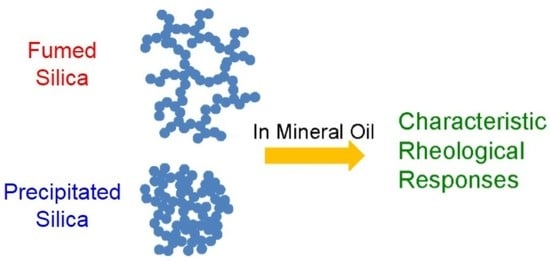
Fumed and Precipitated Hydrophilic Silica Suspension Gels in Mineral Oil: Stability and Rheological Properties
Abstract
:1. Introduction
2. Results and Discussion
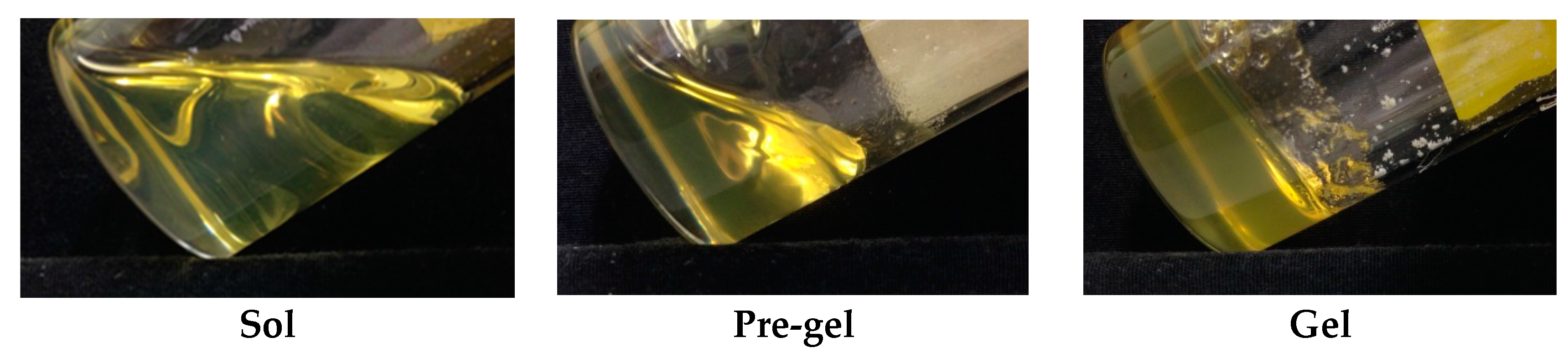
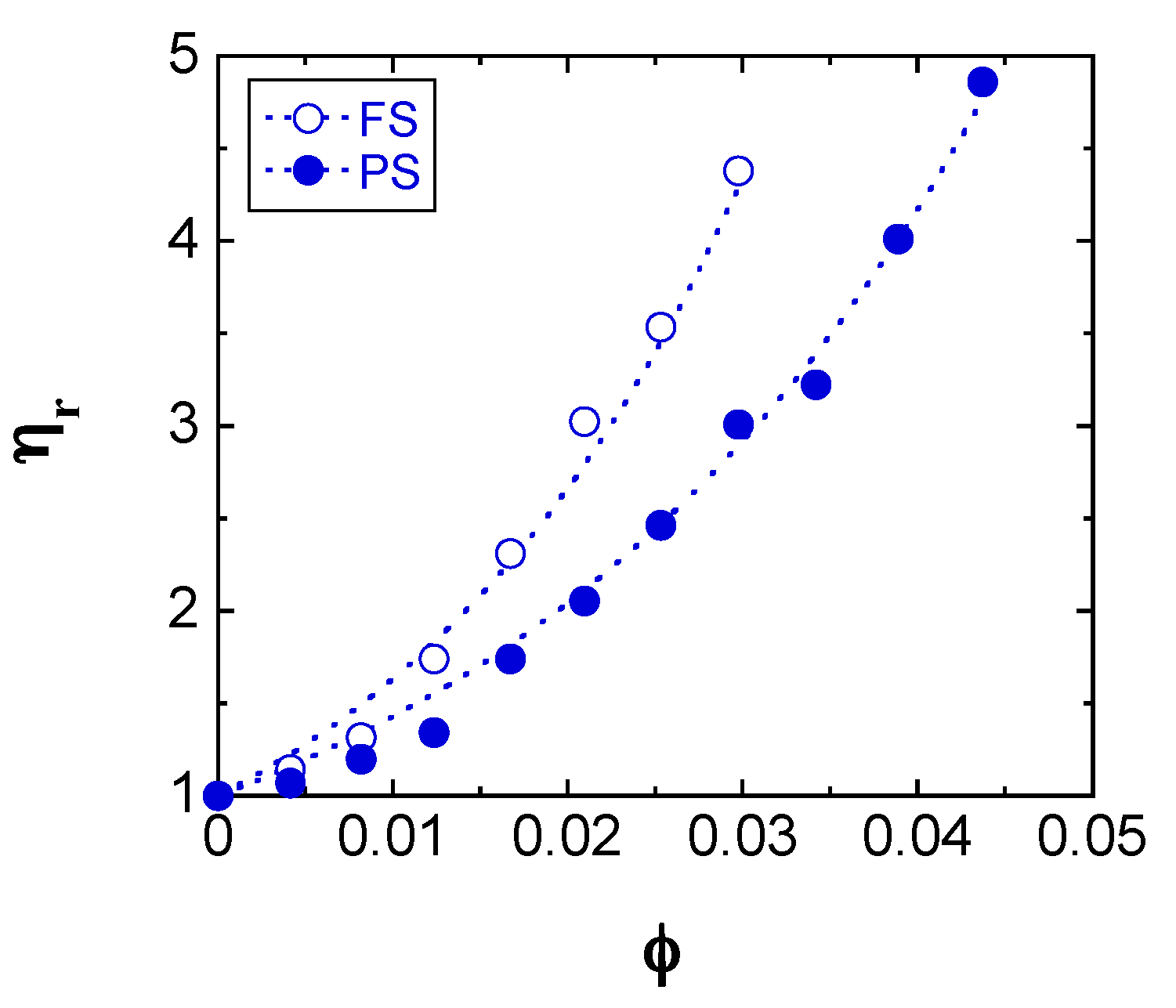
2.1. Sample Classification
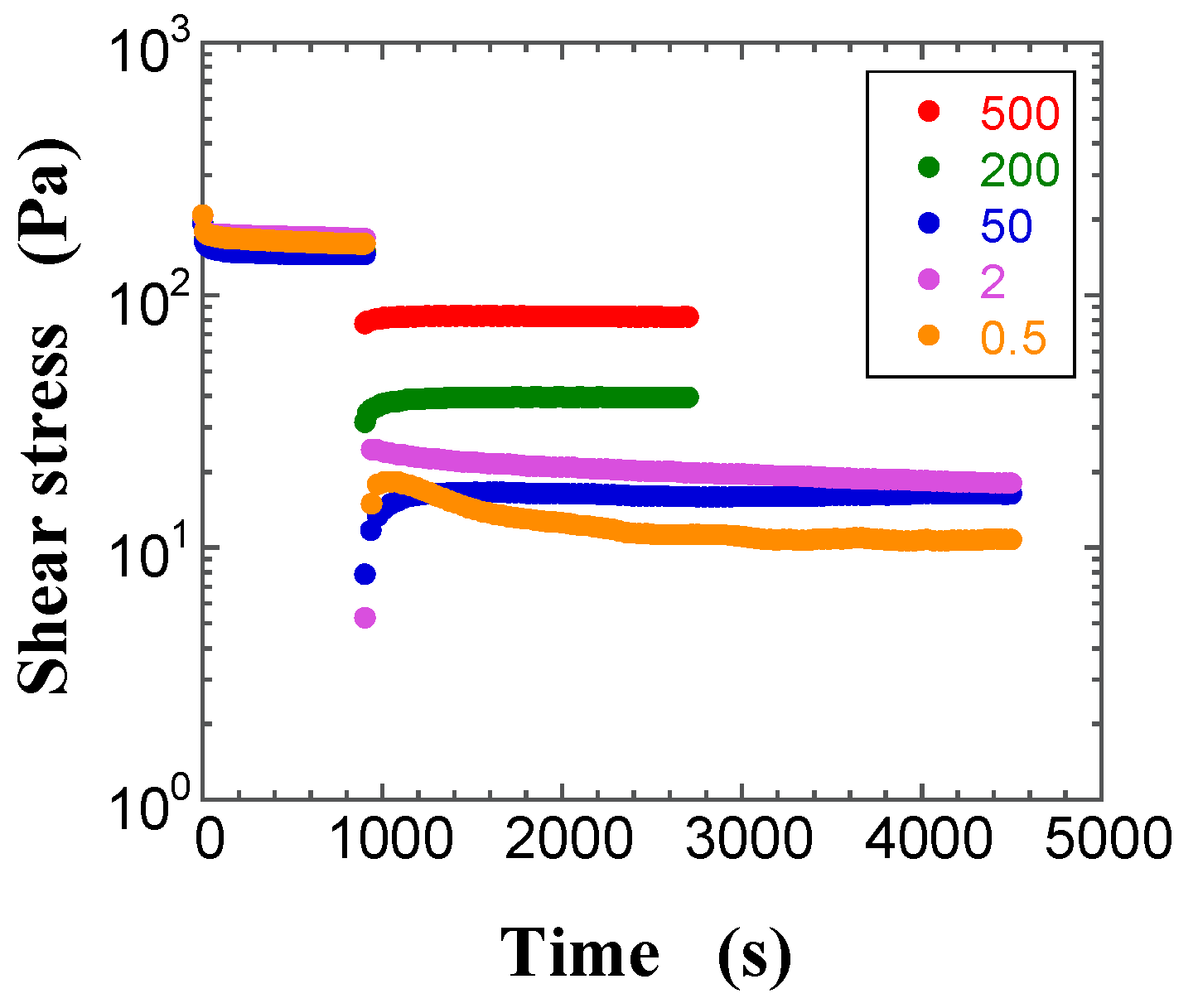
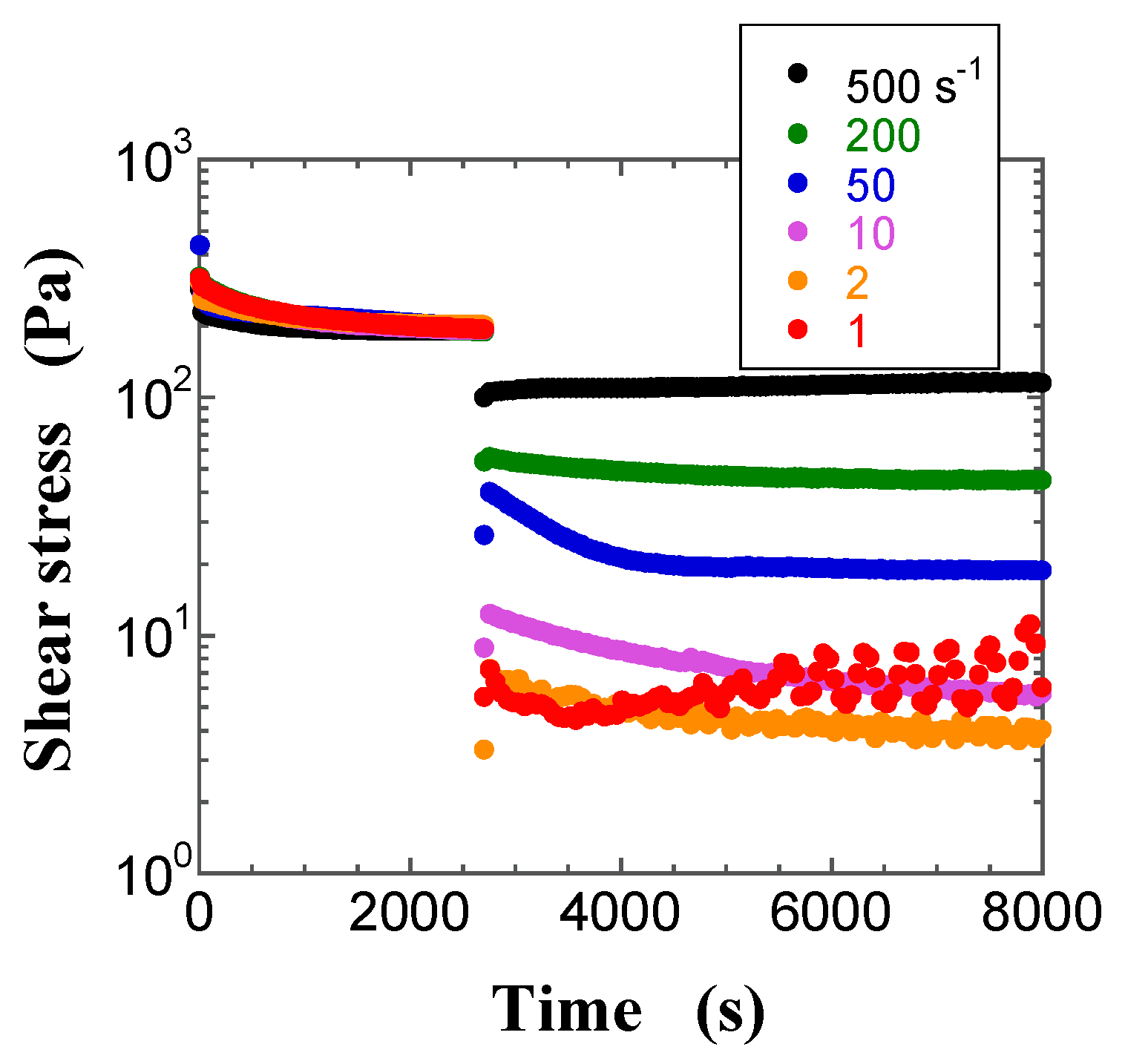
2.2. Transient Shear Stress
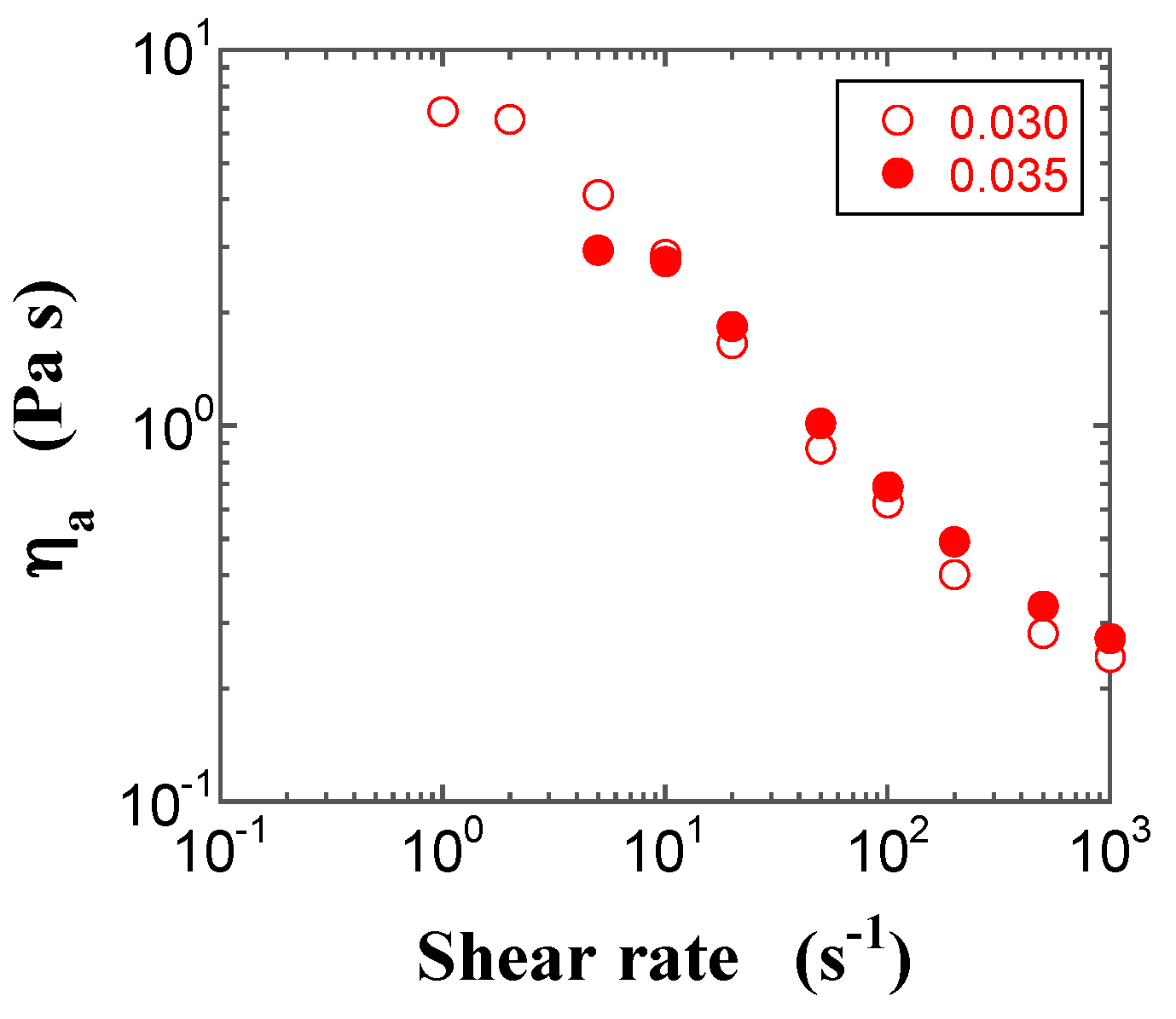
2.3. Steady-State Shear Viscosity
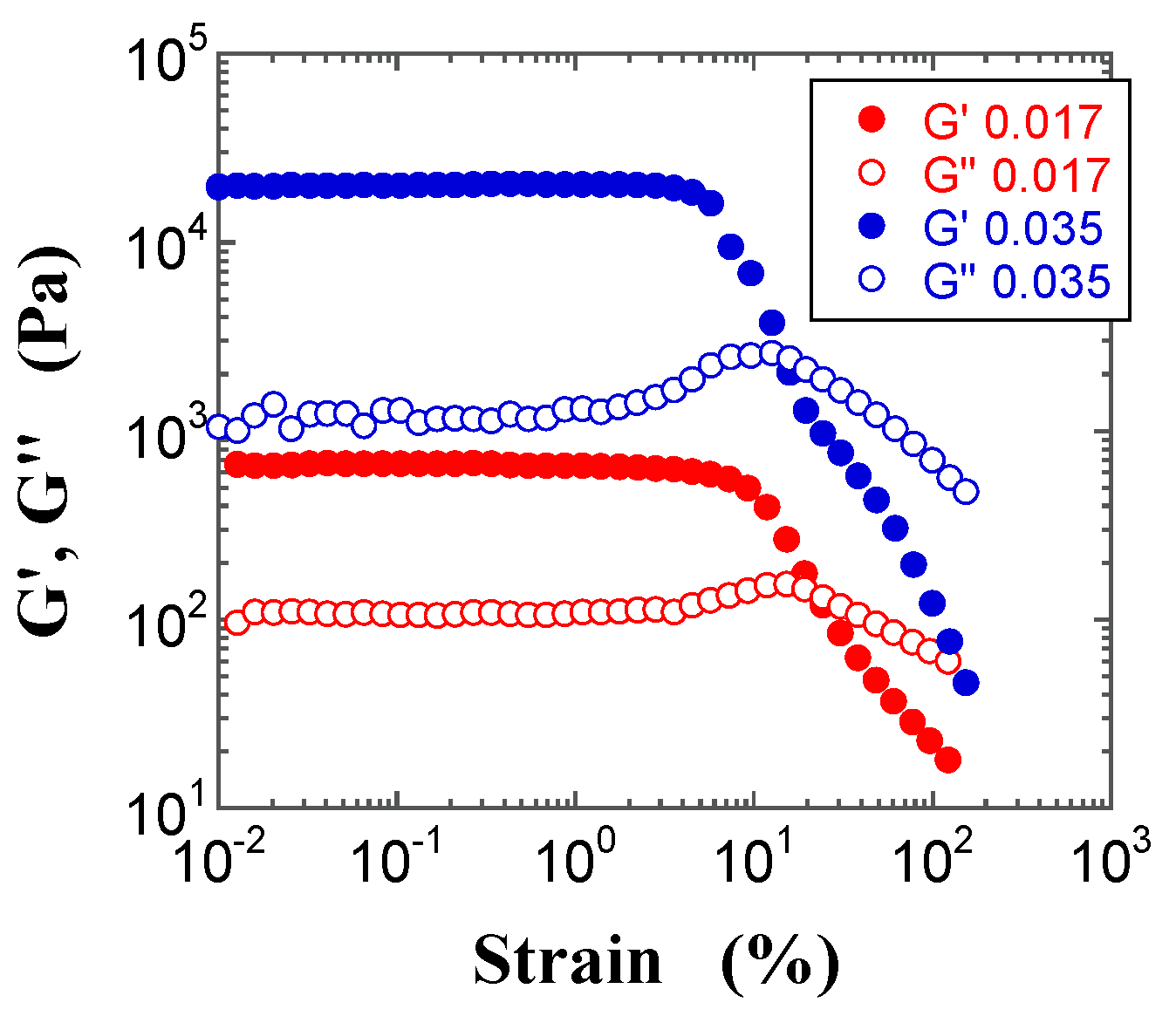
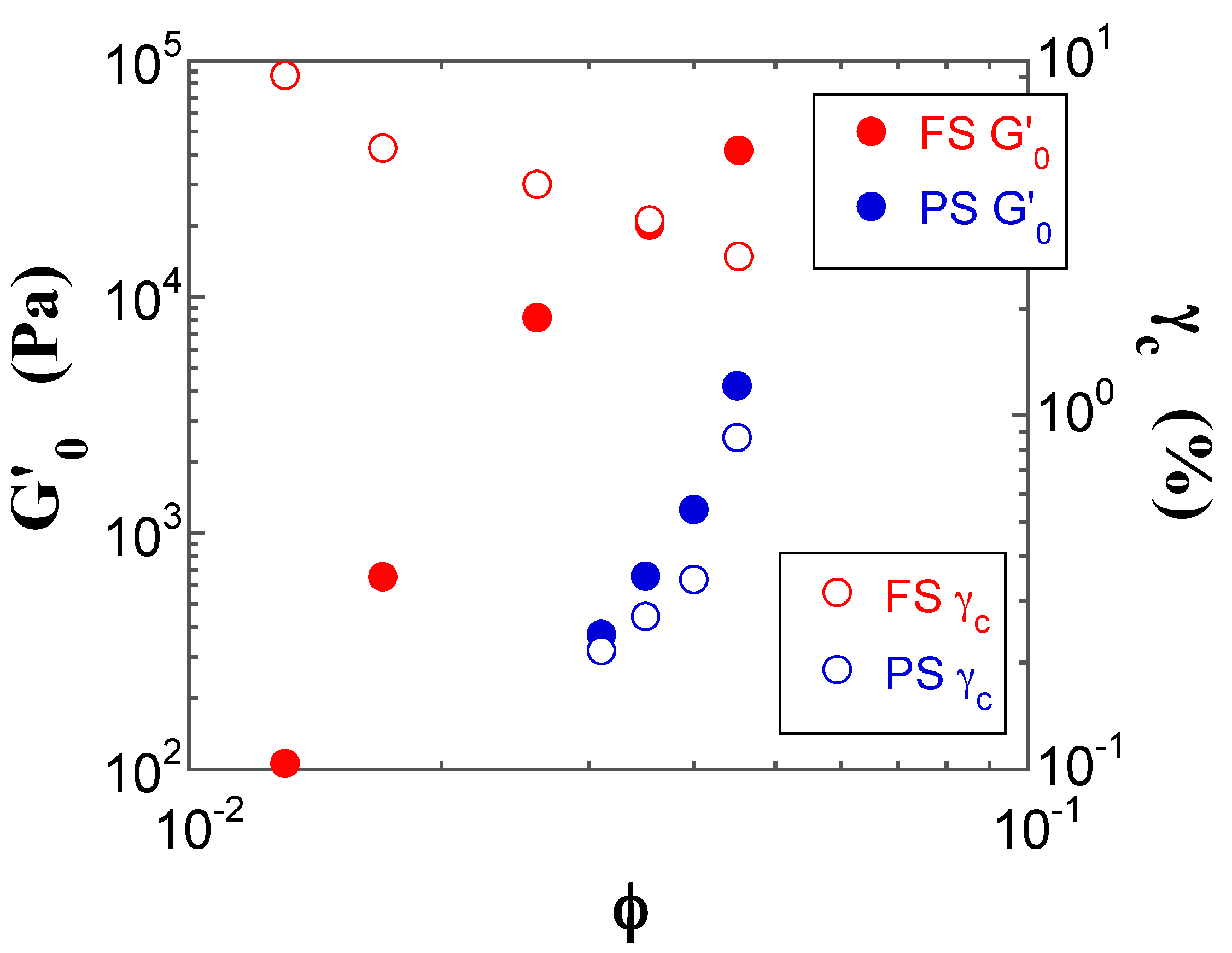
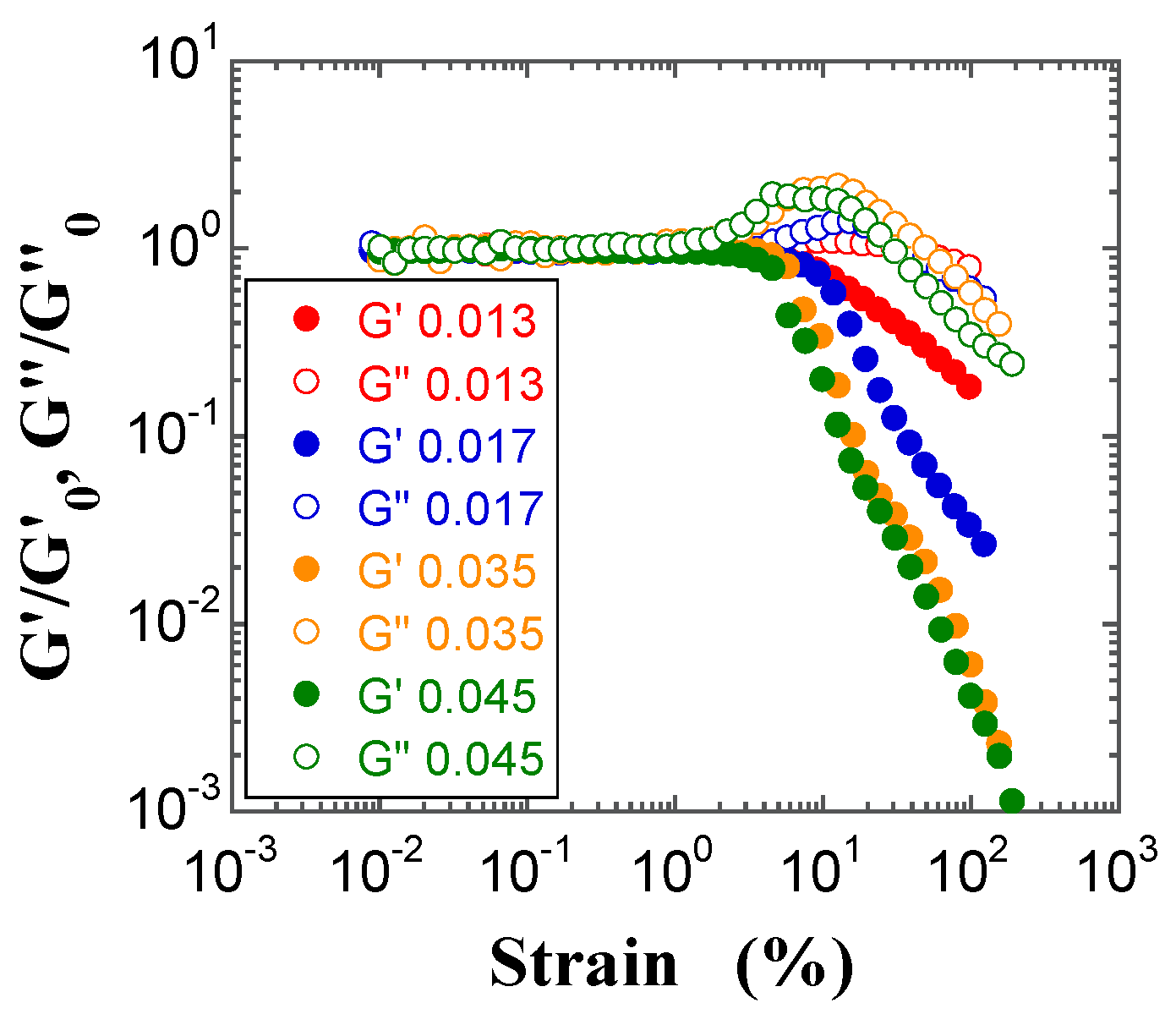
2.4. Dynamic Modulus
3. Experimental Procedures
3.1. Materials
3.2. Preparation of Silica Suspensions
3.3. Rheological Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cohen-Addad, J.P.; Roby, C.; Sauviat, M. Characterization of chain binding to filler in silicone-silica systems. Polymer 1985, 26, 1231–1233. [Google Scholar] [CrossRef]
- Viallat, A.; Cohen-Addad, J.P.; Pouchelon, A. Mechanically induced sorption of siloxane on silica: Experimental and theoretical investigations of chain binding, collective behaviour and multiple-aggregate process. Polymer 1986, 27, 843–848. [Google Scholar] [CrossRef]
- Ziegelbaur, R.S.; Caruthers, J.M. Rheological properties of poly (dimethylsiloxane) filled with fumed silica: I. Hysteresis behavior. J. Nonnewtonian Fluid Mech. 1985, 17, 45–68. [Google Scholar] [CrossRef]
- Kosinski, L.E.; Caruthers, J.M. Rheological properties of poly (dimethylsiloxane) filled with fumed silica: II. Stress relaxation and stress growth. J. Nonnewtonian Fluid Mech. 1985, 17, 69–89. [Google Scholar] [CrossRef]
- Kosinski, L.E.; Caruthers, J.M. The effect of particle concentration on the rheology of polydimethylsiloxane filled with fumed silica. J. Appl. Polym. Sci. 1986, 32, 3393–3406. [Google Scholar] [CrossRef]
- Kosinski, L.E.; Caruthers, J.M. The effect of molecular weight on the rheological properties of poly(dimethylsiloxane) filled with fumed silica. Rheol. Acta 1986, 25, 153–160. [Google Scholar] [CrossRef]
- Aranguren, M.I.; Mora, E.; DeGroot, J.V., Jr.; Macosko, C.W. Effect of reinforcing fillers on the rheology of polymer melts. J. Rheol. 1992, 36, 1165–1182. [Google Scholar] [CrossRef]
- Aranguren, M.I.; Mora, E.; Macosko, C.W. Compounding fumed silicas into polydimethylsiloxane: Bound rubber and final aggregate size. J. Colloid Interface Sci. 1997, 195, 329–337. [Google Scholar] [CrossRef] [PubMed]
- DeGroot, J.V., Jr.; Macosko, C.W. Aging phenomena in silica-filled polydimethylsiloxane. J. Colloid Interface Sci. 1999, 217, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Schaer, E.; Guizani, S.; Choplin, L. Model development for the description of silica particles dispersion in silicone polymer. Chem. Eng. Sci. 2006, 61, 5664–5677. [Google Scholar] [CrossRef]
- Shim, S.E.; Isayev, A.I. Rheology and structure of precipitated silica and poly(dimethyl siloxane) system. Rheol. Acta 2004, 43, 127–136. [Google Scholar] [CrossRef]
- Selomovic, S.; Maynard, S.M.; Hu, Y. Aging effects of precipitated silica in poly(dimethylsiloxane). J. Rheol. 2007, 51, 325–340. [Google Scholar] [CrossRef]
- Khan, S.A.; Zoeller, N.J. Dynamic rheological behavior of flocculated fumed silica suspensions. J. Rheol. 1993, 37, 1225–1235. [Google Scholar] [CrossRef]
- Raghhavan, S.R.; Khan, S.A. Shear-induced microstructural changes in flocculated suspensions of fumed silica. J. Rheol. 1995, 39, 1311–1325. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Khan, S.A. Shear-thickening response of fumed silica suspensions under steady and oscillatory shear. J. Colloid Interface Sci. 1997, 185, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Yziquel, F.; Carreau, P.J.; Tanguy, P.A. Non-linear viscoelastic behavior of fumed silica suspensions. Rheol. Acta 1999, 38, 14–25. [Google Scholar] [CrossRef]
- Chen, S.; Øye, G.; Sjӧblom, J. Rheological properties of silica particle suspensions in mineral oil. J. Dispersion Sci. Tech. 2005, 26, 791–798. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A mechanism for non-Newtonian flow in suspensions of rigid spheres. Trans. Soc. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Hayashi, H.; Kawaguchi, M. Effects of the degree of surface modification on the rheological responses of precipitated silica suspensions in benzyl alcohol. J. Dispersion Sci. Tech. 2017, 38, 737–742. [Google Scholar] [CrossRef]
- Lootens, D.; Damme, H.V.; He´braud, P. Giant Stress Fluctuations at the Jamming Transition. Phys. Rev. Lett. 2003, 90, 178301-1–178301-4. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Kawaguchi, M. Shear-induced changes in rheological responses and neutron scattering properties of hydrophobic silica suspensions at low silica concentrations. J. Dispersion Sci. Technol. 2011, 32, 686–691. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Sood, A.K. Chaotic dynamics in shear-thickening surfactant solutions. Europhys. Lett. 2001, 56, 447–453. [Google Scholar] [CrossRef]
- Ganapathy, R.; Sood, A.K. Intermittency Route to rheochaos in wormlike micelles with flow-concentration coupling. Phys. Rev. Lett. 2006, 96, 108301-1–108301-4. [Google Scholar] [CrossRef] [PubMed]
- Wunenburger, A.S.; Colin, A.; Leng, J.; Arne´odo, A.; Roux, D. Oscillating viscosity in a lyotropic lamellar phase under shear flow. Phys. Rev. Lett. 2001, 86, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.B.; Colin, A.; Roux, D. Dynamical behavior of a complex fluid near an out-of-equilibrium transition: Approaching simple rheological chaos. Phys. Rev. E 2002, 66, 031505-1–031505-12. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.R.; Walls, H.J.; Khan, S.A. Rheology of silica dispersions in organic liquids: New evidence for solvation forces dictated by hydrogen bonding. Langmuir 2000, 16, 7920–7930. [Google Scholar] [CrossRef]
- Liu, X.Q.; Bao, R.Y.; Wu, X.J.; Yang, W.; Xie, B.H.; Yang, M.B. Temperature induced gelation transition of a fumed silica/PEG shear thickening fluid. RSC Adv. 2015, 5, 18367–18374. [Google Scholar] [CrossRef]
- Warren, J.; Offenberger, S.; Toghiani, H.; Pittman, C.U., Jr.; Lacy, T.E.; Kundu, S. Effects of temperature on the shear-thickening behavior of fumed silica suspensions. Appl. Mater. Interfaces 2015, 7, 18650–18661. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Hernández, F.J.; Ayúcar-Rubio, M.F.; Velázquez-Navarro, J.F.; Galindo-Rosales, F.J. Intrinsic viscosity of SiO2, Al2O3 and TiO2 aqueous suspensions. J. Colloid Interface Sci. 2006, 298, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.; Shih, W.Y.; Kim, S.; Liu, J.; Aksay, I.A. Scaling behavior of the elastic properties of colloidal gels. Phys. Rev. A 1990, 42, 4772–4779. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Morbidelli, M. A model relating structure of colloidal gels to their elastic properties. Langmuir 2001, 17, 1030–1036. [Google Scholar] [CrossRef]
- Genovese, D.B. Shear rheology of hard-sphere, dispersed, aggregated suspensions, and filler-matrix composites. Adv. Colloid Interface Sci. 2012, 171/172, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Kawaguchi, M. Effects of solvent power on dynamic moduli of polydimethylsiloxane-treated fumed silica suspension gels. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 436, 1041–1047. [Google Scholar] [CrossRef]
- Marunaka, R.; Kawaguchi, M. Rheological behavior of hydrophobic fumed silica suspensions in different alkanes. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 456, 75–82. [Google Scholar] [CrossRef]
- Marunaka, R.; Kawaguchi, M. Rheological behavior of hydrophobic fumed silica suspensions in aromatic dispersion media. J. Dispersion Sci. Technol. 2017, 38, 223–228. [Google Scholar] [CrossRef]
- Amiri, A.; Øye, G.; Sjӧblom, J. Influence of pH, high salinity and particle concentration on stability and rheological properties of aqueous suspensions of fumed silica. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 349, 43–54. [Google Scholar] [CrossRef]
- Yokoyama, K.; Koike, Y.; Masuda, A.; Kawaguchi, M. Rheological properties of fumed silica suspensions in the presence of potassium chloride. Jpn. J. Appl. Phys. 2007, 46, 328–332. [Google Scholar] [CrossRef]
- Asai, H.; Masuda, A.M.; Kawaguchi, M. Rheological properties of colloidal gels formed from fumed silica suspensions in the presence of cationic surfactants. J. Colloid Interface Sci. 2008, 328, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Kim, S.H.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear as a way to classify the complex fluids. J. Nonnewtonian Fluid Mech. 2002, 107, 51–65. [Google Scholar] [CrossRef]
- Hyun, K.; Nam, J.G.; Wilhellm, M.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear behavior of PEO-PPO-PEO triblock copolymer solutions. Rheol. Acta 2006, 45, 239–249. [Google Scholar] [CrossRef]
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A review of nonlinear oscillatory shear tests: Analysis and application of large amplitude oscillatory shear (LAOS). Prog. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugino, Y.; Kawaguchi, M. Fumed and Precipitated Hydrophilic Silica Suspension Gels in Mineral Oil: Stability and Rheological Properties. Gels 2017, 3, 32. https://doi.org/10.3390/gels3030032
Sugino Y, Kawaguchi M. Fumed and Precipitated Hydrophilic Silica Suspension Gels in Mineral Oil: Stability and Rheological Properties. Gels. 2017; 3(3):32. https://doi.org/10.3390/gels3030032
Chicago/Turabian StyleSugino, Yoshiki, and Masami Kawaguchi. 2017. "Fumed and Precipitated Hydrophilic Silica Suspension Gels in Mineral Oil: Stability and Rheological Properties" Gels 3, no. 3: 32. https://doi.org/10.3390/gels3030032