholar] [CrossRef]
- Accardo, A.; Gentile, F.; Mecarini, F.; Angelis, F.D.; Burghammer, M.; Fabrizio, E.D.; Riekel, C. In Situ X-ray Scattering Studies of Protein Solution Droplets Drying on Micro- and Nanopatterned Superhydrophobic PMMA Surfaces. Langmuir 2010, 26, 15057–15064. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, J.; Sen, D.; Mazumder, S.; Paul, B.; Bhatt, H.; Singh, S.G. Control of Buckling in Colloidal Droplets during Evaporation-Induced Assembly of Nanoparticles. Langmuir 2012, 28, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Sperling, M.; Spiering, V.J.; Velev, O.D.; Gradzielski, M. Controlled Formation of Patchy Anisometric Fumed Silica Supraparticles in Droplets on Bent Superhydrophobic Surfaces. Part. Part. Syst. Charact. 2017, 34, 1600176. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, J.; Gu, Z.; Zhang, G.; Wei, Y.; Yao, X.; Song, Y.; Jiang, L. Controllable Fabrication of Noniridescent Microshaped Photonic Crystal Assemblies by Dynamic Three-Phase Contact Line Behaviors on Superhydrophobic Substrates. ACS Appl. Mater. Interfaces 2015, 7, 22644–22651. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.B.; Kretzschmar, I. Fabrication, Assembly, and Application of Patchy Particles. Macromol. Rapid Commun. 2010, 31, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, Q.; Tripathy, M.; Luijten, E.; Schweizer, K.S.; Granick, S. Janus Particle Synthesis and Assembly. Adv. Mater. 2010, 22, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Muller, A.H.E. Janus particles. Soft Matter 2008, 4, 663–668. [Google Scholar] [CrossRef]
- Perro, A.; Reculusa, S.; Ravaine, S.; Bourgeat-Lami, E.; Duguet, E. Design and synthesis of Janus micro- and nanoparticles. J. Mater. Chem. 2005, 15, 3745–3760. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, S.; Wang, Z.L. Structural colors from Morpho peleides butterfly wing scales. J. Appl. Phys. 2009, 106, 074702. [Google Scholar] [CrossRef]
- Vignolini, S.; Rudall, P.J.; Rowland, A.V.; Reed, A.; Moyroud, E.; Faden, R.B.; Baumberg, J.J.; Glover, B.J.; Steiner, U. Pointillist structural color in Pollia fruit. Proc. Natl. Acad. Sci. USA 2012, 109, 15712. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.J.; Tu, F.; Kim, S.; Yi, G.-R.; Yoo, P.J.; Lee, D. Angle- and strain-independent coloured free-standing films incorporating non-spherical colloidal photonic crystals. Soft Matter 2015, 11, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-S.; Wang, C.-F.; Wang, X.-Q.; Zhang, J.; Tian, Y.; Yin, S.-N.; Chen, S. Tunable Janus colloidal photonic crystal supraballs with dual photonic band gaps. J. Mater. Chem. C 2014, 2, 9431–9438. [Google Scholar] [CrossRef]
- Wang, H.; Yang, S.; Yin, S.-N.; Chen, L.; Chen, S. Janus Suprabead Displays Derived from the Modified Photonic Crystals toward Temperature Magnetism and Optics Multiple Responses. ACS Appl. Mater. Interfaces 2015, 7, 8827–8833. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Velev, O.D. Development and evaluation of realistic microbioassays in freely suspended droplets on a chip. Biomicrofluidics 2007, 1, 014107. [Google Scholar] [CrossRef] [PubMed]
- Maina, J.W.; Cui, J.; Björnmalm, M.; Wise, A.K.; Shepherd, R.K.; Caruso, F. Mold-Templated Inorganic-Organic Hybrid Supraparticles for Codelivery of Drugs. Biomacromolecules 2014, 15, 4146–4151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wise, A.K.; Tan, J.; Maina, J.W.; Shepherd, R.K.; Caruso, F. Mesoporous Silica Supraparticles for Sustained Inner-Ear Drug Delivery. Small 2014, 10, 4244–4248. [Google Scholar] [CrossRef] [PubMed]
- Park, W.M.; Yee, C.M.; Champion, J.A. Self-assembled hybrid supraparticles that proteolytically degrade tumor necrosis factor-α. J. Mater. Chem. B 2016, 4, 1633–1639. [Google Scholar] [CrossRef]
- Sanchez, S.; Soler, L.; Katuri, J. Chemically Powered Micro- and Nanomotors. Angew. Chem. Int. Ed. 2015, 54, 1414–1444. [Google Scholar] [CrossRef] [PubMed]
- Ebbens, S.J.; Howse, J.R. In pursuit of propulsion at the nanoscale. Soft Matter 2010, 6, 726–738. [Google Scholar] [CrossRef]
- Soler, L.; Sanchez, S. Catalytic nanomotors for environmental monitoring and water remediation. Nanoscale 2014, 6, 7175–7182. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, J. The Environmental Impact of Micro/Nanomachines: A Review. ACS Nano 2014, 8, 3170–3180. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Sengupta, S.; Duan, W.; Zhang, H.; Pavlick, R.; Sen, A. Intelligent, self-powered, drug delivery systems. Nanoscale 2013, 5, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.; Takada, T.; Tachibana, M.; Iijima, Y.; Shioi, A.; Yoshikawa, K. Micromotors working in water through artificial aerobic metabolism. Nanoscale 2015, 7, 13186–13190. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xiong, Y.; Zhao, M.; Mao, X.; Liu, Y.; Zhao, H.; Tang, Z. Bioinspired Synthesis of ZnS Supraparticles toward Photoinduced Dechlorination of 2,2′,4,4′,5,5′-Hexachlorobiphenyl. Chem. Asian J. 2013, 8, 1765–1767. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Weng, Z.; Tan, J.; Guo, J.; Wang, C. Hierarchically structured porous organic polymer microspheres with built-in Fe3O4 supraparticles: Construction of dual-level pores for Pt-catalyzed enantioselective hydrogenation. Polym. Chem. 2015, 6, 2892–2899. [Google Scholar] [CrossRef]
- Wang, D.; Möhwald, H. Template-directed colloidal self-assembly—The route to ‘top-down’ nanochemical engineering. J. Mater. Chem. 2004, 14, 459–468. [Google Scholar] [CrossRef]
- Renna, L.A.; Boyle, C.J.; Gehan, T.S.; Venkataraman, D. Polymer Nanoparticle Assemblies: A Versatile Route to Functional Mesostructures. Macromolecules 2015, 48, 6353–6368. [Google Scholar] [CrossRef]
- Kotov, N.A. (Ed.) Nanoparticle Assemblies and Superstructures; Taylor & Francis: Abingdon, UK, 2016. [Google Scholar]
- Kuemin, C.; Huckstadt, K.C.; Lörtscher, E.; Rey, A.; Decker, A.; Spencer, N.D.; Wolf, H. Selective Assembly of Sub-Micrometer Polymer Particles. Adv. Mater. 2010, 22, 2804–2808. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperling, M.; Gradzielski, M. Droplets, Evaporation and a Superhydrophobic Surface: Simple Tools for Guiding Colloidal Particles into Complex Materials. Gels 2017, 3, 15. https://doi.org/10.3390/gels3020015
Sperling M, Gradzielski M. Droplets, Evaporation and a Superhydrophobic Surface: Simple Tools for Guiding Colloidal Particles into Complex Materials. Gels. 2017; 3(2):15. https://doi.org/10.3390/gels3020015
Chicago/Turabian StyleSperling, Marcel, and Michael Gradzielski. 2017. "Droplets, Evaporation and a Superhydrophobic Surface: Simple Tools for Guiding Colloidal Particles into Complex Materials" Gels 3, no. 2: 15. https://doi.org/10.3390/gels3020015