A Bioactive Hydrogel and 3D Printed Polycaprolactone System for Bone Tissue Engineering
Abstract
:1. Introduction
2. Results
2.1. Hydrogel Preparation
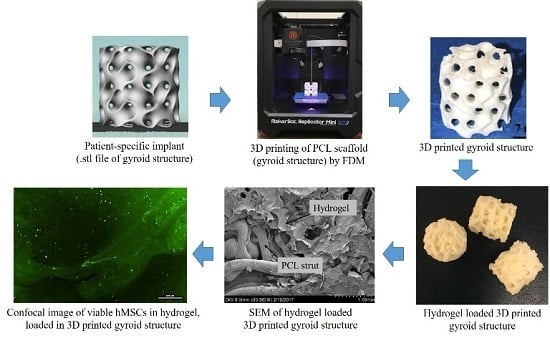
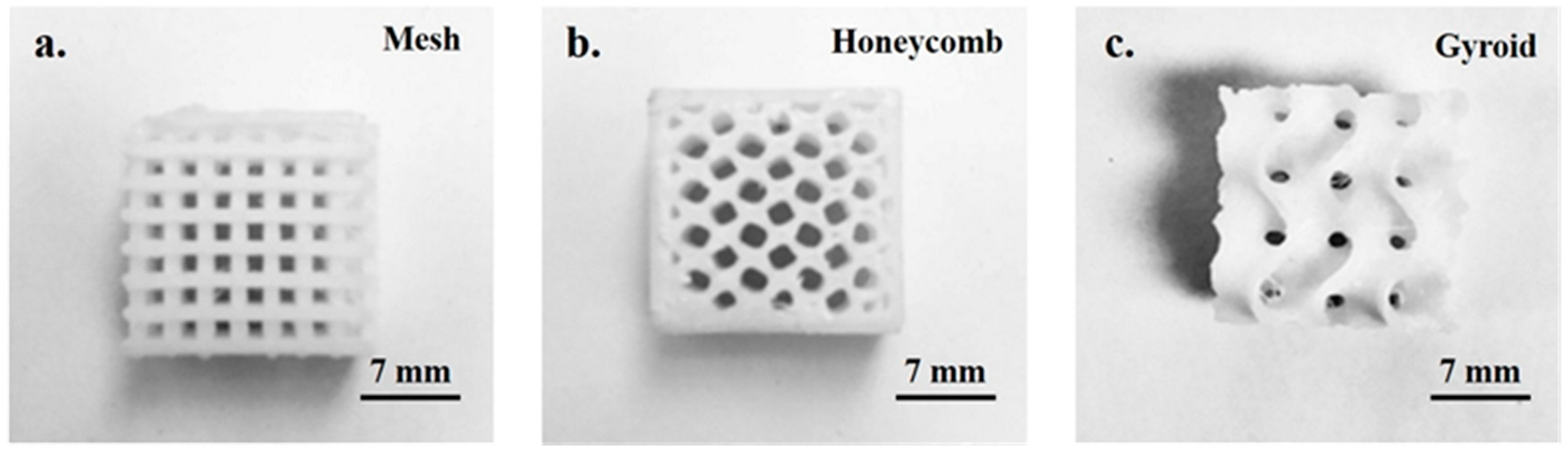
2.2. Rapid Fabrication of the 3D Printed PCL Scaffold
2.3. Hydrogel Retention Capacity of the PCL Scaffolds
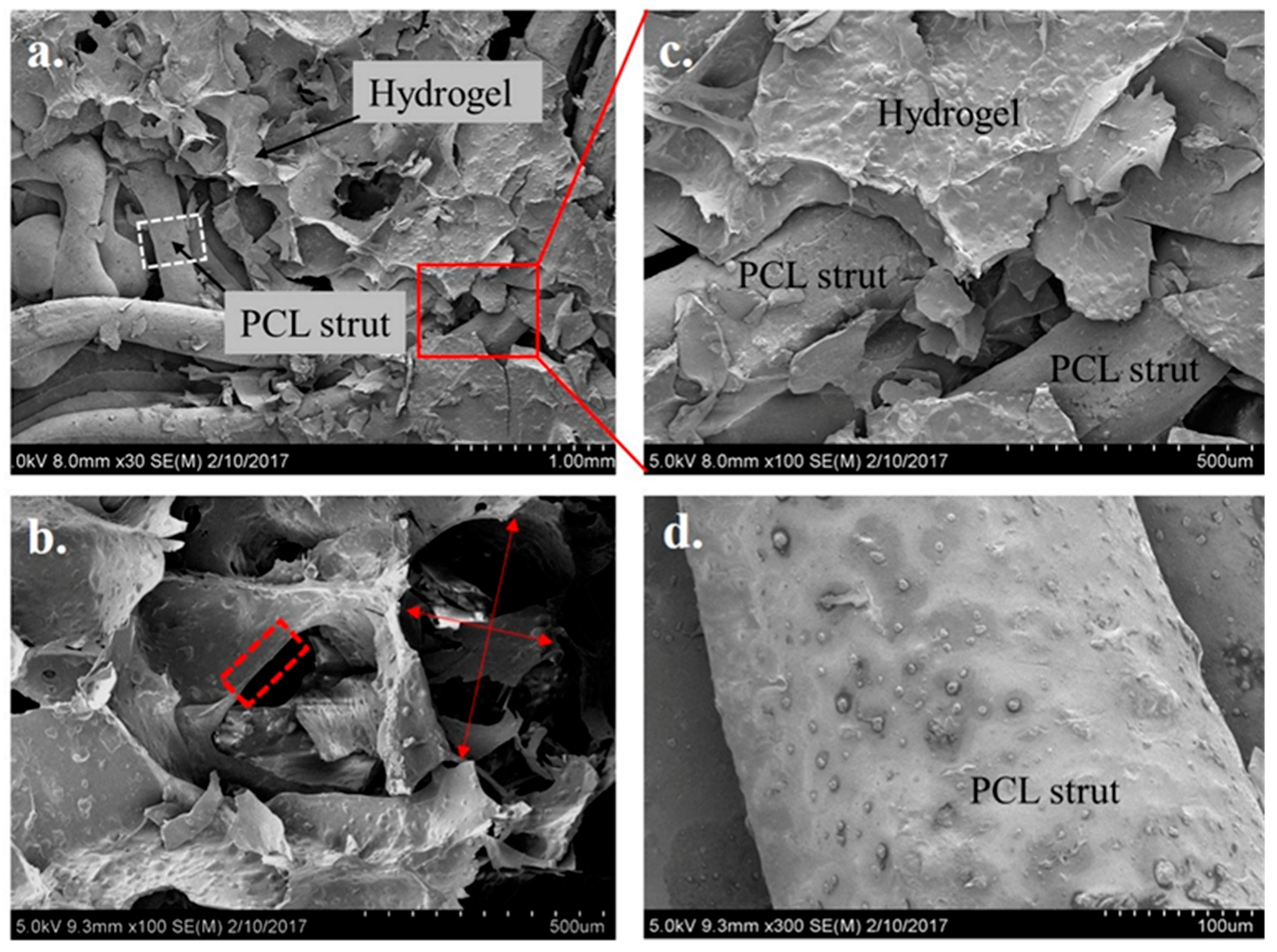
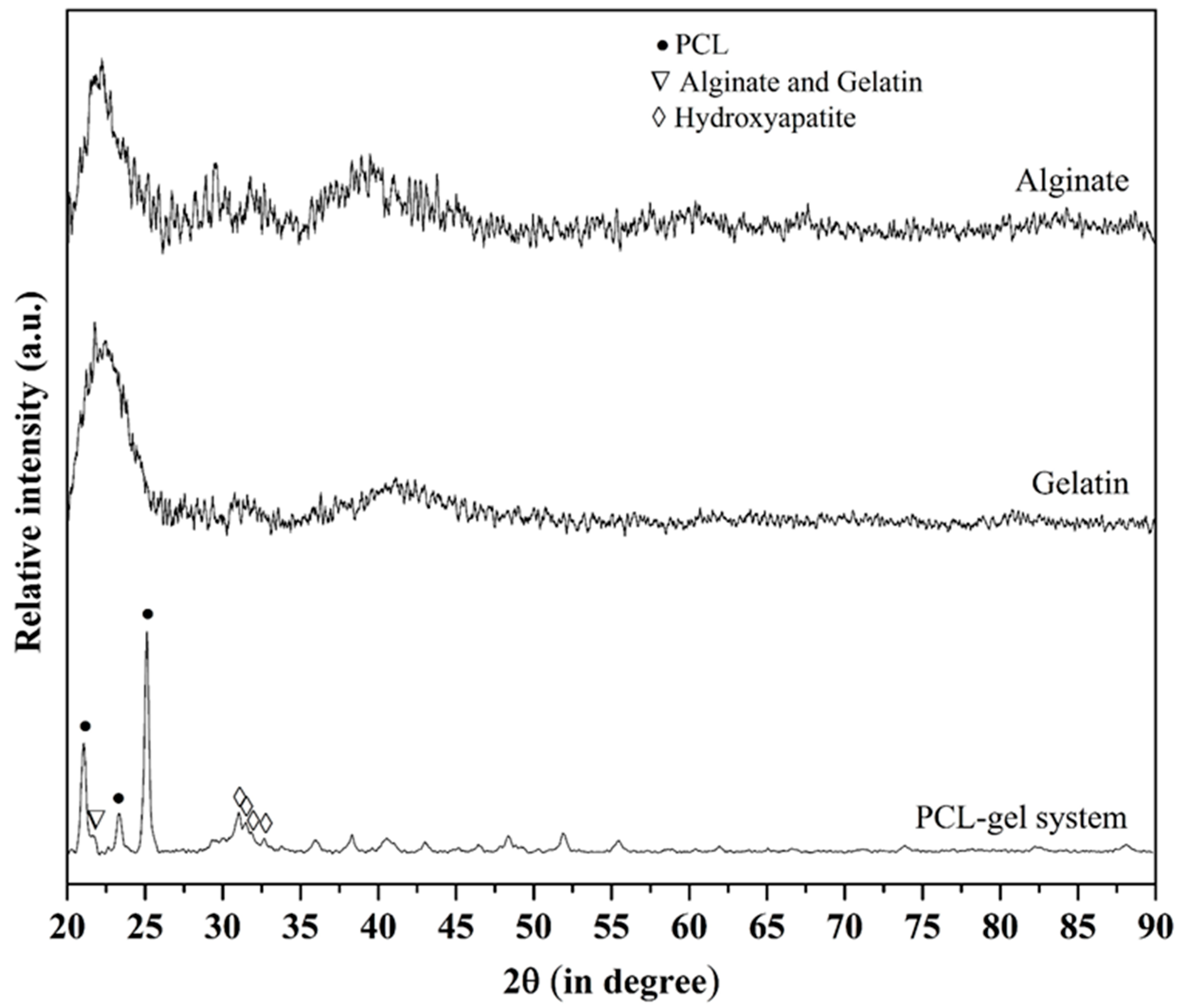
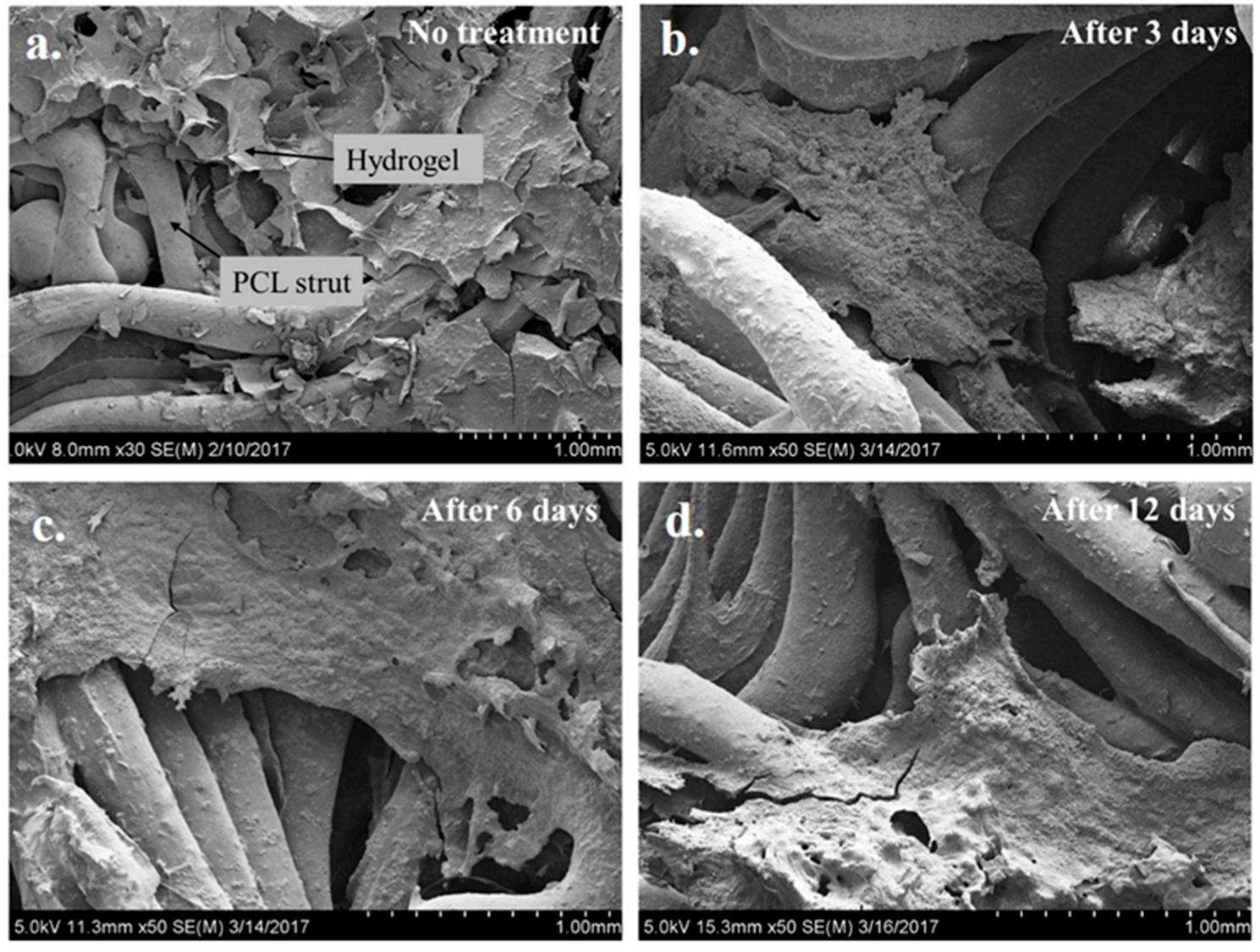
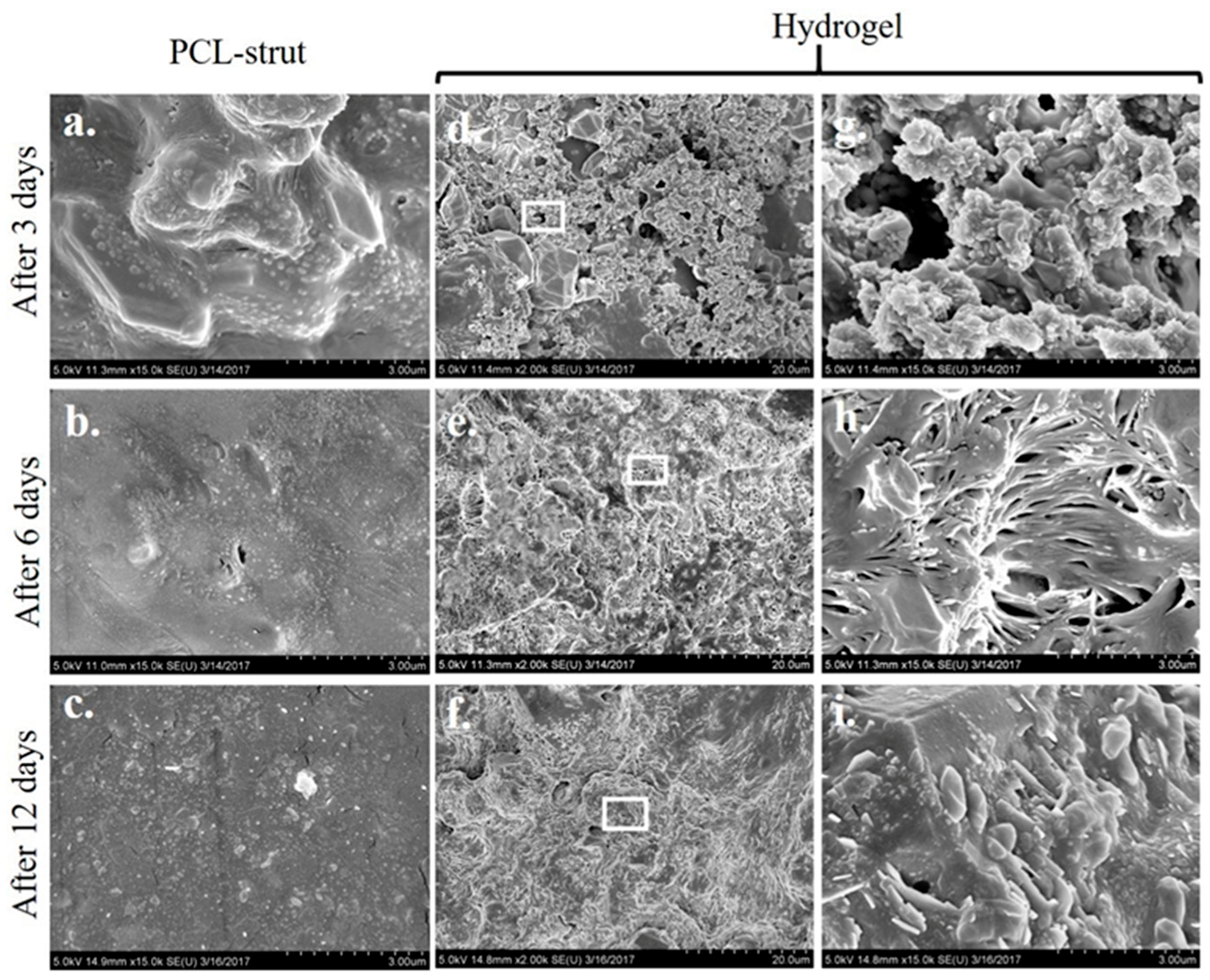
2.4. Microstructure Imaging and Characterization of Phases in the PCL Scaffold and Hydrogel System
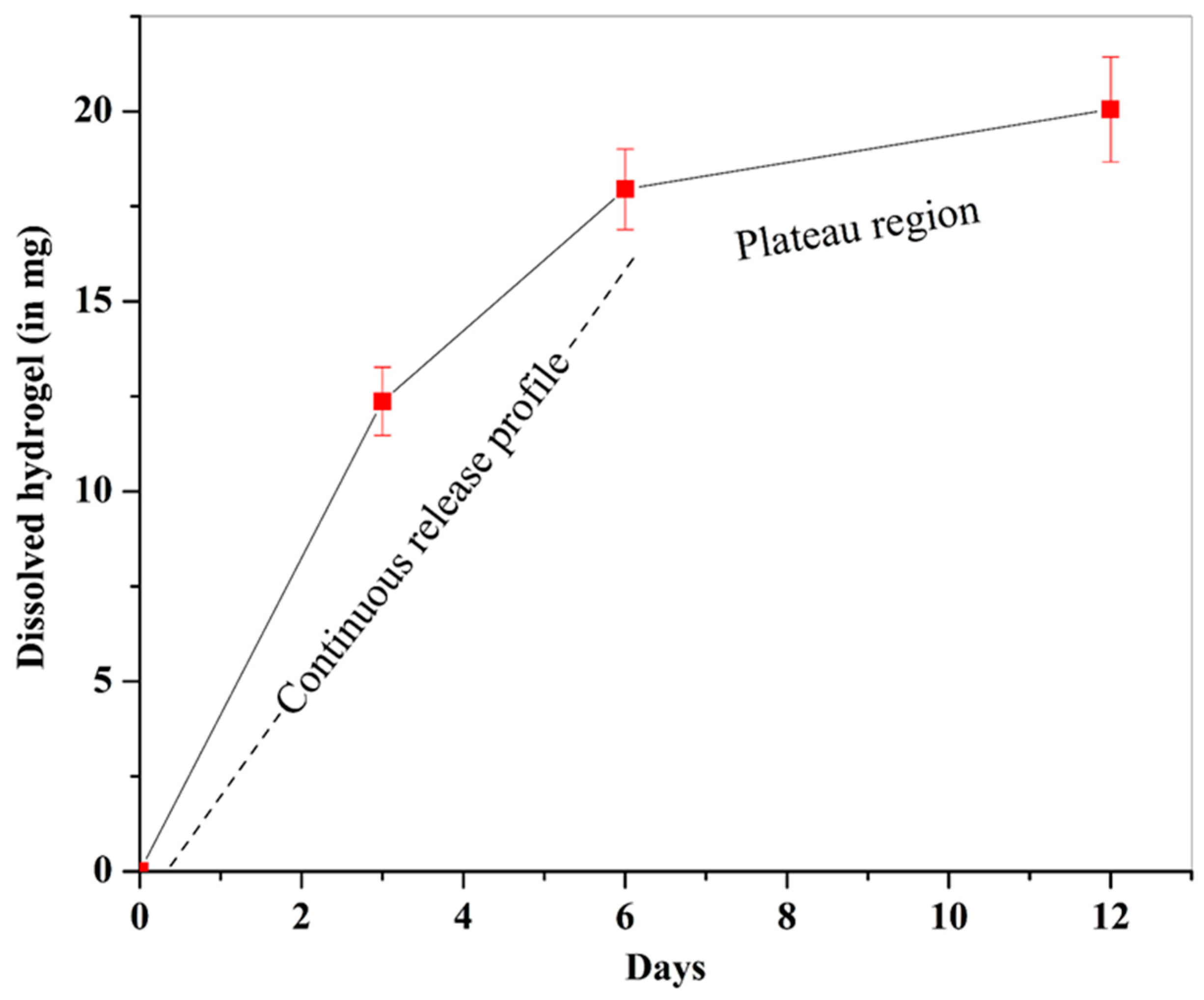
2.5. Sustained Dissolution of Hydrogel in Simulated Body Fluid (SBF)
2.6. Apatite Formation Ability of the PCL/Gel System
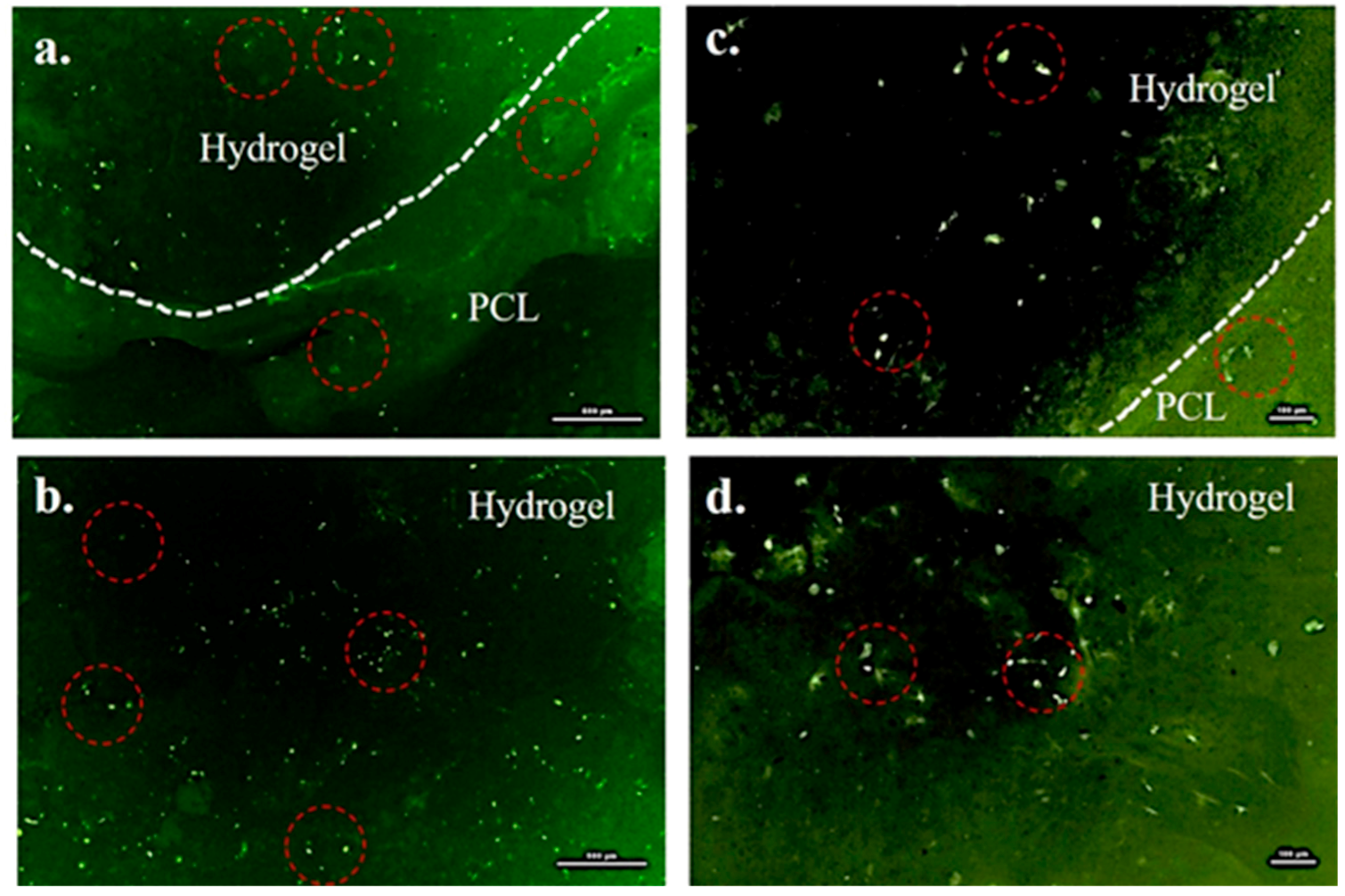
2.7. Cytocompatibility of the PCL/Gel System
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. 3D Printing of PCL Scaffold
5.3. Synthesis of Bioactive Hydrogel Infiltrated with hMSC
5.4. Formation of a Hybrid PCL/Hydrogel System
5.5. Cytocompatibility Assessment
5.6. Dissolution Study and Bioactivity Test
5.6.1. Calculation of the Dissolved Amount of Hydrogel
5.6.2. Apatite Formation on the PCL-Gel Samples
5.7. XRD and SEM Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lichte, P.; Pape, H.C.; Pufe, T.; Kobbe, P.; Fischer, H. Scaffolds for bone healing: Concepts, materials and evidence. Injury 2011, 42, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Brydone, D. Bone grafting, orthopaedic biomaterials and the clinical need for bone engineering. J. Eng. Med. 2010, 224, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, M.; Lorenzo, N.; Diana, C.; Massimo, I. New biomaterials for bone regeneration. Clin. Cases Miner. Bone Metab. 2011, 8, 21–24. [Google Scholar]
- Wang, Z.; Clark, C.C.; Brighton, C.T. Up-regulation of bone morphogenetic proteins in cultured murine bone cells with use of specific electric fields. J. Bone Jt. Surg. 2006, 88, 1053–1065. [Google Scholar] [CrossRef]
- Dupont, K.M.; Sharma, K.; Stevens, H.Y.; Boerckel, J.D.; García, A.J.; Guldberg, R.E. Human stem cell delivery for treatment of large segmental bone defects. Proc. Natl. Acad. Sci. USA 2010, 107, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Huang, Z.; Yu, M.; Zhang, Y.; Chen, H.; Ma, Z.; Liao, H.; Hu, J. An innovative approach for enhancing bone defect healing using plga scaffolds seeded with extracorporeal-shock-wave-treated bone marrow mesenchymal stem cells (BMSCS). Sci. Rep. 2017, 7, 44130. [Google Scholar] [CrossRef] [PubMed]
- Gugala, Z.; Gogolewski, S. Healing of critical-size segmental bone defects in the sheep tibiae using bioresorbable polylactide membranes. Injury 2002, 33, 71–76. [Google Scholar] [CrossRef]
- Yassin, M.A.; Leknes, K.N.; Pedersen, T.O.; Xing, Z.; Sun, Y.; Lie, S.A.; Finne-Wistrand, A.; Mustafa, K. Cell seeding density is a critical determinant for copolymer scaffolds-induced bone regeneration. J. Biomed. Mater. Res. A 2015, 103, 3649–3658. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Hou, T.-Y.; Zhang, Z.-H.; Xie, Z.; Wu, X.-H.; Xu, J.-Z. Effects of initial cell density and hydrodynamic culture on osteogenic activity of tissue-engineered bone grafts. PLoS ONE 2013, 8, e53697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Weir, M.D.; Xu, H.H.K. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 2010, 31, 6502–6510. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, S.; Barui, S.; Vasireddi, R.; Gbureck, U.; Gelinsky, M.; Basu, B. Low temperature additive manufacturing of three dimensional scaffolds for bone-tissue engineering applications: Processing related challenges and property assessment. Mater. Sci. Eng. R. Rep. 2016, 103, 1–39. [Google Scholar] [CrossRef]
- Mercado-Pagán, Á.E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in bone tissue engineering constructs. Ann. Biomed. Eng. 2015, 43, 718–729. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Engbers, G.H.M.; Krijgsveld, J.; Zaat, S.A.J.; Dankert, J.; Feijen, J. Cross-Linking and characterisation of gelatin matrices for biomedical applications. J. Biomater. Sci. Polym. Ed. 2000, 11, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, A.J.; Engbers, G.H.M.; Feijen, J.; de Smedt, S.C.; Meyvis, T.K.L.; Demeester, J.; Krijgsveld, J.; Zaat, S.A.J.; Dankert, J. Characterization of the network structure of carbodiimide cross-linked gelatin gels. Macromolecules 1999, 32, 3325–3333. [Google Scholar] [CrossRef]
- Kokubo, T. Bioceramics and their Clinical Applications; Woodhead Publishing Limited: Cambridge, UK, 2008. [Google Scholar]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 38. [Google Scholar] [CrossRef]
- Holy, C.E.; Shoichet, M.S.; Davies, J.E. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: Investigating initial cell-seeding density and culture period. J. Biomed. Mater. Res. 2000, 51, 376–382. [Google Scholar] [CrossRef]
- Tutak, W.; Kaufman, G.; Gelven, G.; Markle, C.; Maczka, C. Uniform, fast, high concentration delivery of bone marrow stromal cells and gingival fibroblasts by gas-brushing. Biomed. Phys. Eng. Exp. 2016, 2, 35007. [Google Scholar] [CrossRef]
- Wong, R.S.H.; Ashton, M.; Dodou, K. Effect of crosslinking agent concentration on the properties of unmedicated hydrogels. Pharmaceutics 2015, 7, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Hong, Y.; Huber, A.; Takanari, K.; Amoroso, N.J.; Hashizume, R.; Badylak, S.F.; Wagner, W.R. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber–extracellular matrix hydrogel biohybrid scaffold. Biomaterials 2011, 32, 3387–3394. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Burastero, G.; Scarfì, S.; Ferraris, C.; Fresia, C.; Sessarego, N.; Fruscione, F.; Monetti, F.; Scarfò, F.; Schupbach, P.; Podestà, M. The association of human mesenchymal stem cells with bmp-7 improves bone regeneration of critical-size segmental bone defects in athymic rats. Bone 2010, 47, 117–126. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Development of nanocomposites for bone grafting. Compos. Sci. Technol. 2005, 65, 2385–2406. [Google Scholar] [CrossRef]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Rela. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Oonishi, H.; Yamamoto, M.; Ishimaru, H.; Tsuji, E.; Kushitani, S.; Aono, M.; Ukon, Y. The effect of hydroxyapatite coating on bone growth into porous titanium alloy implants. Bone Jt. J. 1989, 71, 213–216. [Google Scholar]
- Kumar, A.; Dhara, S.; Biswas, K.; Basu, B. In vitro bioactivity and cytocompatibility properties of spark plasma sintered Ha-ti composites. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101B, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Webster, T.J.; Biswas, K.; Basu, B. Flow cytometry analysis of human fetal osteoblast fate processes on spark plasma sintered hydroxyapatite–titanium biocomposites. J. Biomed. Mater. Res. A 2013, 101, 2925–2938. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nune, K.C.; Basu, B.; Misra, R.D.K. Mechanistic contribution of electroconductive hydroxyapatite–titanium disilicide composite on the alignment and proliferation of cells. J. Biomater. Appl. 2016, 30, 1505–1516. [Google Scholar] [CrossRef]
- Agulhon, P.; Markova, V.; Robitzer, M.; Quignard, F.O.; Mineva, T. Structure of alginate gels: Interaction of diuronate units with divalent cations from density functional calculations. Biomacromolecules 2012, 13, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Wu, S.-C.; Chang, W.-H.; Dong, G.-C.; Chen, K.-Y.; Chen, Y.-S.; Yao, C.-H. Cell adhesion and proliferation enhancement by gelatin nanofiber scaffolds. J. Bioact. Compat. Polym. 2011, 26, 565–577. [Google Scholar] [CrossRef]
- Rammohan, A.V.; Lee, T.; Tan, V.B.C. A novel morphological model of trabecular bone based on the gyroid. Int. J. Appl. Mech. 2015, 7, 1550048. [Google Scholar] [CrossRef]
- Yan, C.; Hao, L.; Hussein, A.; Young, P. Ti–6al–4v triply periodic minimal surface structures for bone implants fabricated via selective laser melting. J. Mech. Behav. Biomed. Mater. 2015, 51, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Yánez, A.; Herrera, A.; Martel, O.; Monopoli, D.; Afonso, H. Compressive behaviour of gyroid lattice structures for human cancellous bone implant applications. Mater. Sci. Eng. C 2016, 68, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.O.; Vorwald, C.E.; Dreher, M.L.; Mott, E.J.; Cheng, M.H.; Cinar, A.; Mehdizadeh, H.; Somo, S.; Dean, D.; Brey, E.M. Evaluating 3D-printed biomaterials as scaffolds for vascularized bone tissue engineering. Adv. Mater. 2015, 27, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Hafezi, M.; Nezafati, N.; Heasarki, S.; Nadernezhad, A.; Ghazanfari, S.M.H.; Sepantafar, M. Bioinorganics in bioactive calcium silicate ceramics for bone tissue repair: Bioactivity and biological properties. J. Ceram. Sci. Technol. 2014, 5, 1–12. [Google Scholar]
- Ichibouji, T.; Miyazaki, T.; Ishida, E.; Sugino, A.; Ohtsuki, C. Apatite mineralization abilities and mechanical properties of covalently cross-linked pectin hydrogels. Mater. Sci. Eng. C 2009, 29, 1765–1769. [Google Scholar] [CrossRef]
- Heo, S.J.; Kim, S.E.; Wei, J.; Hyun, Y.T.; Yun, H.S.; Kim, D.H.; Shin, J.W.; Shin, J.W. Fabrication and characterization of novel nano-and micro-ha/pcl composite scaffolds using a modified rapid prototyping process. J. Biomed. Mater. Res. A 2009, 89, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Eglin, D.; Maalheem, S.; Livage, J.; Coradin, T. In vitro apatite forming ability of type i collagen hydrogels containing bioactive glass and silica sol-gel particles. J. Mater. Sci. Mater. Med. 2006, 17, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Hyun-Min, K.I.M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Apatite-forming ability of alkali-treated ti metal in body environment. J. Ceram. Soc. Jpn. 1997, 105, 111–116. [Google Scholar]
- Müller, P.; Bulnheim, U.; Diener, A.; Lüthen, F.; Teller, M.; Klinkenberg, E.D.; Neumann, H.G.; Nebe, B.; Liebold, A.; Steinhoff, G. Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells. J. Cell. Mol. Med. 2008, 12, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Grayson, W.L.; Ma, T.; Bunnell, B.; Lu, W.W. Effects of hydroxyapatite in 3-D chitosan–gelatin polymer network on human mesenchymal stem cell construct development. Biomaterials 2006, 27, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.L.; Xu, H.H.K. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate–chitosan composite scaffold. Biomaterials 2009, 30, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Tagai, H.; Aoki, H. Preparation of Synthetic Hydroxyapatite and Sintering of Apatite Ceramics. In Mechanical Properties of Biomaterials, Proceeding of Third Conference on Materials for Use in Medicine and Biology: Mechanical Properties of Biomaterials, Keele University, Keele, UK, 13–15 September 1978; Hastings, G.W., Williams, D.F., Eds.; Wiley: Chichester, UK, 1980. [Google Scholar]
- Kumar, A.; Biswas, K.; Basu, B. On the toughness enhancement in hydroxyapatite-based composites. Acta Mater. 2013, 61, 5198–5215. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. A 2003, 65, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Nune, K.C.; Misra, R.D.K. The functional response of alginate-gelatin-nanocrystalline cellulose injectable hydrogels toward delivery of cells and bioactive molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, I.; Kumar, A.; Joddar, B. A Bioactive Hydrogel and 3D Printed Polycaprolactone System for Bone Tissue Engineering. Gels 2017, 3, 26. https://doi.org/10.3390/gels3030026
Hernandez I, Kumar A, Joddar B. A Bioactive Hydrogel and 3D Printed Polycaprolactone System for Bone Tissue Engineering. Gels. 2017; 3(3):26. https://doi.org/10.3390/gels3030026
Chicago/Turabian StyleHernandez, Ivan, Alok Kumar, and Binata Joddar. 2017. "A Bioactive Hydrogel and 3D Printed Polycaprolactone System for Bone Tissue Engineering" Gels 3, no. 3: 26. https://doi.org/10.3390/gels3030026