Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives
Abstract
:1. Introduction
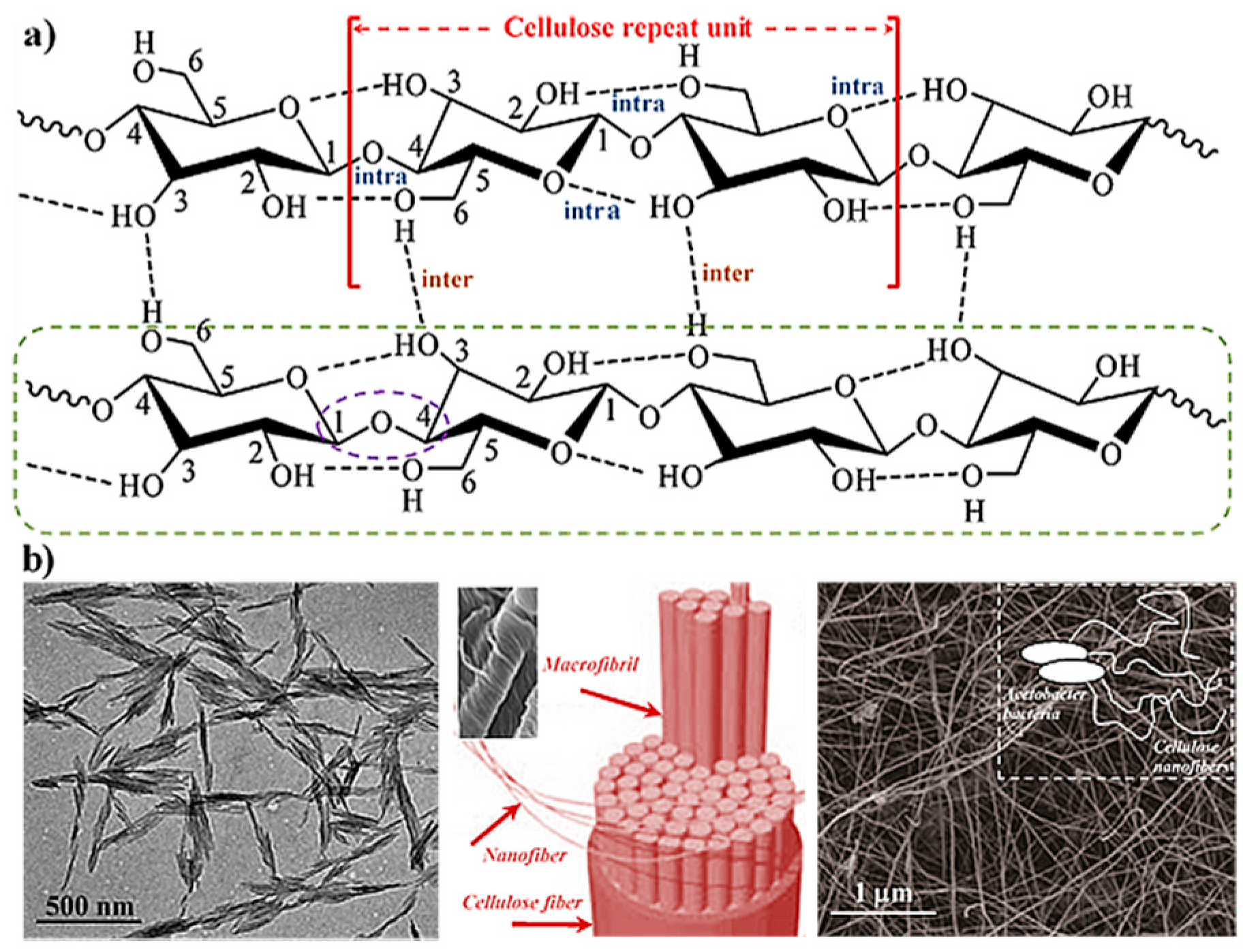
2. Hydrogels Derived from Cellulose
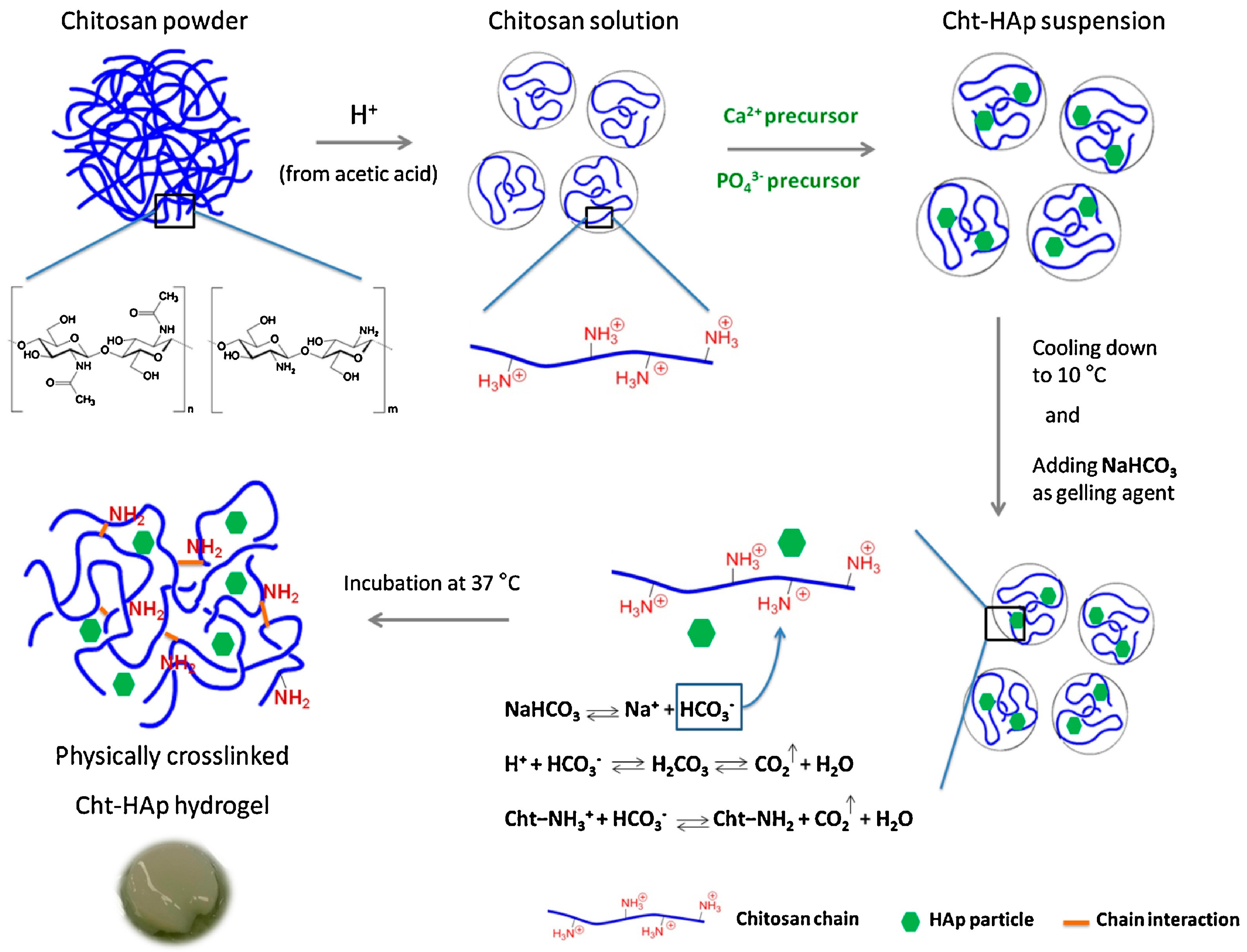
3. Hydrogels Derived from Chitosan
4. Hydrogels Derived from Collagen and Gelatin
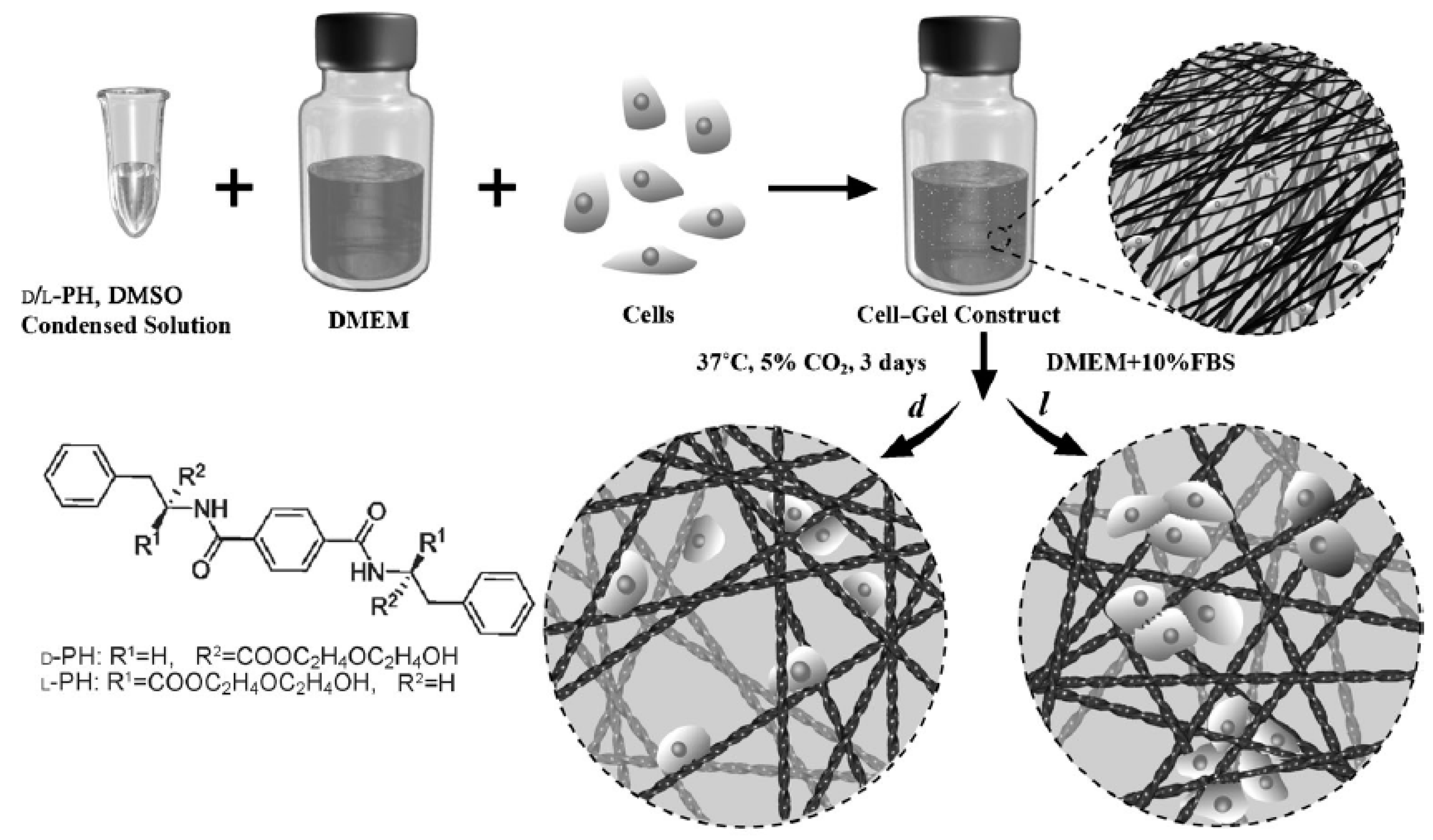
5. Peptide Hydrogels
6. Responsiveness to External Stimuli of Peptide-Derived Hydrogels for 3D Cell Culture
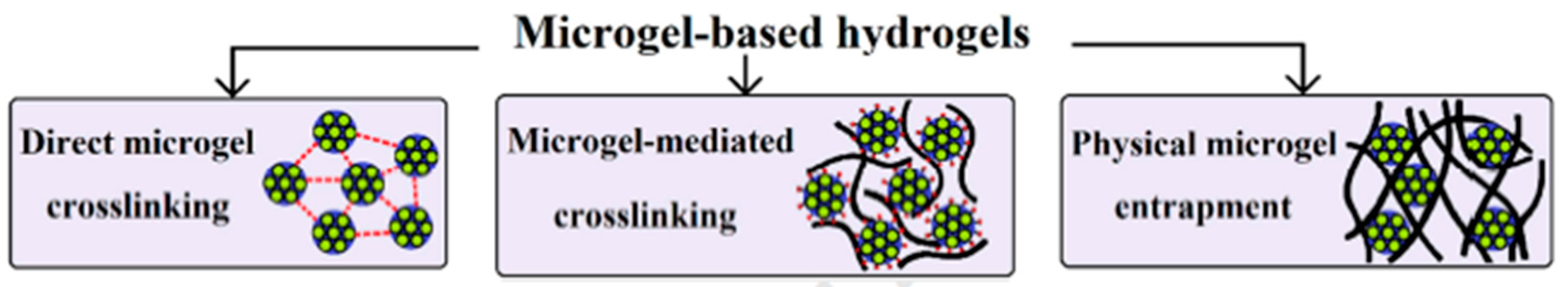
7. Micro- and Nanogels
8. Shape Memory Hydrogels
9. Molecularly Imprinted Hydrogels
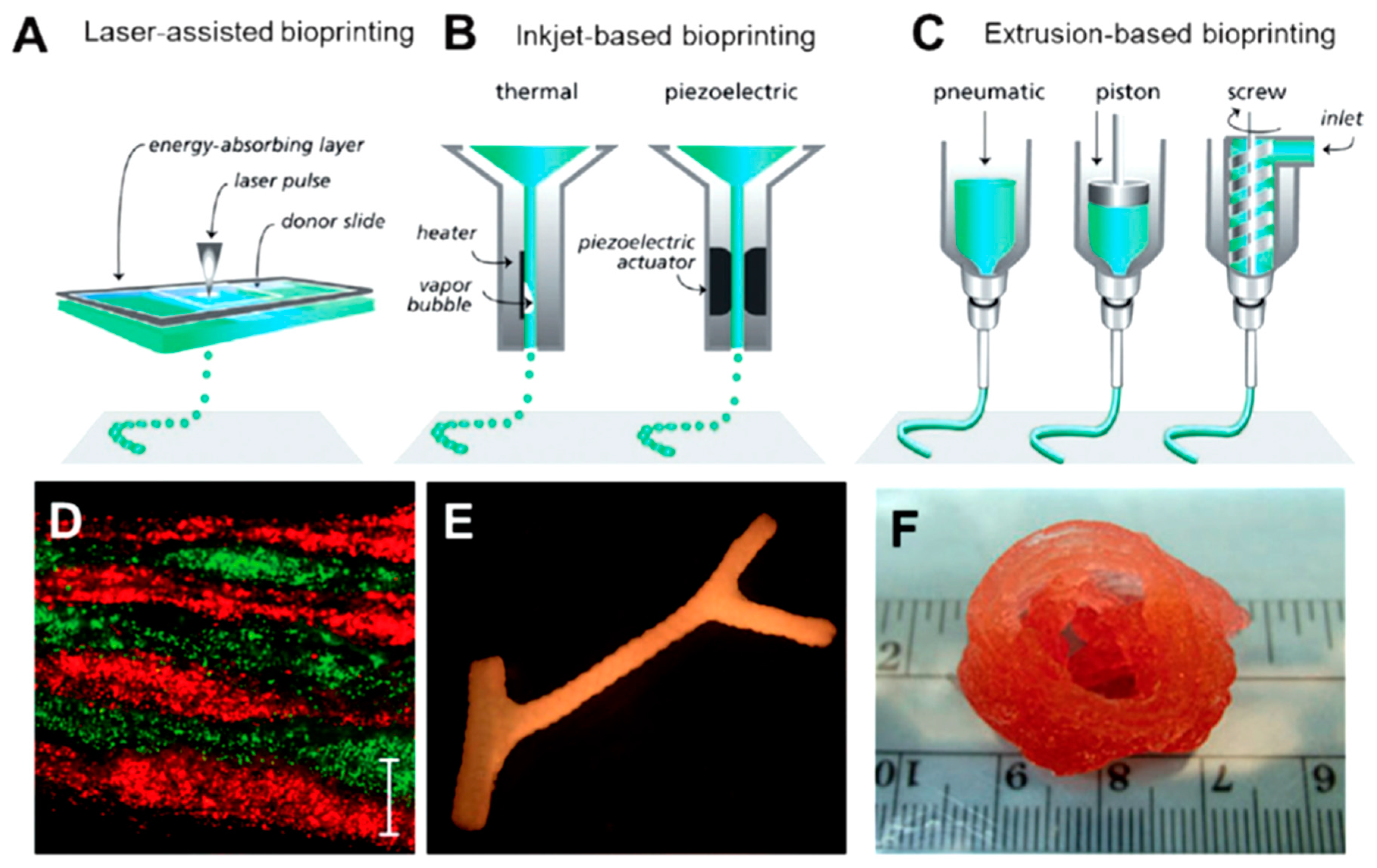
10. Protein-Based Hydrogels for 3D Printing
11. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wichterle, O.; Lím, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Buwalda, S.; Boere, J.K.; Dijksra, P.; Fiejen, J.; Vermoden, T.; Hennink, W. Hydrogels in an historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropylmethylcellulose/glicerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Moreira Teixeira, L.S.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Gyles, D.A.; Castro, L.D.; Carréra Silva, J.O.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Das, N. Preparation methods and properties of hydrogel: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 112–117. [Google Scholar]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Bae, K.H.; Wang, L.S.; Kurisawa, M. Injectable biodegradable hydrogels: Progress and challenges. J. Mater. Chem. B 2013, 1, 5371–5388. [Google Scholar] [CrossRef]
- George, M.; Weiss, R.G. Molecular organogels. Soft matter comprised of low-molecular-mass organic gelators and organic liquids. Acc. Chem. Res. 2006, 39, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Function and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Uchida, K.; Kaneko, Y.; Sakai, K.; Kikuchi, A.; Sakurai, Y.; Okano, T. Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature 1995, 374, 240–242. [Google Scholar] [CrossRef]
- Du, X.W.; Zhou, J.; Xu, B. Supramolecular hydrogels made of basic biological building blocks. Chem. Asian J. 2014, 9, 1446–1472. [Google Scholar] [CrossRef] [PubMed]
- Bakota, E.L.; Sensoy, O.; Ozgur, B.; Sayar, M.; Hartgerink, J.D. Self-assembling multidomain peptide fibers with aromatic cores. Biomacromolecules 2013, 14, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Cheng, W.; Fan, H.; Pei, G. Reconstruction of goat tibial defects usingan injectable tricalcium phosphate/chitosan in combination with autologousplatelet-rich plasma. Biomaterials 2010, 31, 3201–3211. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, Q.; Chen, X.; Sun, Y.; Xu, Q.; Denq, P.; Hu, F.; Yanq, J. Accelerated bony defect healing based on chitosan thermosensitive hydrogel scaffolds embedded with chitosan nanoparticles for the delivery of BMP2 plasmid DNA. J. Biomed. Mater. Res. A 2017, 105A, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Amini, A.A.; Nair, L.S. Injectable hydrogels for bone and cartilage repair. Biomed. Mater. 2012, 7, 24105–24118. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Mishima, S. Steric stabilization of “charge-free” cellulose nanowhiskers by grafting of poly(ethylene glycol). Molecules 2015, 20, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.K.; Pant, H.R.; Tiwari, A.P.; Maharjan, B.; Liao, N.; kim, H.J.; Park, C.H.; Kim, C.S. Three-dimensional cellulose sponge: Fabrication, characterization, biomimetic mineralization, and in vitro cell infiltration. Carbohydr. Polym. 2016, 136, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- Ko, H.F.; Sfeir, C.; Kumta, P.N. Novel synthesis strategies for natural polymer and composite biomaterials as potential scaffolds for tissue engineering. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 1981–1997. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, L.N.; Chang, C.Y.; Cheng, G.Z.; Chen, X.M.; Chu, B.J. Hydrogen-bond-induced inclusion complex in aqueous cellulose/LiOH/urea solution at low temperature. Chem. Phys. Chem. 2007, 8, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, L.N.; Liu, S.L.; Liu, Y.T.; Xu, X.J.; Chen, X.M.; Chu, B.; Guo, X.; Cheng, H.; Han, C.C.; et al. Dynamic self-assembly induced rapid dissolution of cellulose at low temperatures. Macromolecules 2008, 41, 9345–9351. [Google Scholar] [CrossRef]
- Sun, B.Z.; Duan, L.; Peng, G.G.; Li, X.X.; Xu, A.H. Efficient production of glucose by microwave-assisted acid hydrolysis of cellulose hydrogel. Bioresour. Technol. 2015, 192, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Zhang, L.N.; Mao, Y.; Zeng, M.; Li, X.B. Microporous membranes prepared from cellulose in NaOH/thiourea aqueous solution. J. Membr. Sci. 2004, 241, 265–274. [Google Scholar] [CrossRef]
- Takigami, M.; Amada, H.; Nagasawa, N.; Yagi, T.; Kasahara, T.; Takigami, S.; Tamada, M. Preparation and properties of CMC gel. Trans. Mater. Res. Soc. Jpn. 2007, 32, 713–716. [Google Scholar]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Pranger, L.; Tannenbaum, R. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Macromolecules 2008, 41, 8682–8687. [Google Scholar] [CrossRef]
- Čolić, M.; Mihajlović, D.; Mathew, A.; Naser, N.; Kokol, V. Cytocompatibility and immunomodulatory properties of wood based nanofibrillated cellulose. Cellulose 2015, 22, 763–778. [Google Scholar] [CrossRef]
- Grimm, S.; Giesa, R.; Sklarek, K.; Langner, A.; Gosele, U.; Schmidt, H.W.; Steinhart, M. Nondestructive replication of self-ordered nanoporous alumina membranes via cross-linked polyacrylate nanofiber arrays. Nano Lett. 2008, 8, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Korpinen, R.; Mikkonen, K.; Willför, S.; Xu, C. Nanofibrillated cellulose originated from birch sawdust after sequential extractions: A promising polymeric material from waste to films. Cellulose 2014, 21, 2587–2598. [Google Scholar] [CrossRef]
- Syverud, K.; Pettersen, S.; Draget, K.; Chinga-Carrasco, G. Controlling the elastic modulus of cellulose nanofibril hydrogels—Scaffolds with potential in tissue engineering. Cellulose 2015, 22, 473–481. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; Chang, P.R.; Li, J.; Chen, Y.; Wang, D.; Yu, J.; Chen, J. Structure and properties of polysaccharide nanocrystal-doped supramolecular hydrogels based on cyclodextrin inclusion. Polymer 2010, 51, 4398–4407. [Google Scholar] [CrossRef]
- Lin, N.; Bruzzese, C.; Dufresne, A. Tempo-oxidized nanocellulose participating as crosslinking aid for alginate-based sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.M.; Gomes, M.E.; Reis, R.L. The potencial of cellulose nanocrystals in tissue engineering strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Nune, K.; Misra, R. The functional response of alginate-gelatin-nanocrystalline cellulose injectable hydrogels toward delivery of cells and bioactive molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Deepa, B.; Mathew, A.P.; Oksman, K.; Girandon, L. Nanocellulose-based interpenetrating polymer network (IPN) hydrogels for cartilage applications. Biomacromolecules 2016, 17, 3714–3723. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, N.; Huang, J.; Dufresne, A. Highly alkynyl-functionalization ofcellulose nanocrystals and advanced nanocomposites thereof via click chemistry. Polym. Chem. 2015, 6, 4385–4395. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Silva, M.; Gershovich, P.; Betta, S.; Babo, P.; Caridade, S.G.; Mano, J.F.; Motta, A.; Reis, R.L.; Gomes, M.E. Development of injectable hyaluronic acid/cellulose nanocrystals bionanocomposite hydrogels for tissue engineering applications. Bioconjugate Chem. 2015, 26, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- García-Astrain, C.; González, K.; Gurrea, T.; Guaresti, O.; Algar, I.; Eceiza, A.; Gabilondo, N. Maleimide-grafted cellulose nanocrystals as cross-linkers for bionanocomposite hydrogels. Carbohydr. Polym. 2016, 149, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Stella, J.A.; D’Amore, A.; Wagner, W.R.; Sacks, M.S. On the biomechanical function of scaffolds for engineering load-bearing soft tissues. Acta Biomater. 2010, 6, 2365–2381. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.R.; Kuisma, S.W.; Kanninen, L.; Lille, M.; Corlu, A.; GuGuen-Guillouzo, C.; Ikkala, O.; et al. Nanofibrillar cellulose hidrogel promotes three-dimensional liver cell culture. J. Control. Release 2012, 164, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Malinen, M.M.; Kanninen, L.K.; Corlu, A.; Isoniemi, H.M.; Lou, Y.-R.; Yliperttula, M.L.; Urtti, A.O. Differentiation of liver progenitor cell line to functional organotypic cultures in 3D nanofibrillar cellulose and hyaluronan-gelatin hydrogels. Biomaterials 2014, 35, 5110–5121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertaniemi, H.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Gandía, C.; Mäkitie, A.; Partanen, J.; Ikkala, O.; Yliperttula, M. Human stem cell decorated nanocellulose threads for biomedical applications. Biomaterials 2016, 82, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Liu, J.; Chinga-Carrasco, G.; Cheng, F.; Xu, W.; Willför, S.; Syverud, K.; Xu, C. Hemicellulose-reinforced nanocellulose hydrogels for wound healing application. Cellulose 2016, 23, 3129–3143. [Google Scholar] [CrossRef]
- Park, M.; Chang, H.; Jeong, D.H.; Hyun, J. Spatial deformation of nanocellulose hydrogel enhances SERS. Biochip J. 2013, 7, 234–241. [Google Scholar] [CrossRef]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterialcellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.; Khan, S.; Ullah, M.W.; Park, J.K. Bacterial cellulosecomposites: Synthetic strategies and multiple applications in bio-medical and electroconductive fields. Biotechnol. J. 2015, 10, 1847–1861. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Shin, S.; Cheng, J.; Hyun, J. Nanocellulose based asymmetric composite membrane for the multiple functions in cell encapsulation. Carbohydr. Polym. 2017, 158, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, S.; Shen, Y.-I.; Hanjaya-Putra, D.; Mali, P.; Cheng, L.; Gerecht, S. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc. Natl. Acad. Sci. USA 2013, 110, 12601–12606. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Portal, O.; Clark, W.A.; Levinson, D.J. Microbial cellulose wound dressing in the treatment of nonhealing lower extremity ulcers. Wounds 2009, 21, 1–3. [Google Scholar] [PubMed]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Int. Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoparticle- and Microparticle-Based Delivery Systems; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Berthold, A.; Cremer, K.; Kreuter, J. Preparation and characterization of chitosan microspheres as drug carrier for prednisolone sodium phosphate as model for anti-inflammatory drugs. J. Control. Release 1996, 39, 17–25. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Sudheesh Kumar, P.T.; Deepthai, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chtin: Fabrication, properties and applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef]
- Muramatsu, K.; Masuda, S.; Yoshihara, S.; Fujisawa, A. In vitro degradation behavior of freeze-dried carboxymethyl-chitin sponges processed by vacuum-heating and gamma irradiation. Polym. Degrad. Stabil. 2003, 81, 327–332. [Google Scholar] [CrossRef]
- Suzuki, D.; Takahashi, M.; Abe, M.; Sarukawa, J.; Tamura, H.; Tokura, S.; Kurahashi, Y.; Nagano, A. Comparison of various mixtures of β-chitin and chitosan as a scaffold for three-dimensional culture of rabbit chondrocytes. J. Mater. Sci. Mater. Med. 2008, 19, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, R.; Vitello, M.P.; Leitermann, F.; Syldatk, C. Advances in the production of sponge biomass Aplysina aerophoba—A model sponge for ex situ sponge biomass production. J. Biotechnol. 2006, 124, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nagahama, H.; Tokura, S. Preparation of chitin hydrogel under mild conditions. Cellulose 2006, 13, 357–364. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Arunraj, T.; Rejinold, N.S.; Kumar, N.A.; Jayakumar, R. Doxorubicin–chitin–poly(caprolactone) composite nanogel for drug delivery. Int. J. Biol. Macromol. 2013, 62, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.; Rejinold, N.S.; Mangalathillam, S.; Biswas, R.; Nair, S.V.; Jayakumar, R. Fluconazole loaded chitin nanogels as a topical ocular drug delivery agent for corneal fungal infections. J. Biomed. Nanotech. 2013, 9, 1521–1531. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Jayakumar, R. Multifunctional chitin nanogels for simultaneous drug delivery, bioimaging and biosensing. ACS Appl. Mater. Interfaces 2011, 3, 3654–3665. [Google Scholar] [CrossRef] [PubMed]
- Vishnu Priya, M.; Sabitha, M.; Jayakumar, R. Colloidal chitin nanogels: A pletora of applications under one shell. Carbohydr. Polym. 2016, 136, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Agarwal, A.; Negi, Y.S. Review: Chitosan based hydrogel polymeric beads—As drug delivery system. BioResources 2010, 5, 2765–2807. [Google Scholar]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 63, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, M.K.; Jain, S.K. Polysaccharides for colon targeted drug delivery. Drug Deliv. 2004, 11, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, K.; Yoshida, M.; Higaki, K.; Kimura, T.; Shiraishi, K.; Nishikawa, M.; Takakura, Y.; Hashida, M. Hepatic uptake of polystyrene microspheres in rats: Effect of particle size on intrahepatic distribution. J. Control. Release 1999, 59, 15–22. [Google Scholar] [CrossRef]
- Kato, Y.; Onishi, H.; Machida, Y. Biological characteristics of lactosaminated N-succinyl-chitosan as a liver-specific drug carrier in mice. J. Control. Release 2001, 70, 295–307. [Google Scholar] [CrossRef]
- Ruel-Gariepy, E.; Shive, M.; Bichara, A.; Berrada, M.; Le Garrec, D.; Chenite, A.; Leroux, J.C. A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur. J. Pharm. Biopharm. 2004, 57, 53–63. [Google Scholar] [CrossRef]
- Obara, K.; Ishihara, M.; Ozeki, Y.; Ishizuka, T.; Hayashi, T.; Nakamura, S.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; et al. Controlled release of paclitaxel from photocrosslinked chitosan hydrogels and its subsequent effect on subcutaneous tumor growth in mice. J. Control. Release 2005, 110, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Boardman, S.J.; Lad, R.; Green, D.C.; Thornton, P.D. Chitosan hydrogels for targeted dye and protein adsorption. J. Appl. Polym. Sci. 2017, 134, 44846. [Google Scholar] [CrossRef]
- Cao, L.; Cao, B.; Lu, C.; Wang, G.; Yu, L.; Ding, J. An injectable hydrogelformed by in situ cross-linking of glycol chitosan and multi-benzaldehydefunctionalized PEG analogues for cartilage tissue engineering. J. Mater. Chem. B 2015, 3, 1268–1280. [Google Scholar] [CrossRef]
- Yasmeen, S.; Lo, M.K.; Bajracharya, S.; Roldo, M. Injectable scaffolds for bone regeneration. Langmuir 2014, 30, 12977–12985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenite, A.; Buschmann, M.; Wang, D.; Chaput, C.; Kandani, N. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Songkroh, T.; Xie, H.; Yu, W.; Liu, X.; Sun, G.; Xu, X.; Ma, X. Injectable in situ forming chitosan-based hydrogels for curcumin delivery. Macromol. Res. 2015, 23, 53–559. [Google Scholar] [CrossRef]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.K.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.; Ganesh, N.; Selvamurugan, N.; Nair, S.V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation and characterization ofchitosan-gelatin/nanohydroxyapatite composite scaffolds for tissue engineering applications. Carbohydr. Polym. 2010, 80, 687–694. [Google Scholar] [CrossRef]
- Rogina, A.; Ressler, A.; Matíc, I.; Gallego Ferrer, G.; Marijanovic, I.; Ivankovic, M.; Ivankovic, H. Cellular hydrogels based on pH-responsive chitosan-hydroxyapatite system. Carbohydr. Polym. 2017, 166, 173–182. [Google Scholar] [CrossRef] [PubMed]
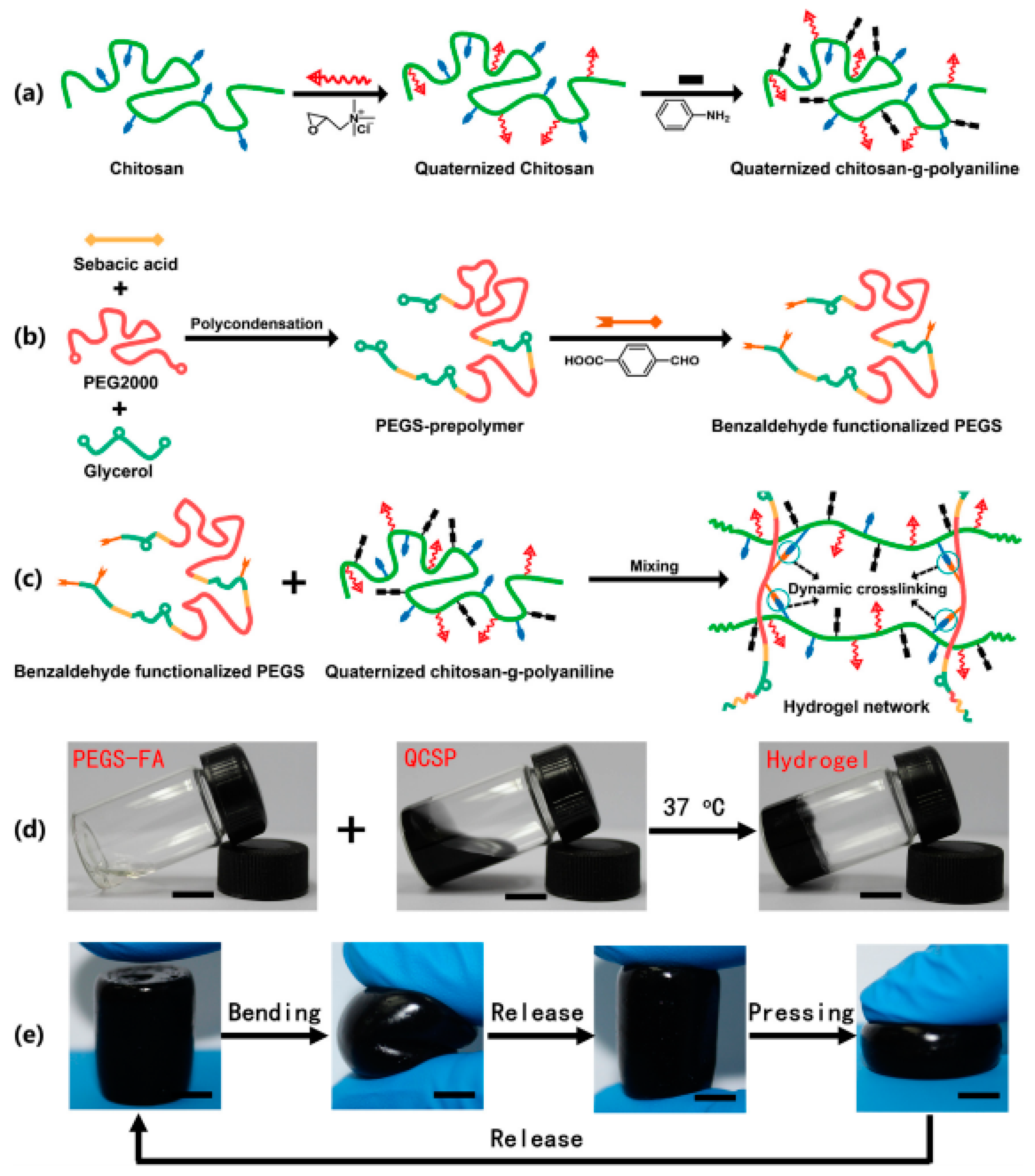
- Zhao, X.; Li, P.; Guo, B.; Ma, P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Kilmartin, P.; Gizdavic-Nikolaidis, M.; Zujovic, Z.; Travas-Sejdic, J.; Bowmaker, G.; Cooney, R. Free radical scavenging and antioxidant properties of conducting polymers examined using EPR and NMR spectroscopies. Synth. Met. 2005, 153, 153–156. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H.; Singh, R.; Lin, P.-C. Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hidrogel. Cellulose 2017, 24, 2215–2228. [Google Scholar]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H. Enhancement of curcumin bioavailability using nanocellulose reinforced chitosan hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, J.; Zhao, N.; Liu, T.; Xu, W.; Suo, A. Novel hydroxyethyl chitosan/cellulose scaffolds with bubble-like porous structure for bone tissue engineering. Carbohydr. Polym. 2017, 167, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, J.; Krishnan, U.M.; Sethuraman, S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol. Adv. 2014, 32, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiao, Z.; Ren, X.; Long, H.; Qian, H.; Ma, K.; Guo, Y. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue derived stromal cells. Peer J 2016, 4, e2497. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Li, S.; Li, P.; Xu, Y.; Wang, L.; Zhao, C.; Ouyang, B.; Tu, B.; Zhang, C.; Luo, L.; et al. A controlled release codelivery system of MSCs encapsulated in dextran/gelatin hydrogel with TGF-β3-loaded nanoparticles for nucleus pulposus regeneration. Stem Cells Int. 2016, 2016, 9042019. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, X.; Sun, H.; Su, J.; Lin, N.; Péault, B.; Song, T.; Yanq, J.; Dai, J.; Hu, Y. Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials 2014, 35, 4888–4900. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Tang, H.; Wu, J.; Hou, X.; Chen, B.; Chen, W.; Zhao, Y.; Shi, C.; Zhou, F.; Yu, W.; et al. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials 2015, 69, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Hu, X.Y.; Zeng, W.; Huang, J.H.; Zhang, Y.G.; Luo, Z.J. Rapid sciatic nerve regeneration of rats by a surface modified collagen-chitosan scaffold. Injury 2013, 44, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Blanco, M.D.; Davidenko, N.; Cameron, R.E. Tailoring chitosan/collagen scaffolds for tissue engineering: Effect of composition and different crosslinking agents on scaffold properties. Carbohydr. Polym. 2015, 132, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Zuber, M.; Zia, F.; Zia, K.M.; Tabasum, S.; Salman, M.; Sultan, N. Collagen based polyurethanes: A review of recent advances and perspective. Int. J. Biol. Macromol. 2015, 80, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Stegemann, J.P. Thermogelling chitosan and collagen composite hydrogels initiated with β-glycerophosphate for bone tissueengineering. Biomaterials 2010, 31, 3976–3985. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Stegemann, J.P. Glyoxal crosslinking of cell-seeded chitosan/collagen hydrogels for bone regeneration. Acta Biomater. 2011, 7, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Li, L.; Yan, X.; Zhang, W.; Zhang, Y.; Wang, Y.; Liu, T. Characterizationof human adipose tissue-derived stem cells in vitro culture and in vivo differentiation in a temperature-sensitive chitosan/β-glycerophosphate/collagen hybrid hydrogel. Mater. Sci. Eng. C 2017, 70, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Liu, K.; Zhang, Z.; Liu, C.; Liu, X.; Xin, Y.; Cheng, X.; Xu, T.; Cha, D.; Fan, B. Fabrication and evaluation of thermosensitive chitosan/collagen/β-glycerophosphate hydrogels for tissue regeneration. Carbohydr. Polym. 2017, 167, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High mechanical strength double-network hydrogel with bacterial cellulose. Adv. Funct Mater. 2004, 14, 1124–1128. [Google Scholar] [CrossRef]
- Xu, D.; Huang, J.; Zhao, D.; Ding, B.; Zhang, L.; Cai, J. High-flexibility, high-toughness double-cross-linked chitin hydrogels by sequential chemicaland physical cross-linkings. Adv. Mater. 2016, 28, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huang, J.; Zhong, Y.; Li, K.; Zhang, L.; Cai, J. High-strength and high-toughness double-cross-linked cellulose hydrogels: A new strategy usingsequential chemical and physical cross-linking. Adv. Funct. Mater. 2016, 26, 6279–6287. [Google Scholar] [CrossRef]
- Lim, H.-S.; Kwon, E.; Lee, M.; Lee, Y.M.; Suh, K.-D. One-pot template-free synthesis of monodisperse hollow hydrogel microspheres and their resulting properties. Macromol. Rapid Comm. 2013, 34, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Kakegawa, T.; Osaki, T.; Enomoto, J.; Ito, T.; Nittami, T.; Fukuda, J. Rapid engineering of endothelial cell-lined vascular-like structures in situ crosslinkable hydrogels. Biofabrication 2014, 6, 025006. [Google Scholar] [CrossRef] [PubMed]
- McClendon, M.T.; Stupp, S.I. Tubular hydrogels of circumferentially aligned nanofibers to encapsulate and orient vascular cells? Biomaterials 2012, 33, 5713–5722. [Google Scholar] [CrossRef] [PubMed]
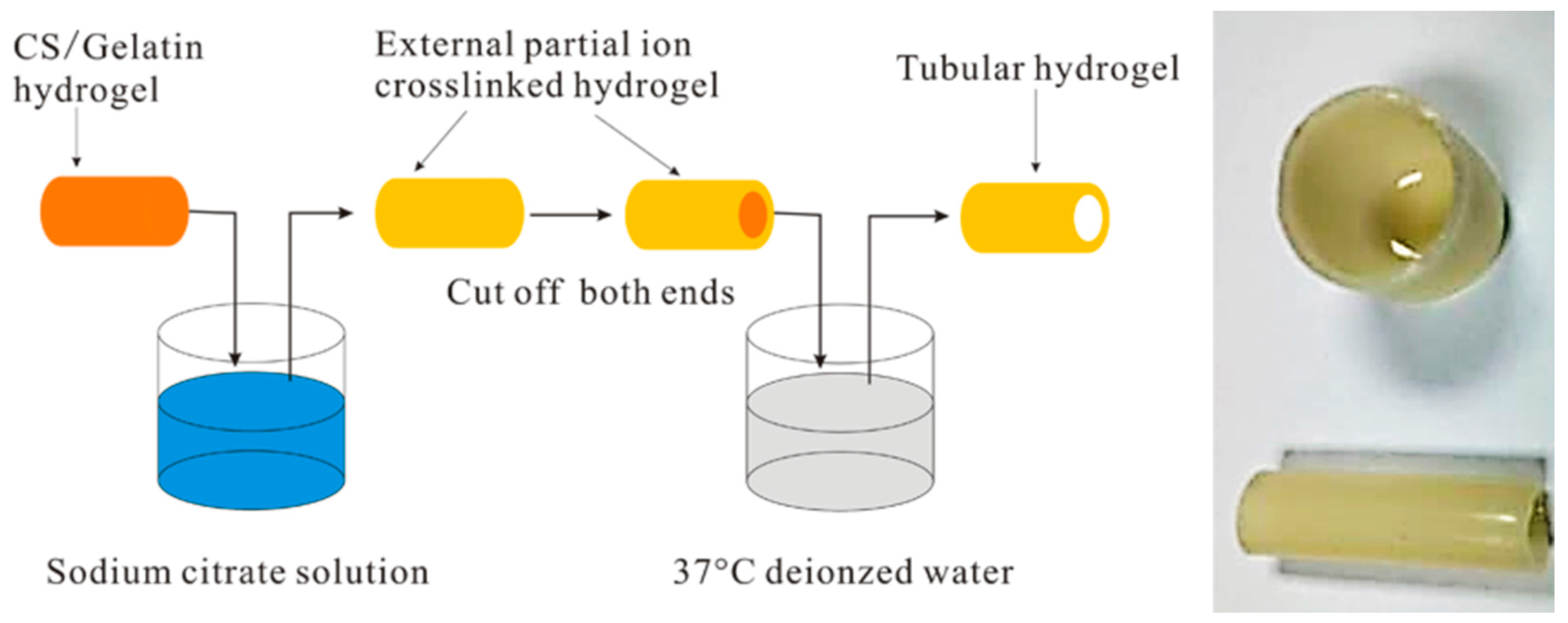
- Wu, S.; Dong, H.; Li, Q.; Wang, G.; Cao, X. High strength, biocompatible hydrogels with designable shapes and special hollow-formed character using chitosan and gelatin. Carbohydr. Polym. 2017, 168, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Pedron, S.; van Lierop, S.; Horstman, P.; Penterman, R.; Broer, D.J.; Peeters, E. Stimuli responsive delivery vehicles for cardiac microtissue transplantation. Adv. Funct. Mater. 2011, 21, 1624–1630. [Google Scholar] [CrossRef]
- Saini, H.; Navaei, A.; van Putten, A.; Nikkhah, M. 3D cardiac microtissues encapsulated with the co-culture of cardiomyocytes and cardiac fibroblasts. Adv. Healthc. Mater. 2015, 4, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Navaei, A.; Saini, H.; Christenson, W.; Sulliva, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, V.; Cellot, G.; Toma, F.M.; Long, C.S.; Caldwell, J.H.; Zentilin, L.; Giacca, M.; Turco, A.; Prato, M.; Ballerini, L.; et al. Carbon nanotubes promote growth and spontaneous electrical activity in cultured cardiac myocytes. Nano Lett. 2012, 12, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Firme, C.P.; Bandaru, P.R. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 245–256. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroglu, E.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized carbon nanotubes: Biomedical applications. Int. J. Nanomed. 2012, 7, 5361–5374. [Google Scholar]
- Koppes, A.N.; Keating, K.W.; McGregor, A.L.; Koppes, R.A.; Kearns, K.R.K.; Ziemba, A.M.; McKay, C.A.; Zuidema, J.M.; Rivet, C.J.; Gilbert, C.J.; et al. Robust neurite extension following exogenous electrical stimulation within single walled carbon nanotube-composite hydrogels. Acta Biomater. 2016, 39, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Gelain, F. Peptidic Biomaterials: From self-assembling to regenerative medicine. Trends Biotechnol. 2017, 35, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Holmes, T.C.; DiPersio, C.M.; Hynes, R.O.; Su, X.; Rich, A. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials 1995, 16, 1385–1393. [Google Scholar] [CrossRef]
- Zhang, S.; Altman, M. Peptide self-assembly in functional polymer science and engineering. React. Funct. Polym. 1999, 41, 91–102. [Google Scholar] [CrossRef]
- Ramachandran, S.; Tseng, Y.; Ye, Y.B. Repeated Rapid Shear-Responsiveness of Peptide Hydrogels with Tunable Shear Modulus. Biomacromolecules 2005, 6, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Raspa, A.; Saracino, G.A.A.; Pugliese, R.; Silva, D.; Cigognini, D.; Vescovi, A.; Gelain, F. Complementary Co-assembling Peptides: From In Silico Studies to In Vivo Application. Adv. Funct. Mater. 2014, 24, 6317–6328. [Google Scholar] [CrossRef]
- Tarek, M. Molecular dynamics investigation of an oriented cyclic peptide nanotube in DMPC bilayers. Biophys. J. 2003, 85, 2287–2298. [Google Scholar] [CrossRef]
- Van Bommel, K.J.C.; van der Pol, C.; Kuizebelt, I.; Friggeri, A.; Heeres, A.; Meetsma, A.; Feringa, B.L.; van Esch, J. Responsive cyclohexane-based low-molecular-weight hydrogelators with modular architecture. Angew. Chem. Int. Ed. 2004, 43, 1663. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Webber, M.J.; Stupp, S.I. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Pept. Sci. 2010, 94, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Xu, H. Hydrophobic-region-induced transitions in self-assembled peptide nanostructures. Langmuir 2009, 25, 4115–4123. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.G.; Grodzinsky, A.J. Self-assembling peptide hydrogel fodters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc. Natl. Acad. Sci. USA 2002, 99, 9996–10001. [Google Scholar] [CrossRef] [PubMed]
- Mujeeb, A.; Miller, A.F.; Saiani, A.; Gough, J.E. Self-assembled octapeptide scaffolds for in vitro chondrocyte culture. Acta Biomater. 2013, 9, 4609–4617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Greenfied, M.A.; Mata, A.; Palmer, L.C.; Bitton, R.; Mantei, J.R.; Aparicio, C.; de la Cruz, M.O.; Stupp, S.I. A self-assembly pathway to aligned monodomain gels. Nat. Mater. 2010, 9, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.F.; Devgun, J.M.; Collier, J.H. Fibrillized peptide microgels for cell ancapsulation and 3D cell culture. Soft Matter 2011, 7, 6005–6011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yokoi, H.; Tanaka, M.; Kinoshita, T.; Tan, T. Self-assembled pH-responsive hydrogels composed of the RATEA16 peptide. Biomacromolecules 2008, 9, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Smith, A.M.; Das, A.K.; Hodson, N.W.; Collins, R.F.; Ulijn, R.V.; Gough, J.E. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 2009, 30, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.-Q.; Feng, C.-L. Amino Acids and Peptide-Based Supramolecular Hydrogels for Three-Dimensional Cell Culture. Adv. Mater. 2017, 29, 1604062. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.F.; Megley, K.A.; Viswanathan, N.; Krogstad, D.V.; Drews, L.B.; Kade, M.J.; Qian, Y.C.; Tirrell, M.V. pH-Responsive Branched Peptide Amphiphile Hydrogel Designed for Applications in Regenerative Medicine with Potential as Injectable Tissue Scaffolds. J. Mater. Chem. 2012, 22, 19447–19454. [Google Scholar] [CrossRef]
- Panda, J.J.; Dua, R.; Mishra, A.; Mittra, B.; Chauhan, V.S. 3D cell growth and proliferation on RGD functionalized nanofibrillar hydrogel based on a conformationally restricted residue containing dipeptide. ACS Appl. Mater. Interfaces 2010, 2, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Qu, Y.; Waddington, L.J.; Easton, C.D.; Glattauer, V.; Lithgow, T.J.; McLean, K.M.; Forsythe, J.S.; Hartley, P.G. Self-assembly of ciprofloxacin and a tripeptide into an antimicrobial nanostructured hydrogel. Biomaterials 2013, 34, 3678–3687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Gao, Y.; Shi, J.F.; Browdy, H.M.; Xu, B. Novel anisotropic supramolecular hydrogel with high stability over a wide pH range. Langmuir 2011, 27, 1510–1512. [Google Scholar] [CrossRef] [PubMed]
- Orbach, R.; Adler-Abramovich, L.; Zigerson, S.; Mironi-Harpaz, I.; Seliktar, D.; Gazit, E. Self-assembled Fmoc-peptides as a platform for the formation of nanostructures and hydrogel. Biomacromolecules 2009, 10, 2646–2651. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Tsukiji, S.; Ikeda, M.; Hamachi, I. Stiff, multistimuli-responsive supramolecular hydrogels as unique molds for 2D/3D microarchitectures of live cells. Chem. Asian J. 2011, 6, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Du, X.W.; Gao, Y.; Shi, J.F.; Xu, B. Aromatic-aromatic interactions enhance interfiber contacts for enzymatic formation of a spontaneously aligned supramolecular hydrogel. J. Am. Chem. Soc. 2014, 136, 2970–2973. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Zhang, D.; Feng, C.L. Control of three-dimensional cell adhesion by the chirality of nanofibers in hydrogels. Angew. Chem. Int. Ed. 2014, 53, 7789–7793. [Google Scholar] [CrossRef] [PubMed]
- Funke, W.; Okay, O.; Joos-Muller, B. Microgels—Intramolecularly crosslinked macromolecules with a globular structure. Adv. Polym. Sci. 1998, 136, 139–234. [Google Scholar]
- Shewan, H.M.; Stokes, J.R. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. Fabrication methods of biopolymeric microgels and microgel-based hydrogels. Food Hydrocoll. 2017, 62, 262–272. [Google Scholar] [CrossRef]
- Gonçalves, V.L.; Laranjeira, M.C.M.; Fávere, V.T.; Pedrosa, R.C. Effect of crosslinking agents on chitosan microspheres in controlled release of diclofenac sodium. Polímeros 2005, 15, 6–12. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, L.; Tian, Z.; Chen, L.; Subirade, M. Preparation and in vitro evaluation of calcium-induced soy protein isolate nanoparticles and their formation mechanism study. Food Chem. 2012, 133, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, T.; Durand, D. Controlled food protein aggregation for new functionality. Curr. Opin. Colloid Interface Sci. 2013, 18, 249–256. [Google Scholar] [CrossRef]
- Nicolai, T. Formation and functionality of self-assembled whey protein microgels. Colloids Surf. B 2016, 137, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Hu, J.; Pan, X.; Yao, P.; Jiang, M. Stable and pH-sensitive nanogels prepared by self-assembly of chitosan and ovalbumin. Langmuir 2006, 22, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Takashima, R. Mechanical properties of the capsules of chitosan–soy globulin polyelectrolyte complex. Food Hydrocoll. 2003, 17, 885–888. [Google Scholar] [CrossRef]
- Thomas, W.R. Carrageenan. In Thickenning and Gelling Agents for Food; Imeso, A.P., Ed.; Springer: Hong Kong, China, 1997; pp. 45–59. [Google Scholar]
- Gabriele, A.; Spyropoulos, F.; Norton, I.T. Kinetic study of fluid gel formation and viscoelastic response with kappa-carrageen. Food Hydrocoll. 2009, 23, 2054–2061. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Transglutaminase-induced caseinate gelation for the microencapsulation of probiotic cells. Int. Dairy J. 2009, 19, 77–84. [Google Scholar] [CrossRef]
- Madadlou, A.; Jaberipour, S.; Eskandari, M.H. Nanoparticulation of enzymatically cross-linked whey proteins to encapsulate caffeine via microemulsification/heat gelation procedure. LWT—Food Sci. Technol. 2014, 57, 725–730. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Kulkarni, A.R.; Aminabhavi, T.M. Crosslinked chitosan microspheres for encapsulation of diclofenac sodium: Effect of crosslinking agent. J. Microencapsul. 2002, 19, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Gazme, B.; Madadlou, A. Fabrication of whey protein–pectin conjugate particles through laccase-induced gelation of microemulsified nanodroplets. Food Hydrocoll. 2014, 40, 189–195. [Google Scholar] [CrossRef]
- Shu, X.Z.; Zhu, K.J. Chitosan/gelatin microspheres prepared by modified emulsification and ionotropic gelation. J. Microencapsul. 2001, 18, 237–245. [Google Scholar] [PubMed]
- Jiang, S.; Chen, Q.; Tripathy, M.; Luijten, E.; Schweizer, K.S.; Granick, S. Janus particle synthesis and assembly. Adv. Mater. 2010, 22, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Marquis, M.; Davy, J.; Cathala, B.; Fang, A.; Renard, D. Microfluidics assisted generation of innovative polysaccharide hydrogel microparticles. Carbohydr. Polym. 2015, 116, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Ma, G.H.; Su, Z.G. Preparation of uniform sized chitosan microspheres by membrane emulsification technique and application as a carrier of protein drug. J. Control. Release 2005, 106, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Gu, Y.H.; Zhou, Q.Z.; Ma, G.H.; Wan, Y.H.; Su, Z.G. Preparation and characterization of uniform-sized chitosan microspheres containing insulin by membrane emulsification and a two-step solidification process. Colloids Surf. B 2006, 50, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Farjami, T.; Madadlou, A.; Labbafi, M. Characteristics of the bulk hydrogels made of the citric acid cross-linked whey protein microgels. Food Hydrocoll. 2015, 50, 159–165. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, Z. Microgel reinforced composite hydrogels with pH-responsive, self-healing properties. Colloids Surf. A 2015, 468, 327–332. [Google Scholar] [CrossRef]
- Sivakumaran, D.; Maitland, D.; Hoare, T. Injectable microgel-hydrogel composites for prolonged small-molecule drug delivery. Biomacromolecules 2011, 12, 4112–4120. [Google Scholar] [CrossRef] [PubMed]
- Xie, T. Tunable polymer multi-shape memory effect. Nature 2010, 464, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Qi, H.J.; Xie, T. Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding. Prog. Polym. Sci. 2015, 49–50, 79–120. [Google Scholar] [CrossRef]
- Habault, D.; Zhang, H.J.; Zhao, Y. Light-triggered self-healing and shape-memory polymers. Chem. Soc. Rev. 2013, 42, 7244–7256. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Yu, C.J.; Wang, S.D.; Malyarchuk, V.; Xie, T.; Rogers, J.A. Deformable, programmable, and shape-memorizing micro-optics. Adv. Funct. Mater. 2013, 23, 3299–3306. [Google Scholar] [CrossRef]
- Small, W.; Singhal, P.; Wilson, T.S.; Maitland, D.J. Thermo-moisture responsive polyurethane shape-memory polymer and composites: A review. J. Mater. Chem. 2010, 20, 3356–3366. [Google Scholar] [PubMed]
- Lu, W.; Le, X.; Zhang, J.; Huang, Y.; Chen, T. Supramolecular shape memory hydrogels: A new bridge between stimuli-responsive polymers and supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.F.; Lv, F.C.; Cao, L.J.; Liu, L.; Zhang, Y.; Lu, Z.G. Multistimuli-Responsive, moldable supramolecular hydrogels croos-linked by ultrafast complexation of metal ions and biopolymers. Angew. Chem. Int. Ed. 2015, 54, 7944–7948. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Q.; Cao, Y.; Yuan, Q.J.; Wang, Y.F.; Li, J.H.; Li, B.J.; Zhang, S. Redox- and glucose-induced shape-memory polymers. Macromol. Rapid Commun. 2013, 34, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.X.; Babalhavaeji, A.; Samanta, S.; Beharry, A.A.; Woolley, G.A. Red-shifting azobenzene photoswitches for in vivo use. Acc. Chem. Res. 2015, 48, 2662–2670. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. Photoresponsive gelators. Chem. Commun. 2016, 52, 8196–8206. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Ouyang, G.; Qin, L.; Liu, M. Supra-dendron gelator based on azobenzene–cyclodextrin host–guest interactions: Photoswitched optical and chiroptical reversibility. Chem. Eur. J. 2016, 22, 18208–18214. [Google Scholar] [CrossRef] [PubMed]
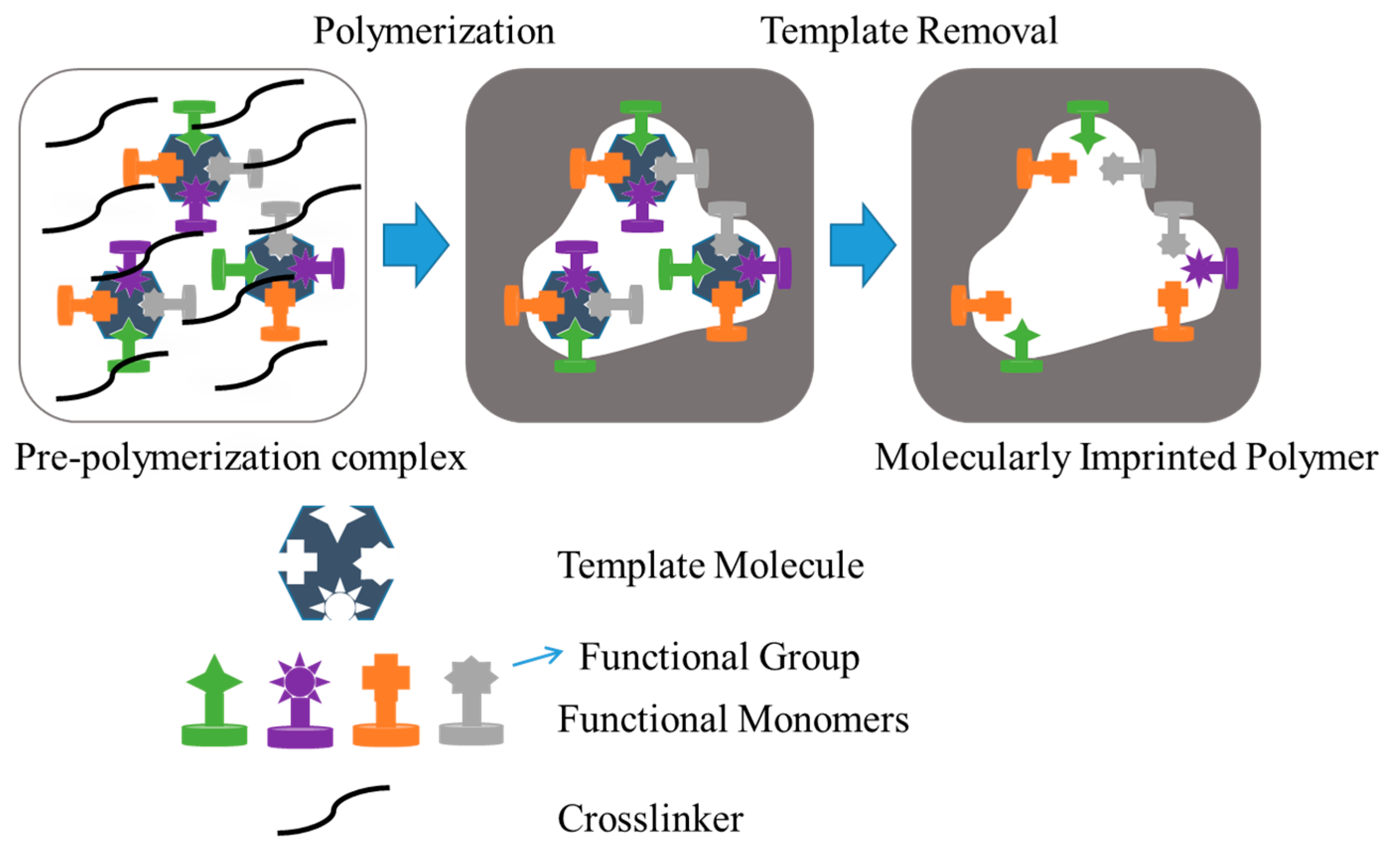
- Neves, M.I.; Wechsler, M.E.; Gomes, M.E.; Reis, R.L.; Granja, P.L.; Peppas, N.A. Molecularly imprinted intelligent scaffolds for tissue engineering applications. Tissue Eng. B 2017, 23, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Kryscio, D.R.; Peppas, N.A. Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater. 2012, 8, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Culver, H.R.; Daily, A.D.; Khademhosseini, A.; Peppas, N.A. Intelligent recognitive systems in nanomedicine. Curr. Opin. Chem. Eng. 2014, 4, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Cheng, G.; Huang, J.; Ying, X. Rebinding and recognition properties of protein-macromolecularly imprinted calcium phosphate/alginate hybrid polymer microspheres. React. Funct. Polym. 2008, 68, 732–741. [Google Scholar] [CrossRef]
- Zhao, K.Y.; Wei, J.F.; Zhou, J.Y.; Zhao, Y.P.; Cheng, G.X. The rebinding properties of bovine serum albumin imprinted calcium phosphate/polyacrylate/alginate hybrid polymer microspheres. Adv. Mater. Res. 2010, 152, 1636–1644. [Google Scholar] [CrossRef]
- Zhu, D.W.; Chen, Z.; Zhao, K.Y.; Kan, B.H.; Liu, L.X.; Dong, X.; Wang, H.; Zhang, C.; Leng, X.G.; Zhang, L.H. Polypropylene non-woven supported fibronectin molecular imprinted calcium alginate/polyacrylamide hydrogel filmfor cell adhesion. Chin. Chem. Lett. 2015, 26, 807–992. [Google Scholar] [CrossRef]
- Fukazawa, K.; Ishihara, K. Fabrication of a cell adhesive protein imprinting surface with an artificial cell membrane structure for cell capturing. Biosens. Bioelectron. 2009, 25, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.K.; Singh, M.; Singh, M. Biopolymeric receptor for peptide recognition by molecular imprinting approach—Synthesis, characterization and application. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.Q.; Guo, T.Y.; Song, M.D.; Zhang, B.H.; Zhang, B.L. Hemoglobin recognition by imprinting in semiinterpenetrating polymer network hydrogel based on polyacrylamide and chitosan. Biomacromolecules 2005, 6, 2601–2606. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhao, S.; Hao, Y.; Zhang, L.; Cui, X.; Liu, D.; Tang, Y. Facile and green synthesis of polysaccharide-based magnetic molecularly imprinted nanoparticles for protein recognition. RSC Adv. 2015, 5, 88436–88444. [Google Scholar] [CrossRef]
- DePorter, S.M.; Lui, I.; McNaughton, B.R. Programmed cell adhesion and growth on cell-imprinted polyacrylamide hydrogels. Soft Matter 2012, 8, 10403–10408. [Google Scholar] [CrossRef]
- Włodarczyk-Biegun, M.K.; del Campo, A. 3D Bioprinting of structural proteins. Biomaterials 2017, 134, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Arslan-Yildiz, A.; El Assal, R.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards artificial tissue models: Past, present, and future of 3D bioprinting. Biofabrication 2016, 8, 014103. [Google Scholar] [CrossRef] [PubMed]
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A review of three-dimensional printing in tissue engineering. Tissue Eng. B 2016, 22, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Wang, X. Biodegradable polymers and stem cells for bioprinting. Molecules 2016, 21, 539. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Gantelius, J.; Svahn, H.A. 3D Bioprinting of tissue/organ models. Angew. Chem. Int. Ed. Engl. 2016, 55, 4650–4665. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Moon, S.; Emre, A.E.; Lien, C.; Turali, E.S.; Demirci, U. Cell Bioprinting as a Potential High-Throughput Method for Fabricating Cell-Based Biosensors (CBBs). In Proceedings of the IEEE Sensors, Christchurch, New Zealand, 25–28 October 2009; pp. 387–391. [Google Scholar]
- Lee, W.; Debasitis, J.C.; Lee, V.K.; Lee, J.H.; Fischer, K.; Edminster, K.; Park, J.K.; Yoo, S.S. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009, 30, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Pinckney, J.; Lee, V.; Lee, J.H.; Fischer, K.; Polio, S.; Park, J.K.; Yoo, S.S. Three-dimensional bioprinting of rat embryonic neural cells. Neuroreport 2009, 20, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef] [PubMed]
- Gruene, M.; Unger, C.; Koch, L.; Deiwick, A.; Chichkov, B. Dispensing pico to nanolitre of a natural hydrogel by laser-assisted bioprinting. Biomed. Eng. Online 2011, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. A 2013, 101, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Lee, J.-S.; Chun, W.; Kim, G.H. An innovative collagen-based cell-printing method for obtaining human adipose stem cell-laden structures consisting of core–sheath structures for tissue engineering. Biomacromolecules 2016, 17, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.S.; Dai, G.; Karande, P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. C 2014, 20, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Atala, A. Biomaterials for integration with 3-D bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746. [Google Scholar] [CrossRef] [PubMed]
- De la Puente, P.; Ludeña, D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp. Cell. Res. 2014, 322, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Polio, S.; Lee, W.; Dai, G.; Menon, L.; Carroll, R.S.; Yoo, S.S. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp. Neurol. 2010, 223, 645–652. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Valle, L.J.; Díaz, A.; Puiggalí, J. Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives. Gels 2017, 3, 27. https://doi.org/10.3390/gels3030027
Del Valle LJ, Díaz A, Puiggalí J. Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives. Gels. 2017; 3(3):27. https://doi.org/10.3390/gels3030027
Chicago/Turabian StyleDel Valle, Luís J., Angélica Díaz, and Jordi Puiggalí. 2017. "Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives" Gels 3, no. 3: 27. https://doi.org/10.3390/gels3030027