Assembly of a Tripeptide and Anti-Inflammatory Drugs into Supramolecular Hydrogels for Sustained Release
Abstract
:1. Introduction
2. Results and Discussion
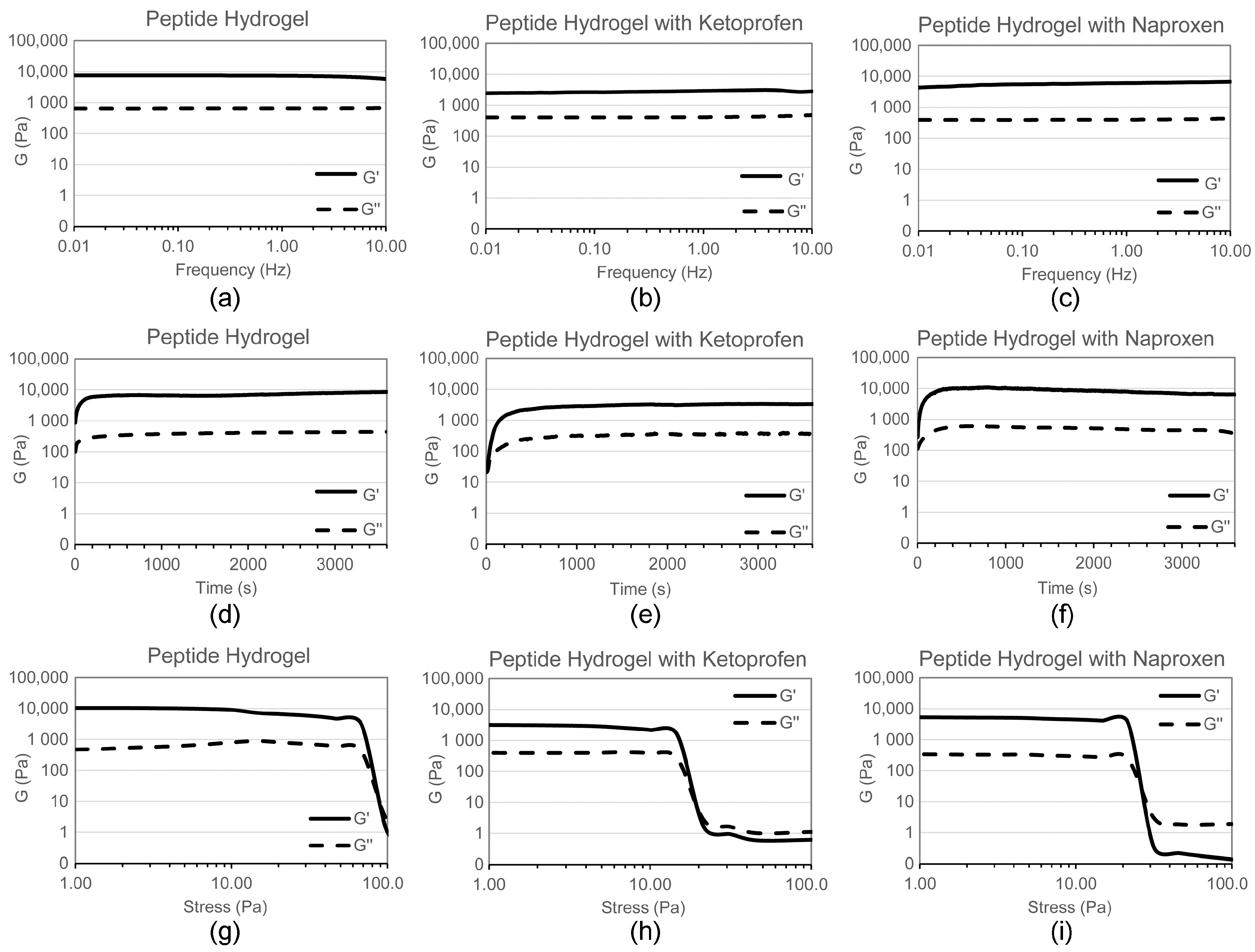
2.1. Hydrogel Characterisation
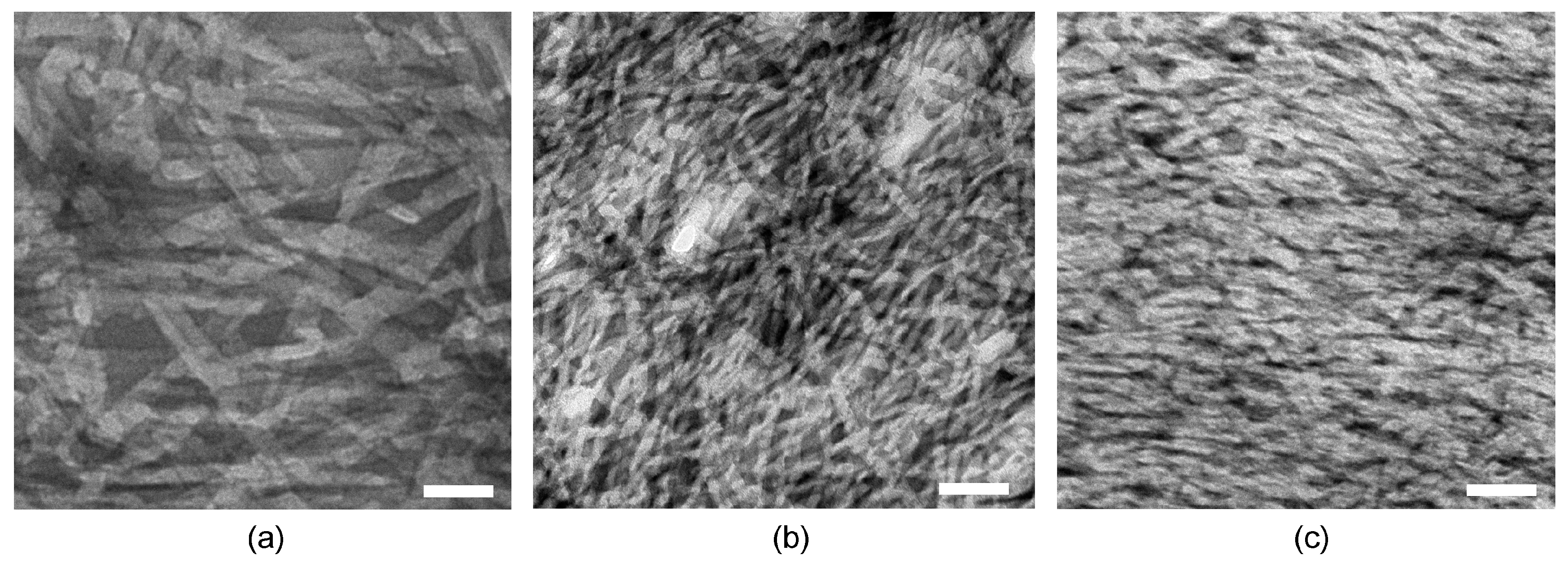
2.2. Nanostructure Morphology
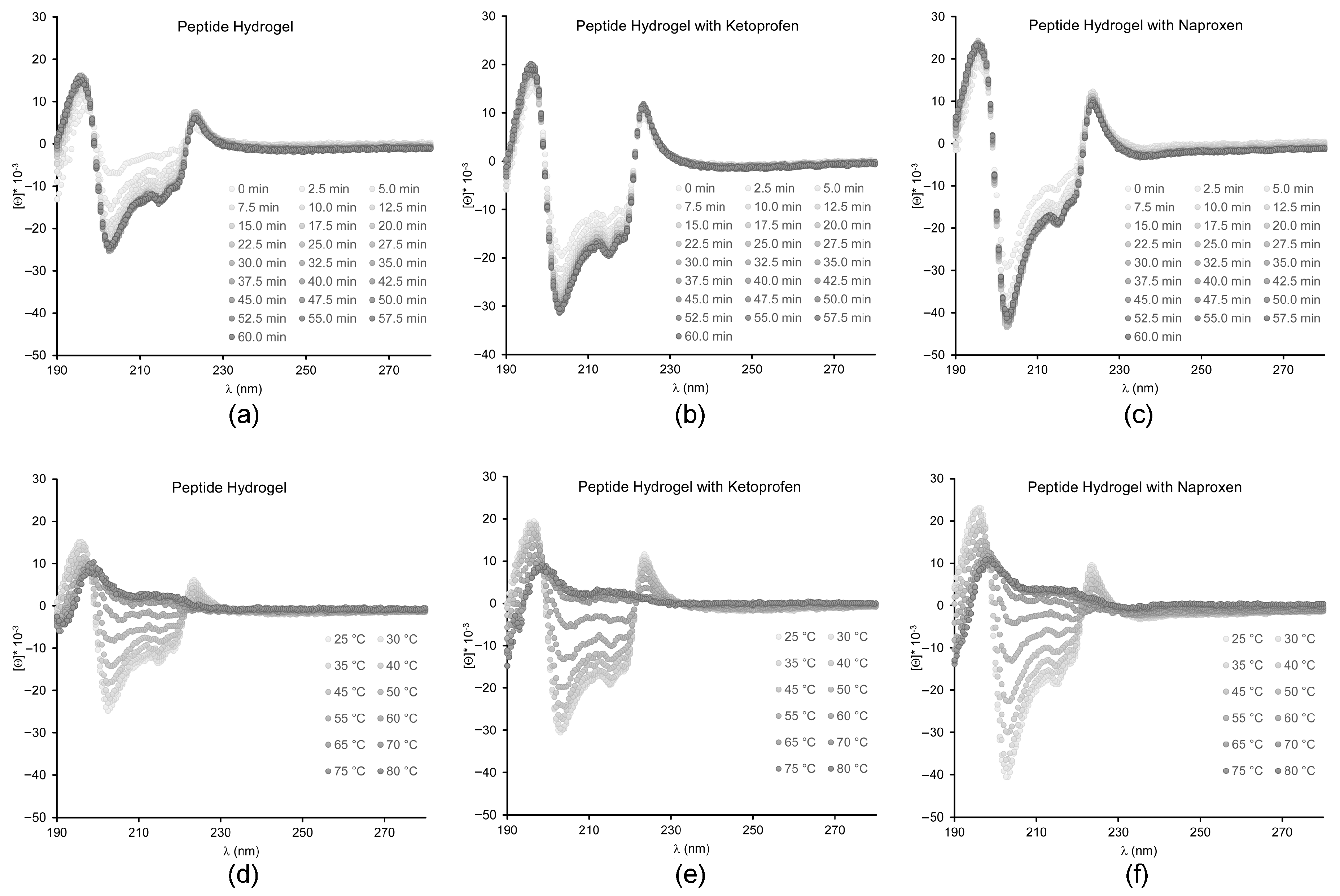
2.3. Spectroscopic Study of Peptide Conformation
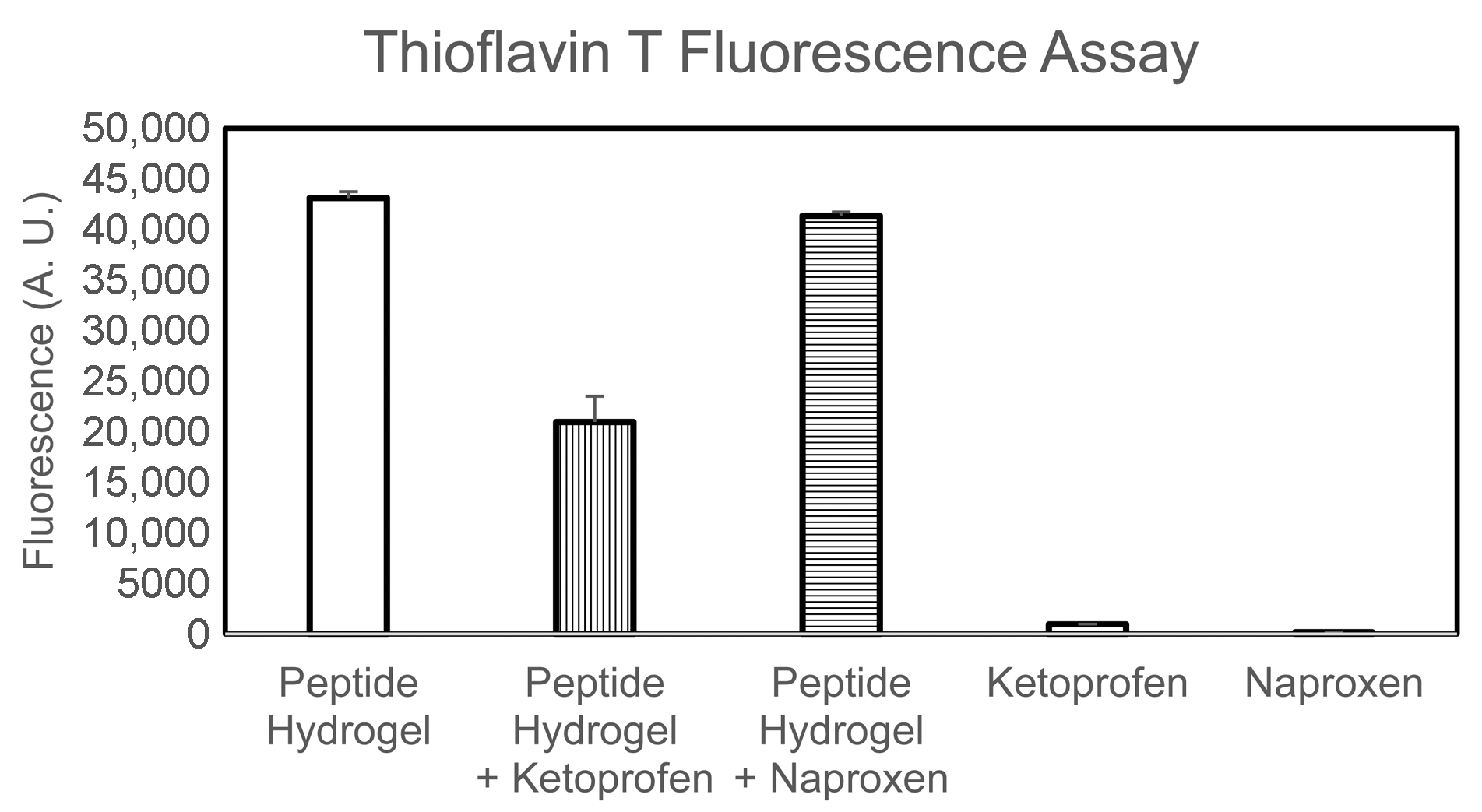
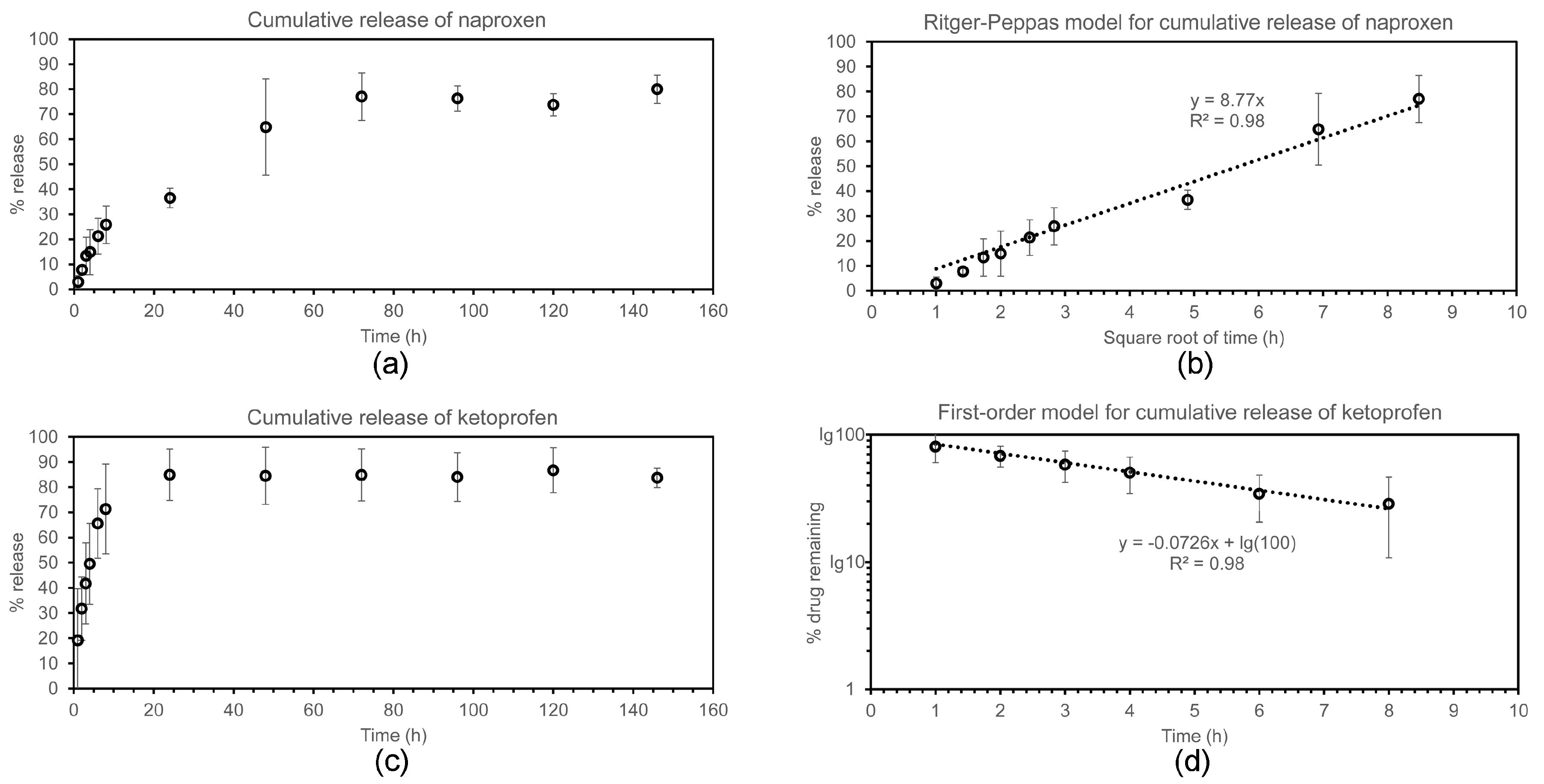
2.4. Drug Release Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Peptide Synthesis and Purification
4.3. Hydrogel Preparation
4.4. Oscillatory Rheometry
4.5. Transmission Electron Microscopy
4.6. Circular Dichroism
4.7. Fourier-Transformed Infrared Spectroscopy
4.8. Thioflavin T Fluorescence Assay
4.9. Drug Release Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, J.; Du, X.; Xu, B. Supramolecular biofunctional materials. Biomaterials 2017, 129, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Dipeptide and tripeptide conjugates as low-molecular-weight hydrogelators. Macromol. Biosci. 2011, 11, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, M.; Styan, K.E.; Marchesan, S. The unexpected advantages of using d-amino acids for peptide self- assembly into nanostructured hydrogels for medicine. Curr. Top. Med. Chem. 2016, 16, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Styan, K.E.; Easton, C.D.; Waddington, L.; Vargiu, A.V. Higher and lower supramolecular orders for the design of self-assembled heterochiral tripeptide hydrogel biomaterials. J. Mater. Chem. B 2015, 3, 8123–8132. [Google Scholar] [CrossRef] [Green Version]
- Vargiu, A.V.; Iglesias, D.; Styan, K.E.; Waddington, L.J.; Easton, C.D.; Marchesan, S. Design of a hydrophobic tripeptide that self-assembles into amphiphilic superstructures forming a hydrogel biomaterial. Chem. Commun. 2016, 52, 5912–5915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, A.M.; Kurbasic, M.; Kralj, S.; Melchionna, M.; Marchesan, S. A biocatalytic and thermoreversible hydrogel from a histidine-containing tripeptide. Chem. Commun. 2017, 53, 8110–8113. [Google Scholar] [CrossRef] [PubMed]
- Orbach, R.; Mironi-Harpaz, I.; Adler-Abramovich, L.; Mossou, E.; Mitchell, E.P.; Forsyth, V.T.; Gazit, E.; Seliktar, D. The rheological and structural properties of fmoc-peptide-based hydrogels: The effect of aromatic molecular architecture on self-assembly and physical characteristics. Langmuir 2012, 28, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Morris, K.; Laybourn, A.; Elias, D.; Hicks, M.R.; Rodger, A.; Serpell, L.; Adams, D.J. Self-assembly mechanism for a naphthalene-dipeptide leading to hydrogelation. Langmuir 2010, 26, 5232–5242. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.D.; Wojciechowski, J.P.; Warren, H.; in het Panhuis, M.; Thordarson, P. Effect of heterocyclic capping groups on the self-assembly of a dipeptide hydrogel. Soft Matter 2016, 12, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Vilaca, H.; Pereira, G.; Castro, T.G.; Hermenegildo, B.F.; Shi, J.; Faria, T.Q.; Micaelo, N.; Brito, R.M.M.; Xu, B.; Castanheira, E.M.S.; et al. New self-assembled supramolecular hydrogels based on dehydropeptides. J. Mater. Chem. B 2015, 3, 6355–6367. [Google Scholar] [CrossRef]
- Vilaca, H.; Hortelao, A.C.; Castanheira, E.M.; Queiroz, M.J.; Hilliou, L.; Hamley, I.W.; Martins, J.A.; Ferreira, P.M. Dehydrodipeptide hydrogelators containing naproxen n-capped tryptophan: Self-assembly, hydrogel characterization, and evaluation as potential drug nanocarriers. Biomacromolecules 2015, 16, 3562–3573. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Rambhia, K.J.; Ma, P.X. Controlled drug release for tissue engineering. J. Control. Release 2015, 219, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Qu, Y.; Waddington, L.J.; Easton, C.D.; Glattauer, V.; Lithgow, T.J.; McLean, K.M.; Forsythe, J.S.; Hartley, P.G. Self-assembly of ciprofloxacin and a tripeptide into an antimicrobial nanostructured hydrogel. Biomaterials 2013, 34, 3678–3687. [Google Scholar] [CrossRef] [PubMed]
- Howe, E.J.; Okesola, B.O.; Smith, D.K. Self-assembled sorbitol-derived supramolecular hydrogels for the controlled encapsulation and release of active pharmaceutical ingredients. Chem. Commun. 2015, 51, 7451–7454. [Google Scholar] [CrossRef] [PubMed]
- Haldar, D. Two component hydrogel with γ-amino butyric acid as potential receptor and neurotransmitter delivery system. Tetrahedron 2008, 64, 186–190. [Google Scholar] [CrossRef]
- Castelletto, V.; Hamley, I.W.; Stain, C.; Connon, C. Slow-release rgd-peptide hydrogel monoliths. Langmuir 2012, 28, 12575–12580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, J.J.; Mishra, A.; Basu, A.; Chauhan, V.S. Stimuli responsive self-assembled hydrogel of a low molecular weight free dipeptide with potential for tunable drug delivery. Biomacromolecules 2008, 9, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Yang, Z.; Zhang, R.; Li, L.; Fan, Y.; Kuang, Y.; Gao, Y.; Wang, T.; Lu, W.W.; Xu, B. Supramolecular hydrogel of a d-amino acid dipeptide for controlled drug release in vivo. Langmuir 2009, 25, 8419–8422. [Google Scholar] [CrossRef] [PubMed]
- Ischakov, R.; Adler-Abramovich, L.; Buzhansky, L.; Shekhter, T.; Gazit, E. Peptide-based hydrogel nanoparticles as effective drug delivery agents. Bioorg. Med. Chem. 2013, 21, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.A.; Escuder, B.; Miravet, J.F. Supramolecular hydrogels for enzymatically triggered self-immolative drug delivery. Tetrahedron 2010, 66, 2614–2618. [Google Scholar] [CrossRef]
- Sun, Y.; Kaplan, J.A.; Shieh, A.; Sun, H.-L.; Croce, C.M.; Grinstaff, M.W.; Parquette, J.R. Self-assembly of a 5-fluorouracil-dipeptide hydrogel. Chem. Commun. 2016, 52, 5254–5257. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Kuang, Y.; Gao, Y.; Du, X.; Shi, J.; Xu, B. Self-delivery multifunctional anti-hiv hydrogels for sustained release. Adv. Healthc. Mater. 2013, 2, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, Y.; Kuang, Y.; Shi, J.; Du, X.; Zhou, J.; Wang, H.; Yang, Z.; Xu, B. Dephosphorylation of d-peptide derivatives to form biofunctional, supramolecular nanofibers/hydrogels and their potential applications for intracellular imaging and intratumoral chemotherapy. J. Am. Chem. Soc. 2013, 135, 9907–9914. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, J.; Yang, C.; Zhao, H.; Li, D.; Yin, Z.; Yang, Z. The inhibition of tumor growth and metastasis by self-assembled nanofibers of taxol. Biomaterials 2012, 33, 5848–5853. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, Y.; Gao, Y.; Du, X.; Shi, J.; Xu, B. d-amino acids boost the selectivity and confer supramolecular hydrogels of a nonsteroidal anti-inflammatory drug (NSAID). J. Am. Chem. Soc. 2013, 135, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Waddington, L.; Easton, C.; Kushkaki, F.; McLean, K.; Forsythe, J.; Hartley, P. Tripeptide self-assembled hydrogels: Soft nanomaterials for biological applications. BioNanoScience 2013, 3, 21–29. [Google Scholar] [CrossRef]
- Sanderson, H.; Thomsen, M. Comparative analysis of pharmaceuticals versus industrial chemicals acute aquatic toxicity classification according to the united nations classification system for chemicals. Assessment of the (q)sar predictability of pharmaceuticals acute aquatic toxicity and their predominant acute toxic mode-of-action. Toxicol. Lett. 2009, 187, 84–93. [Google Scholar] [PubMed]
- Cringoli, M.C.; Kralj, S.; Kurbasic, M.; Urban, M.; Marchesan, S. Luminescent supramolecular hydrogels from a tripeptide and nitrogen-doped carbon nanodots. Beilsten J. Nanotechnol. 2017, 8, 1553–1562. [Google Scholar] [CrossRef]
- Shea, J.E.; Wu, C.; Biancalana, M.; Koide, S. Binding modes of thioflavin-t to the single-layer beta-sheet of the peptide self-assembly mimics. J. Mol. Biol. 2009, 394, 627–633. [Google Scholar]
- Amdursky, N.; Erez, Y.; Huppert, D. Molecular rotors: What lies behind the high sensitivity of the thioflavin-t fluorescent marker. Acc. Chem. Res. 2012, 45, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, G.E.; Muller-Goymann, C.C. Ketoprofen sodium: Preparation and its formation of mixed crystals with ketoprofen. J. Pharm. Sci. 1997, 86, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Méndez del Río, J.R.; Rousseau, R.W. Solubility and prediction of the heat of solution of sodium naproxen in aqueous solutions. J. Pharm. Sci. 2005, 94, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Waddington, L.; Easton, C.D.; Winkler, D.A.; Goodall, L.; Forsythe, J.; Hartley, P.G. Unzipping the role of chirality in nanoscale self-assembly of tripeptide hydrogels. Nanoscale 2012, 4, 6752–6760. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Adler-Abramovich, L.; Lampel, A.; Bram, Y.; Lipstman, S.; Gazit, E. Formation of functional super-helical assemblies by constrained single heptad repeat. Nat. Commun. 2015, 6, 8615. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, M.R.; Sambashivan, S.; Nelson, R.; Ivanova, M.I.; Sievers, S.A.; Apostol, M.I.; Thompson, M.J.; Balbirnie, M.; Wiltzius, J.J.W.; McFarlane, H.T.; et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 2007, 447, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Jayawarna, V.; Smith, A.; Gough, J.E.; Ulijn, R.V. Three-dimensional cell culture of chondrocytes on modified di-phenylalanine scaffolds. Biochem. Soc. Trans. 2007, 35, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, G.; Wang, L.; Xu, B. Using a kinase/phosphatase switch to regulate a supramolecular hydrogel and forming the supramolecular hydrogel in vivo. J. Am. Chem. Soc. 2006, 128, 3038–3043. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurbasic, M.; Romano, C.D.; Garcia, A.M.; Kralj, S.; Marchesan, S. Assembly of a Tripeptide and Anti-Inflammatory Drugs into Supramolecular Hydrogels for Sustained Release. Gels 2017, 3, 29. https://doi.org/10.3390/gels3030029
Kurbasic M, Romano CD, Garcia AM, Kralj S, Marchesan S. Assembly of a Tripeptide and Anti-Inflammatory Drugs into Supramolecular Hydrogels for Sustained Release. Gels. 2017; 3(3):29. https://doi.org/10.3390/gels3030029
Chicago/Turabian StyleKurbasic, Marina, Chiara D. Romano, Ana M. Garcia, Slavko Kralj, and Silvia Marchesan. 2017. "Assembly of a Tripeptide and Anti-Inflammatory Drugs into Supramolecular Hydrogels for Sustained Release" Gels 3, no. 3: 29. https://doi.org/10.3390/gels3030029