1. Introduction
Deficient bioavailability due to certain physicochemical properties of some active substances is still a challenge in pharmaceutical science. Thus, new formulation strategies are required to overcome this problem—for example for poorly water-soluble compounds [
1].
In the last years, the study of formulations to deliver water-insoluble molecules has increased. Emulsions and solid lipid nanoparticles (SLN) are up to now the most studied systems to deliver this type of molecule, and are unstable and have limited loading capacity. Therefore, there is still a demand for readily stable hydrophobic carriers with a higher loading capacity. Colloidal dispersions of gelled lipid nanoparticles (GLN) present an attractive alternative to surpass these limitations, since they may act as hydrophobic reservoirs [
2,
3].
Organogels are semi-solid systems (soft materials) in which a three-dimensional network composed of cross-linked gelator fibers immobilizes an organic liquid [
4]. Organogels are principally classified based on the type of organogelator used, but also based on the nature of the organic liquid and its intermolecular interactions [
5,
6]. These organogelators can be divided into polymeric organic gelators (POG) and low molecular-mass organic gelators (LMOG) [
5]. POG are high molecular-mass molecules which are capable of jellifying organic solvents, forming physical crosslinking points. These crosslinking points can be due to the formation of hydrogen bonds (inducing the formation of α-helices and β-sheets) for polypeptide polymers or π−π stacking interactions for p-conjugated polymers. LMOG are relatively small organic molecules (molecular weight < 2000 g/mol, usually even smaller than 500 g/mol) which are capable of forming a three-dimensional network around the organic liquid, thus gelling the organic liquid. LMOG have several advantages over POG: better biocompatibility, lower toxicity, easy to prepare, stronger stability, and high gelling capacities needing less quantities of gelator (<1 wt %) [
5,
6].
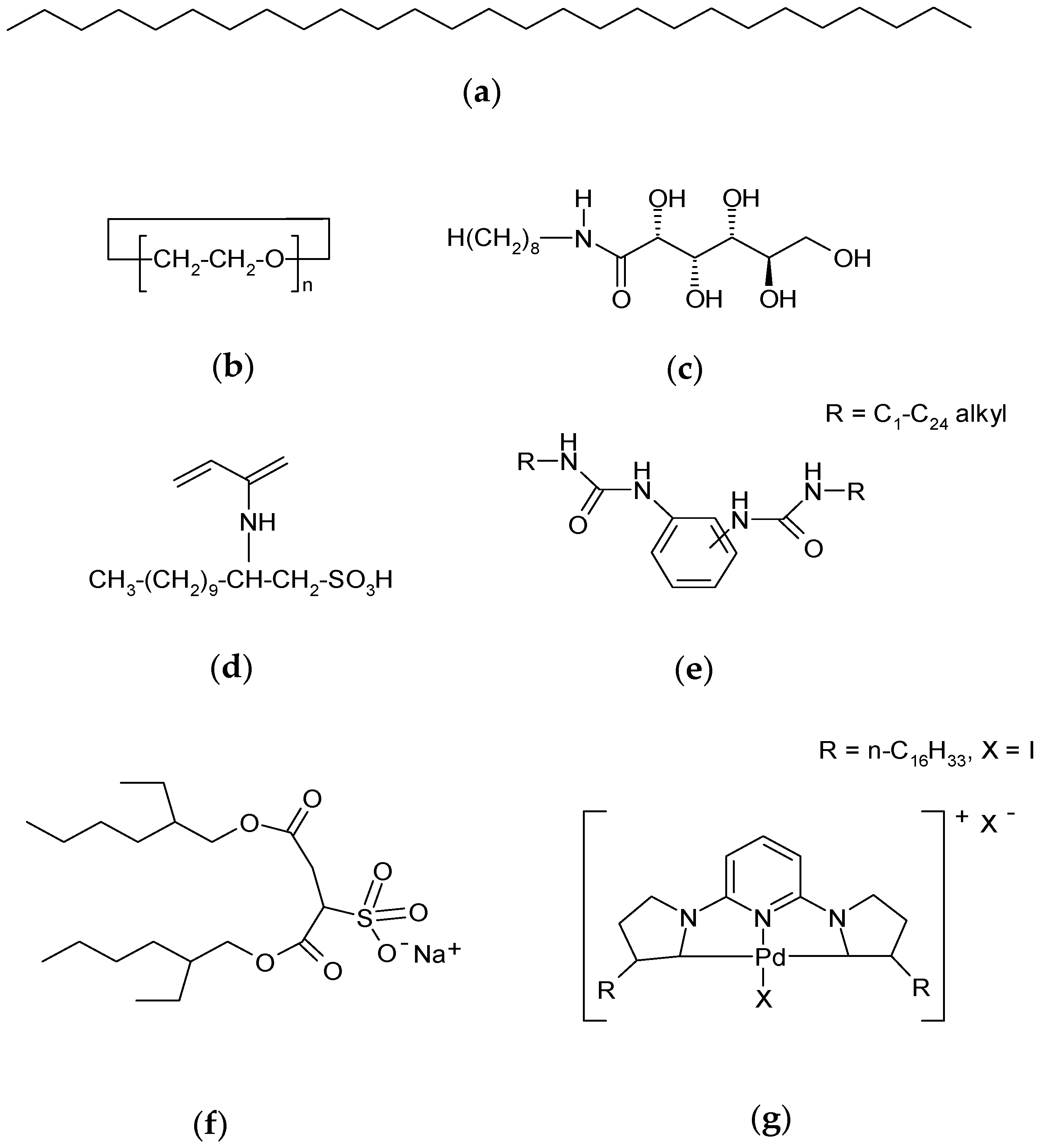
The LMOG are separated into different classes according to criteria based on their molecular structure. For example, they can be classified as alkane organic gelators, as n-octacosane (
Figure 1a), organic gelators with one heteroatom as ethers (e.g., 60-crown-20-macrocycle;
Figure 1b), organic gelators with two heteroatoms (e.g.,
N-octyl-
d-gluconamide;
Figure 1c), organic gelators with three heteroatoms (e.g., racemic 2-acryloylamide-dedecane-1-sulfonic acid or ADSA;
Figure 1d), polymerizable organic gelators such as bis-urea gelators (
Figure 1e), two-component organic gelators such as bis-(2-ethylhexyl) sodium sulfosuccinate or AOT (
Figure 1f), and organometallic gelators such as palladium-CNC pincer bis(carbene) (
Figure 1g) [
5,
6].
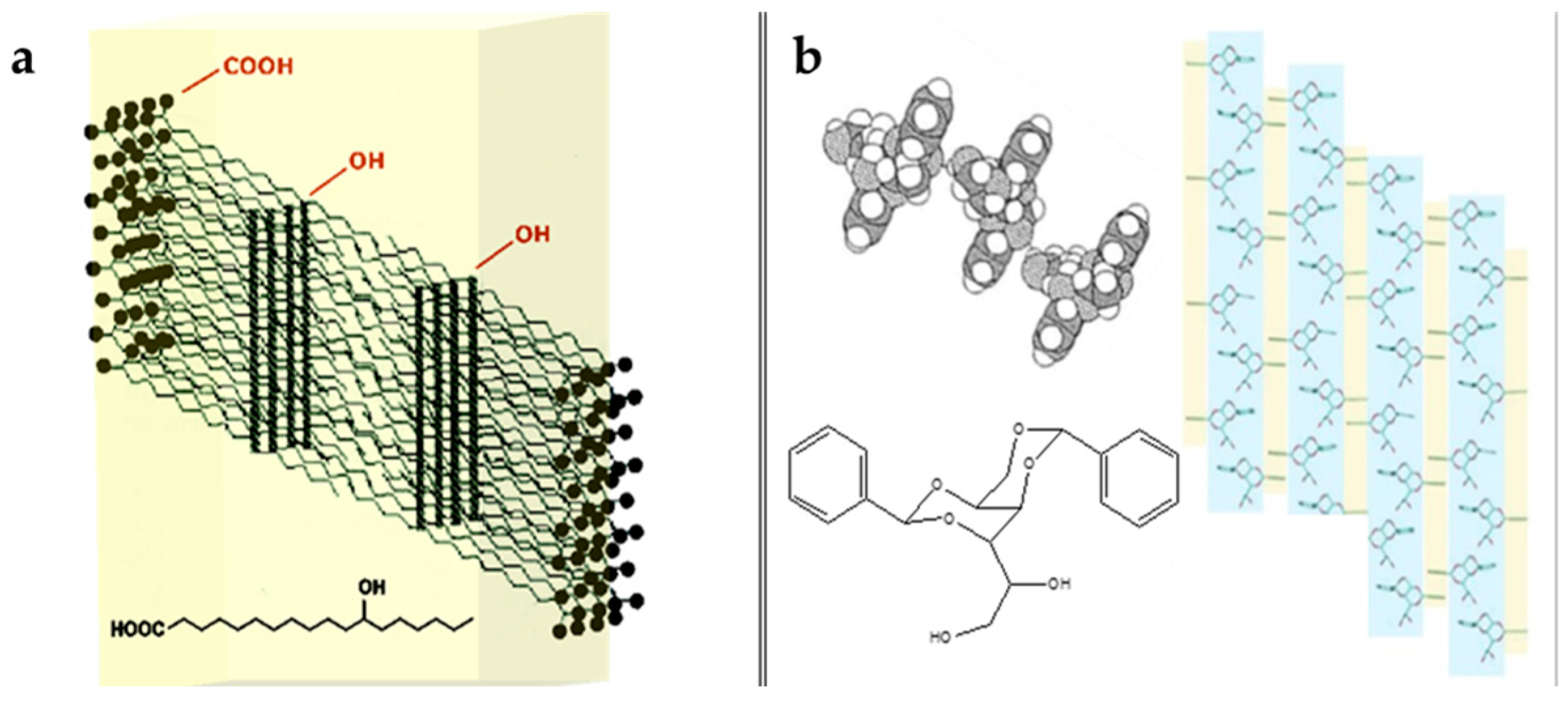
Derived from this classification, several chemical compounds are used in cosmetics, such as 12-hydroxystearic acid (HSA) (
Figure 2a), 1,3:2,4-di-
O-benzylidene-
d-sorbitol (DBS) (
Figure 2b), sterols, lecithin, mono- and diglycerides, lecithin mixtures with sorbitan esters, fatty acids, fatty alcohols, waxes, and wax esters [
5,
6].
Plant metabolites—many of which are very structurally complicated—present attractive candidates as new LMOGs because they are available in renewable supply without extensive synthetic effort. Moreover, LMOG organogels can be classified into two groups depending on the kinetic properties of aggregates: “strong” organogel (solid fiber network) and “weak” organogel (fluid fiber network) [
7,
8,
9].
In solution, LMOG self-assemble to form fibrous structures responsible for the gelation phenomenon [
4]. Typically, the intermolecular forces that drive aggregation include hydrogen-bonding, π−π stacking, dipole−dipole, and London dispersion forces; however, hydrogen-bonding is a very important component of the self-assembly driving force in a large fraction of reported organogelators [
10]. These physical gels are usually thermoreversible (reversible gel–sol/sol–gel phase transition by heating and cooling, respectively), depending on the molecular structure of the gelling agent and the fluid which is rigidified. It is possible to form nanoscale superstructures such as nanofibres, nanoribbons, nanosheets, nanoparticles, etc., which are of interest for materials and nanostructures conception. The interconversion between the gel and solution/sol phases depends only on the disassembly and assembly of the constituent molecules [
7,
11].
LMOGs are mostly used, since a limited number of synthetic polymers (e.g., polypeptides and polyesters) with these organogelation properties have been reported [
12,
13,
14].
2. Preparation of LMOG Organogel Self-Assembled Systems
The formulation of stable colloidal dispersions of GLN includes two successive steps. The preparation of the organogel from an organic liquid (vegetable or emollient oil) and an organic gelator as the first step. After organogel preparation, aqueous dispersions of GLN are obtained by their dispersion in a water phase containing a stabilizing and/or emulsifying agent(s) [
12] (
Figure 3).
2.1. Gelation Process
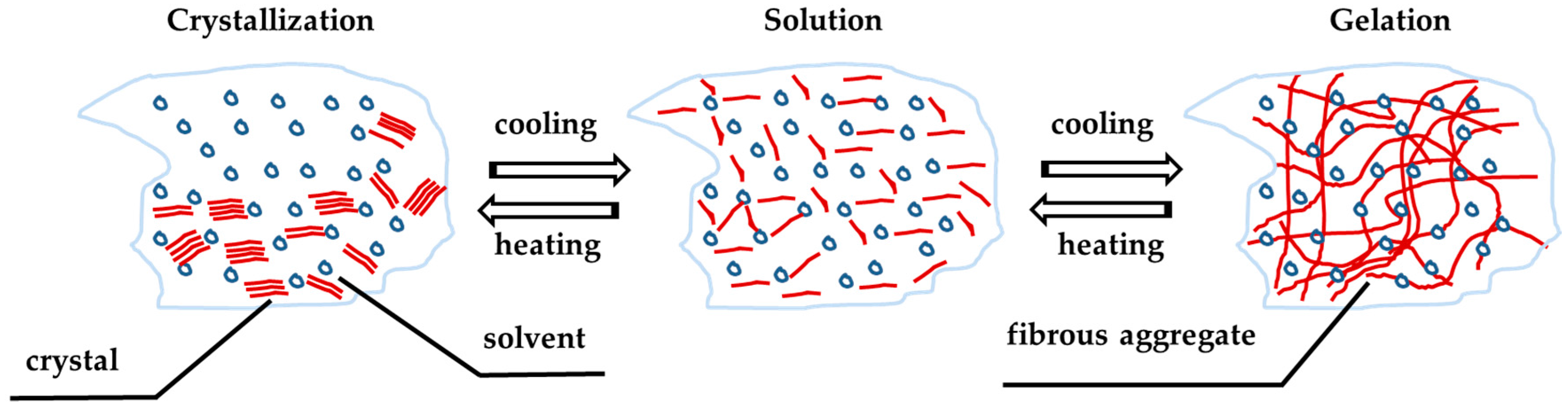
LMOG organogels are obtained by dissolving a small amount of the organogelator at high temperature to obtain an isotropic solution and then cooling this solution under its characteristic gelation transition temperature, known as Tgel. The mixture must be completely solubilized and homogenized before being cooled below its Tgel, which represents the temperature from which the organogel formation process started. The Tgel has proven to be dependent on the concentration of the organogelator, the properties of the organic liquid (or oil), such as its polarity or viscosity, and in some cases of the cooling process. Gelation occurs as the hot solutions cools, while the self-assembled networks are formed. Since LMOGs form a physical gel (unlike chemical gels), these organogels are thermally reversible organic gel-like materials. The gelation process in this type of organogel is represented in
Figure 4 [
12].
It is important note that organogels formed by LMOGs tend to crystallize, leading to a more metastable organogel. It is possible to adhere a polymer as a gelation-driven compound with covalent bonds, converting it into a “polymer-based gelator” or supramolecular crosslinkable polymer organogelator that can form a more stable gel. For example, polysiloxanes, polyethers, and polycarbonates are useful polymers to confer a more stable characteristic to LMOGs [
15]. Moreover, lecithin—a common organogelator usually used as stabilizing and dispersing agent to obtain stable organogel microemulsions—is expensive and difficult to obtain with high purity. When using lecithin of lesser purity, it can be used in the presence of synthetic polymers in the preparation of gel microemulsions in order to obtain the same stabilizing properties [
16].
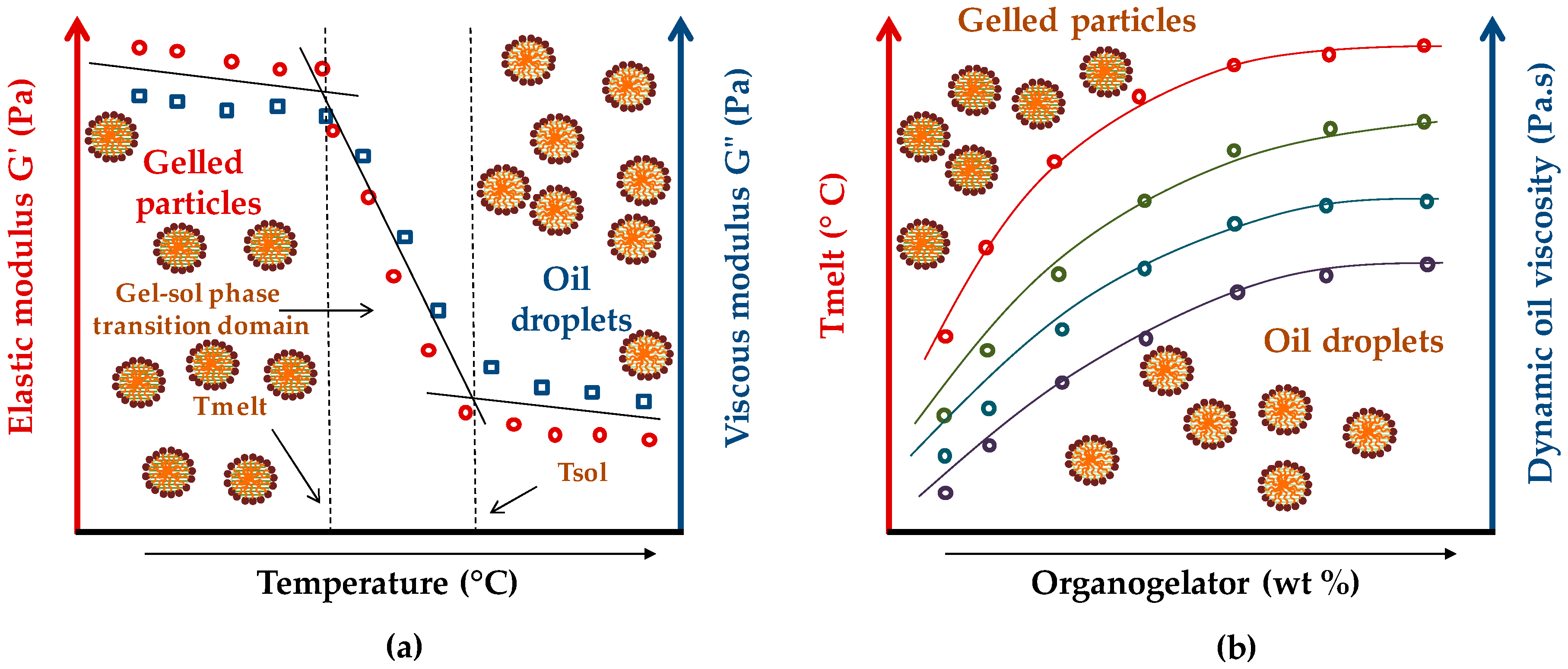
2.2. Phase Transition Parameters
The primary concept to consider in organogels is their phase transition parameters. These parameters are determined by rheology, as following the evolution of the elastic (G′) and viscous (G″) moduli versus temperature increase or decrease. The viscoelastic moduli permit the determination of the phase transition parameters of an organogel, such as gel–sol or sol–gel phase transition domains (PTDs), Tmelt (organogel melting temperature), Tgel, Tsol (organogel liquefaction temperature), as well as Tform (organogel formation temperature) [
5,
6].
Figure 5 shows the viscoelastic behavior of an LMOG organogel during its melting process (
Figure 5a) and during its formation process (
Figure 5b).
The rheological behavior of an LMOG organogel versus temperature presents two phases: the gel phase and the sol phase, separated by a transitional phase, known as PTD. According to the considered process, there is a gel–sol PTD (organogel liquefaction) or sol–gel PTD (organogel formation). The Tmelt corresponds to an abrupt decrease of the viscoelastic moduli and characterizes the beginning of the organogel melting process. This process is progressive, and takes place over a temperature range determined by the gel–sol PTD, and from a certain temperature (known as Tsol—organogel liquefaction), the organogel is completely liquefied. Concerning the sol–gel process (organogel formation), the Tgel corresponds to the abrupt increase of the viscoelastic moduli. Tgel indicates the beginning of the gelation process, contrary to the Tform which indicates the organogel formation temperature, above which the material shows constant viscoelastic properties with the temperature decrease. The sol–gel PTD is closed between Tgel and Tform.
The phase transition parameters of organogels obtained from HSA and various oils (emollients and vegetables) are reported in
Table 1. The data show that Tgel and Tmelt values increase with increasing HSA proportion, regardless of the nature of the oil. Concerning Tsol and Tform, these parameters also increase when the HSA wt % increases and show higher values with vegetable oils, which is related to their important dynamic viscosity. The PTD range variation depends on the considered process. In the case of sol–gel PTD, it is closer than those of the gel–sol one. Moreover, this difference decreases as the proportions of HSA are increased for both types of oils (emollients and vegetable oils).
It is observed that when the proportion of HSA is increased, the sol–gel PTD increases and the gel–sol PTD decreases. The formation or destruction of the three-dimensional network of HSA fibers involves two very different processes. In the case of gelling, it is a highly cooperative process that is very energetically favorable. Moreover, during the melting process, there is a progressive breakdown of the network and the fluidization is complete only when all the HSA molecules are dissociated. These two processes are also very sensitive to the strength of the network junctions; that is, to their degrees of crystallization. The increase in HSA content increases the number of junctions in the network, which widens the sol–gel PTD because the network has a higher degree of interconnectivity. At low HSA wt %, a low density of junctions is observed, but these are strongly crystallized. Moreover, when the HSA content increases, the fiber network is more entangled, but the junction zones are more disordered and therefore break more rapidly during the gelling process.
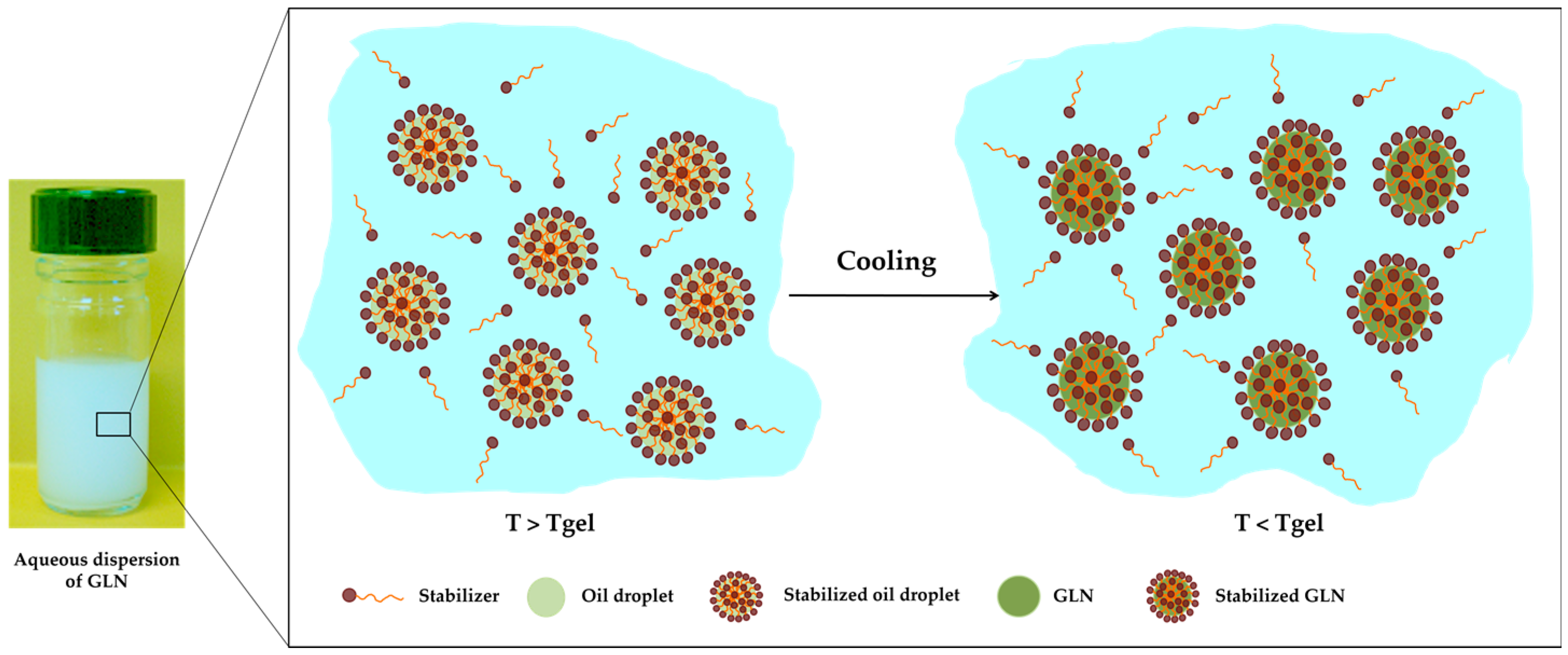
2.3. Organogel Nanoparticle Dispersion
Tgel has an important part in the preparation of the GLN dispersions from a LMOG. As observed in
Figure 6, the formation of these one depends on this parameter.
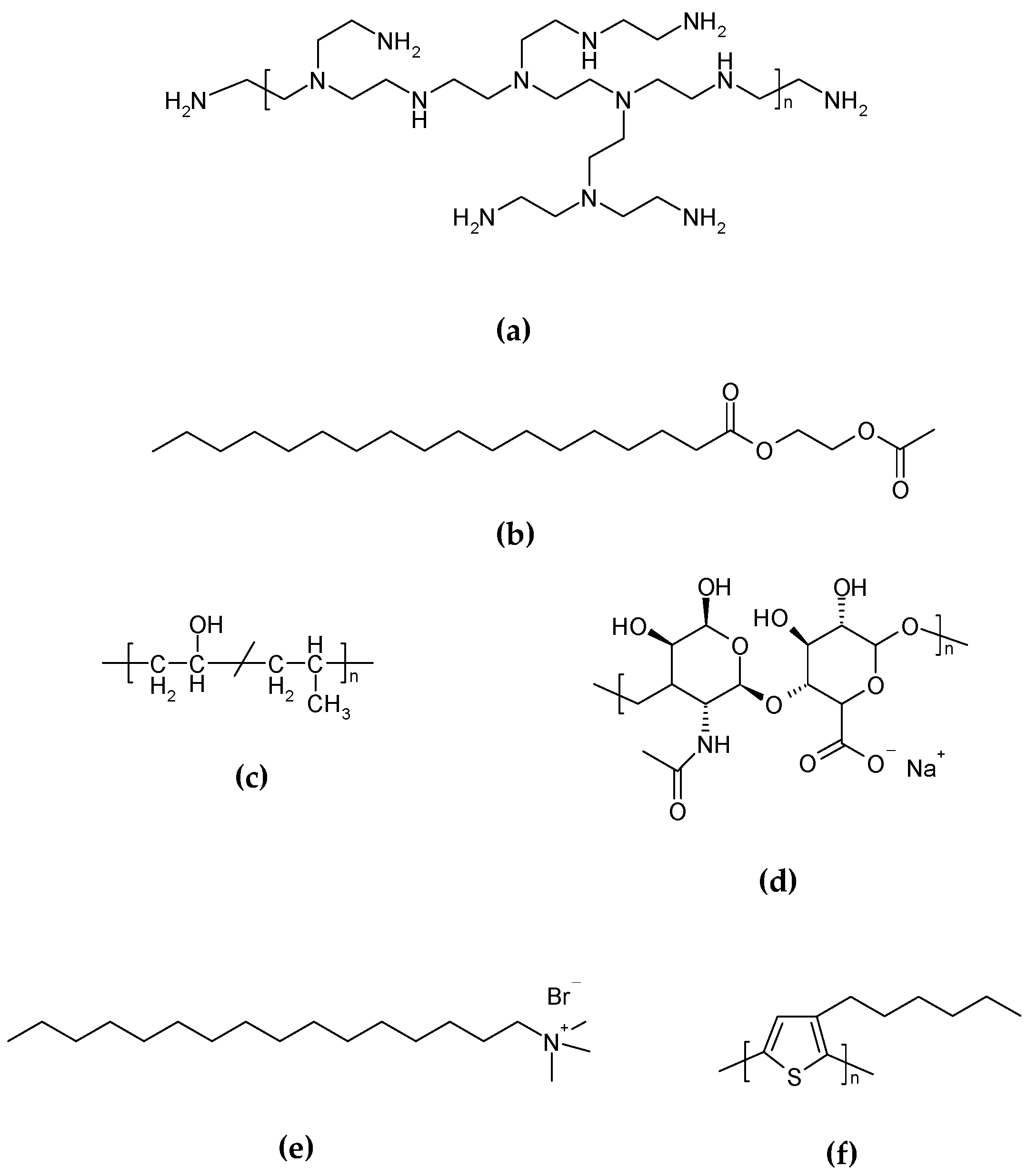
The mixture must be heated over the characteristic organogel Tgel, and then homogenized while still hot by ultrasound probe or using mechanical homogenization in the presence of emulsifying, thickening, or stabilizing agents. For example, polyethylenenimine (PEI) (
Figure 7a) [
17], acetylated glycol stearate (AGS) (
Figure 7b), and polyvinyl alcohol (PVA) (
Figure 7c) are used as stabilizing agents, and sodium hyaluronate (SH) as a thickening one (
Figure 7d) [
4]. Another common surfactant used as a stabilizing agent for these preparations is cetyltrimethylammonium bromide (CTAB) (
Figure 7e) [
12]. Even though both homogenization methods have been reported, sonication with ultrasound probe has proven to produce a more stable aqueous dispersion obtained from vegetable oils and HSA as organogelator [
4], as well as for organogel dispersions with poly(3-hexylthiophene) (P3HT) (
Figure 7f) [
18].
4. GLN Potential Applications
Organogel dispersions of GLN are a suitable formulation for the preparation of new systems which could be used for cosmetic applications or as smart nanoparticles for drug delivery [
4,
12]. According to the data base, one of the first patent applications where the term organogel appeared was deposited organic solvent [
21]. However, it was not until 1996 that the organogel dispersion concept appeared in European application patents. Notably, in the dermo-cosmetic field, companies like L’Oréal have patented dispersions of gel particles in a stabilized aqueous phase. [
22].
Besides the cosmetic industry, organogel dispersions have been used as drug delivery systems and have proved their ability to enhance the permeation and improve drug release in dermal and transdermal formulations (
Figure 9). It has been shown that organogel dispersions are effective in the improvement of sun protection factor (SPF) value, increasing the ability to absorb UV rays. This could be related to the optical properties of the particles and diffusing capacity provided by the organogelator. There is a synergistic improvement of two factors: the photo-protective ability and the photo-stability of the UVB filter [
4]. Moreover, it has been used for topical treatments of early stages of skin cancers. In addition, these systems could be used for intravenous applications by adapting the formulation for this aim [
3].
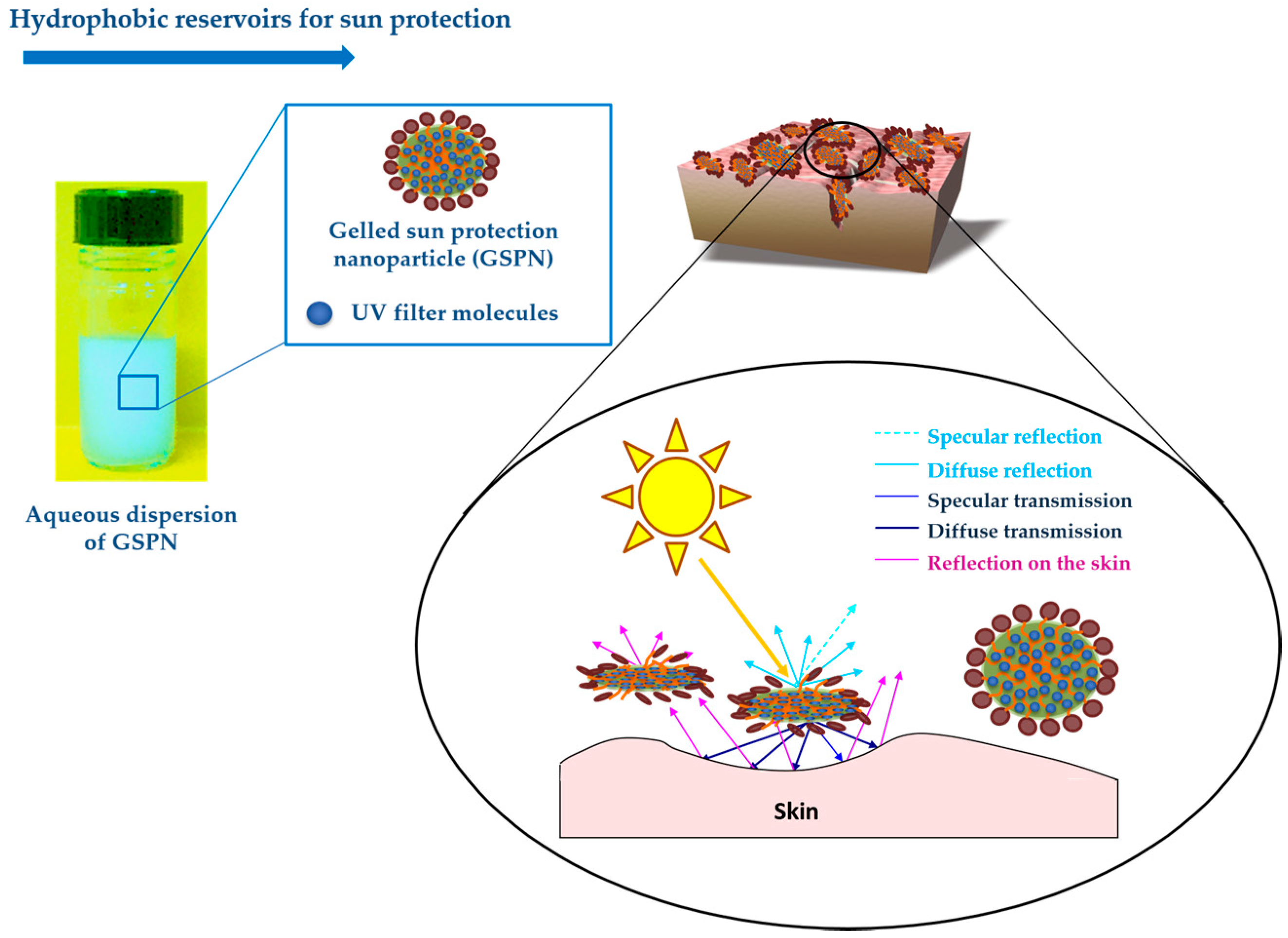
4.1. Hydrophobic Reservoirs for Sun Protection
Gelled sun protection nanoparticles (GSPN) in water could be employed as a nanocarrier for dermo-cosmetic applications or as hydrophobic reservoirs for photoprotection (
Figure 10) [
4,
5,
6,
17,
23].
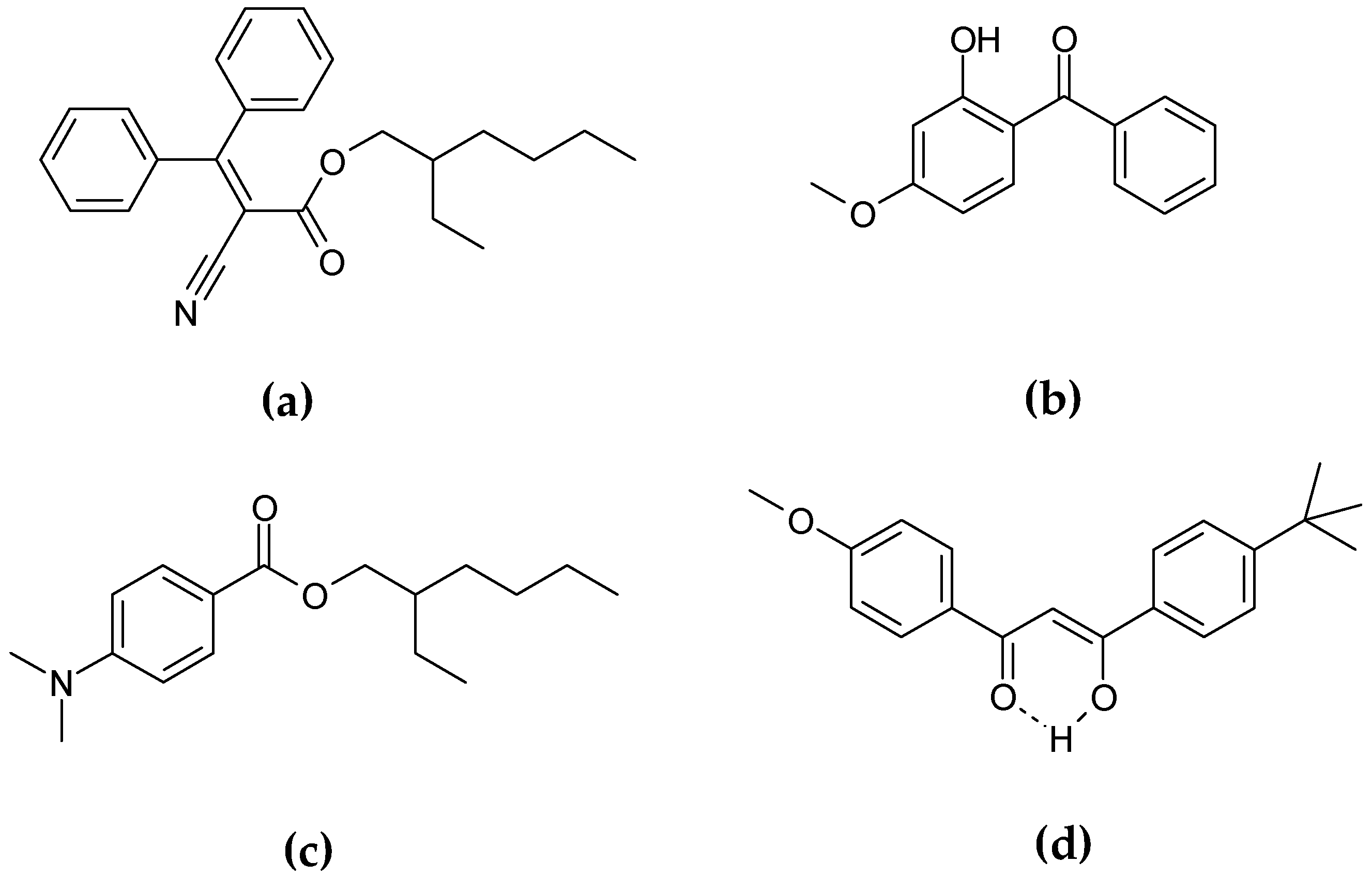
The preparation process and physicochemical properties of these GSPN aqueous dispersions have been described previously by Chaouat et al. [
24]. Gelled particle dispersions of sunscreen organic mixture–1-(4-methoxyphenyl)-3-(4-
tert-butylphenyl)propane-1,3-dione or avobenzone (UVA solar filter) (
Figure 11a) dissolved in 2-ethylhexyl 2-cyano-3,3-diphenyl-2-propenoate or octocrylene (UVB solar filter, viscous liquid) (
Figure 11b) and HSA as organic gelator were obtained by emulsification at high temperature and cooling at room temperature, using PVA as a stabilizing and dispersing agent. The results confirmed that the GSPN size was in agreement with the necessity of keeping the particles on the skin surface, avoiding their penetration through the
stratum corneum. TEM observation of the dispersion showed spherical particles with a mean diameter of 600 nm—a size in accordance with the DLS measurements. The UV spectroscopy observations showed more important absorption of the gelled particles compared to that of corresponding emulsion droplets. A rheological investigation allowed the particle sol-gel phase transition parameters to be determined, confirming their gelled state. A comparative aging study of the emulsion and corresponding dispersion showed greatly enhanced physical stability after gelation. This last point was also proved by ζ-potential measurements.
According to Kirilov et al., aqueous dispersions of GSPN were also obtained with two organic liquids [
4]. Vaseline and almond oils were used as organic media for the GSPN preparation. Gelled nanoparticles containing a model sunscreen molecule with mean size of 450 nm, ζ-potential value above −30 mV, and PDI of 0.18 were obtained by the ultrasound probe homogenization method. A comparative study of their dispersion aging showed a greatly enhanced GSPN stability after gelation. According to the UVB absorption evaluation, GSPN improved the photoprotective ability and the photostability of the immobilized UVB blocker as 2-ethylhexyl-p-dimethylaminobenzoate or EHMAB (
Figure 11c). In addition, GSPN showed a high water resistance (~83%), even after 40 min of immersion.
Actually, studies concerning the immobilization of benzophenone-3 or BP-3—a commonly used UVA and UVB absorbed molecule—as GSPN are being carried out (
Figure 11d).
The obtained results demonstrate the interest in GSPN and their aqueous dispersion as a concept for the preparation of original sun protection formulations with improved diffusion properties and waterproof ability.
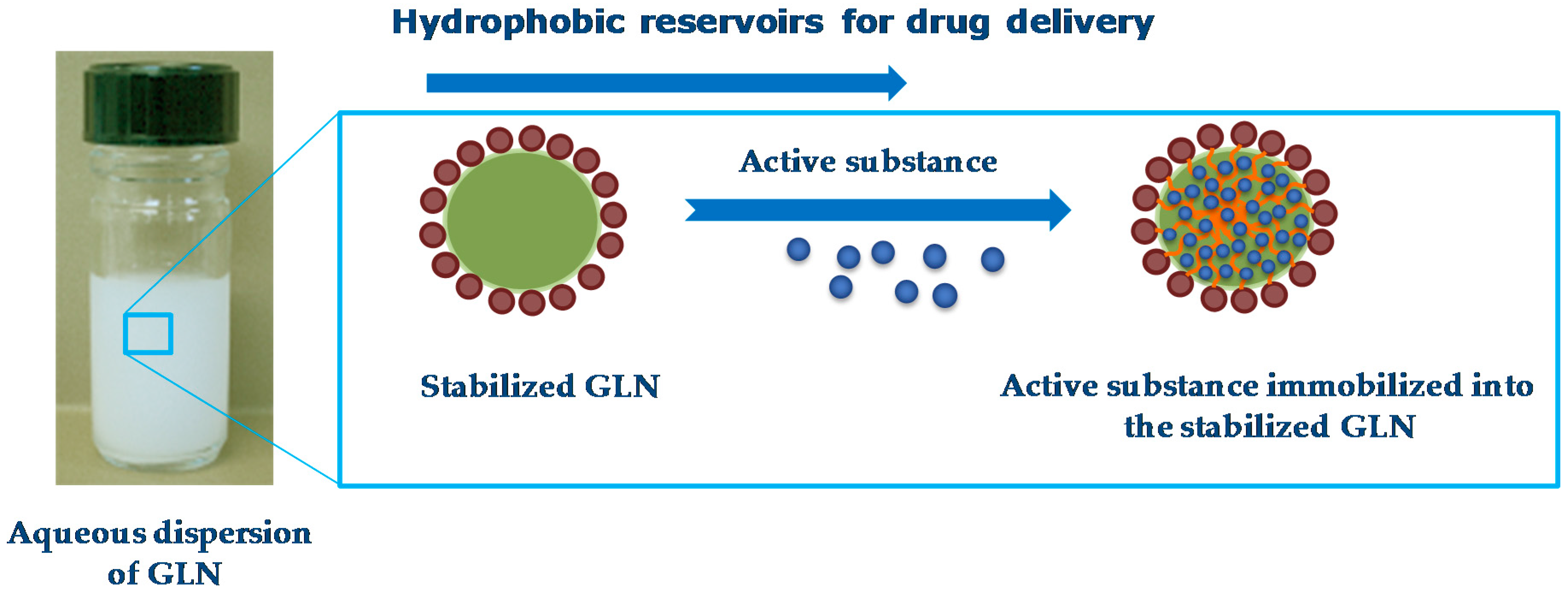
4.2. Hydrophobic Reservoirs for Drug Delivery
Other studies demonstrated the interest of colloidal dispersions of GLN for the encapsulation and delivery of lipophilic active compounds (
Figure 12).
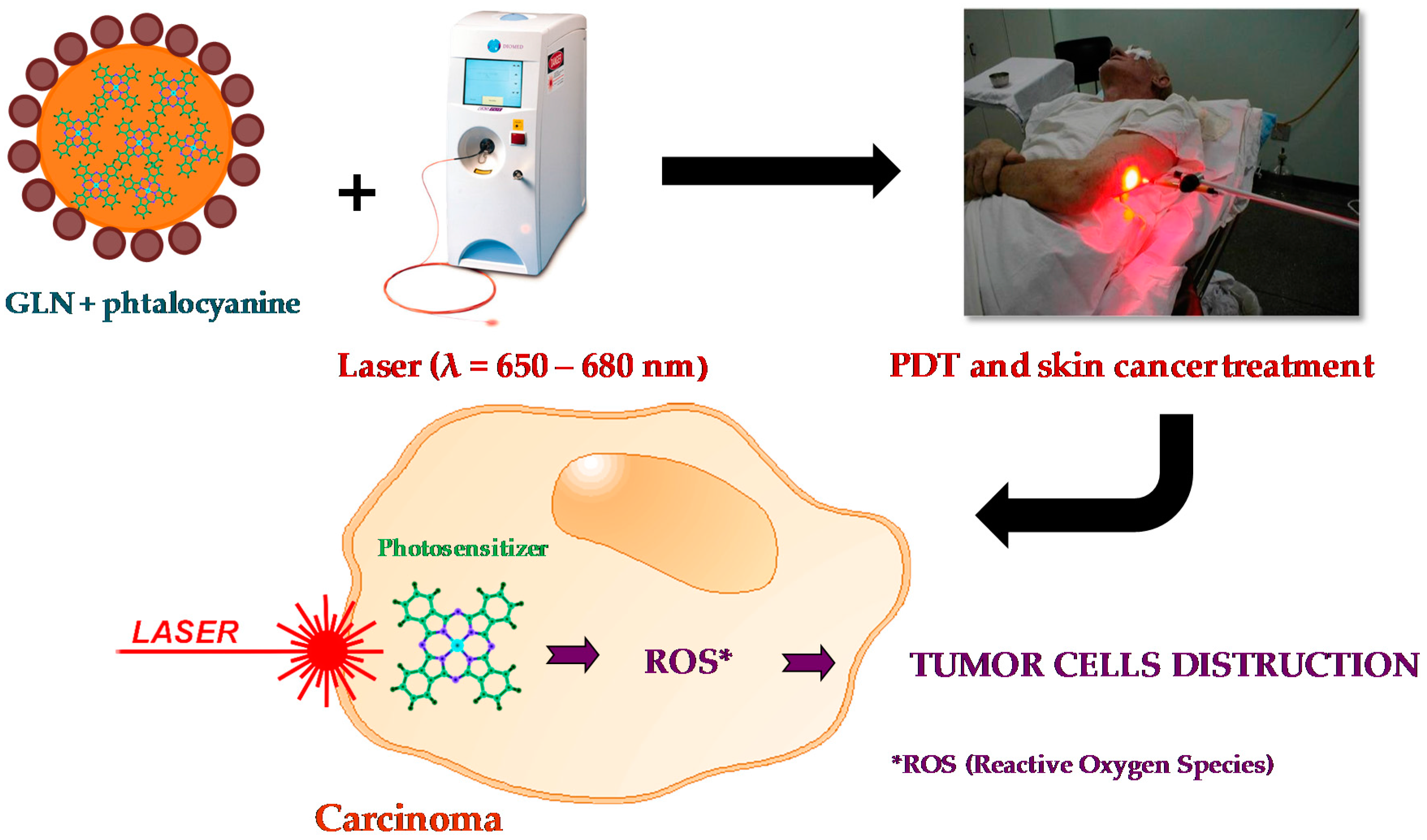
Sigueira-Mourra et al. prepared stable dispersions of soybean oil GLN with excellent chloroaluminum phthalocyanine (ClAlPc) encapsulation efficiency. The obtained ClAlPc-loaded GLN formulation could be a very useful system for photodynamic therapy (PDT) procedures (
Figure 13).
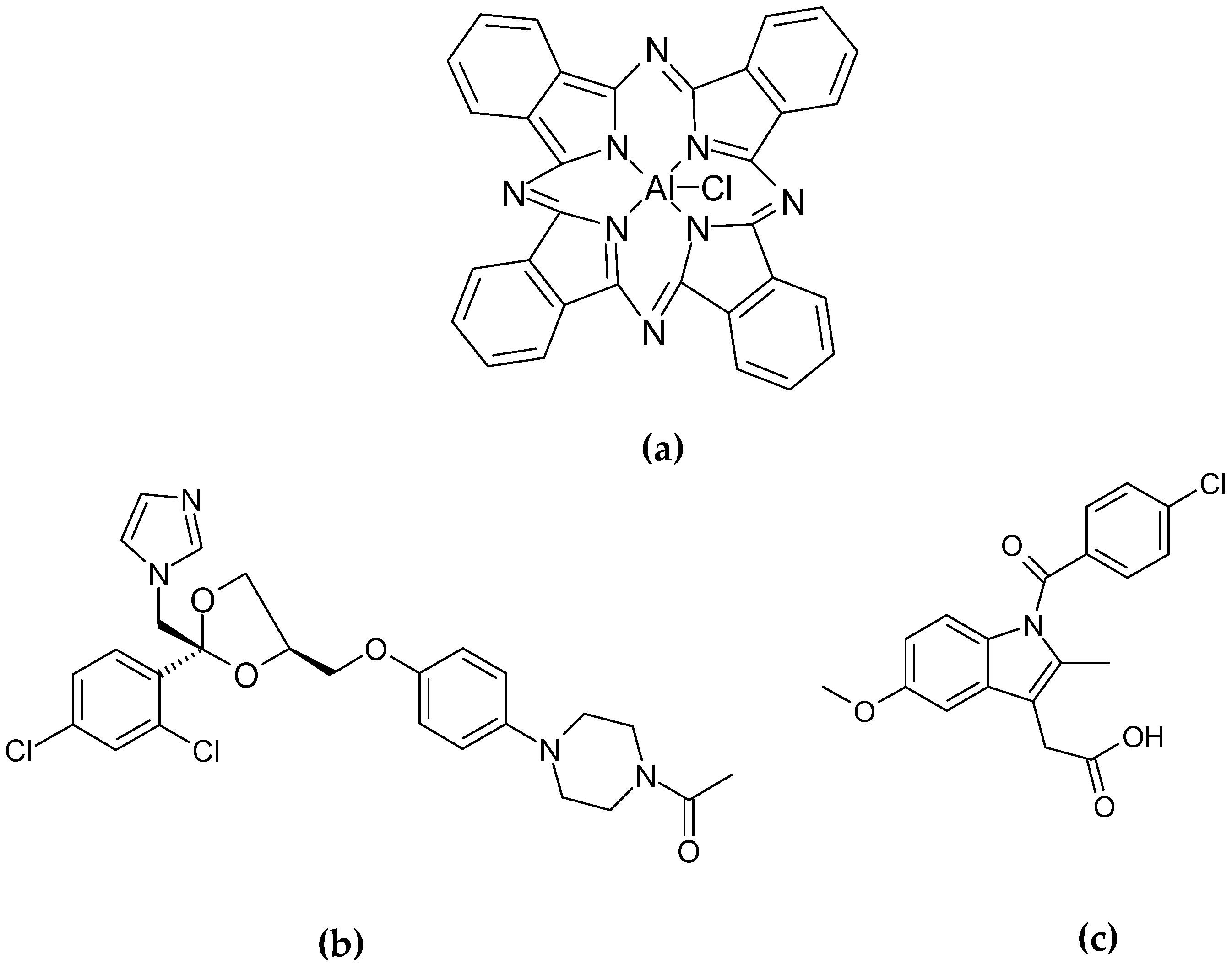
Authors have efficiently encapsulated ClAlPc (
Figure 14a)—a second-generation photosensitizer agent usually used in PDT—into soybean oil GLN dispersed in aqueous medium. Nanoparticles were jellified and stabilized by HSA and PEI, respectively. They were obtained by hot homogenization using ultrasound probe and then cooling at room temperature until obtaining a stable GLN dispersion. Nanoparticles showed mean diameter of 280 nm, narrow size distribution (PDI ~ 0.3), positive ζ potential value (~+50 mV), and spherical morphology (
Table 2). Dispersions exhibited pronounced physical stability after a 6-month aging period. The PEI and HSA proportions allowed the GLN size, charge, and stability to be controlled, resulting from the complex electrostatic interactions between the positively-charged PEI and the negatively-charged HSA fibers present on the gelled particles’ surface on the one hand and stearic repulsions between positively-charged GLN on the other hand.
Martin et al. studied the release profile and efficiency of GLN obtained by the same preparation process as Siqueira-Mourra et al. [
24]. In the first step, they used DLS and differential scanning calorimetry (DSC) to select the most suitable formulations by optimizing the proportion of ingredients (HSA, PVA, castor oil) to obtain particles of the smallest size and greatest stability. Two lipophilic drug models—indomethacin (
Figure 14b) and ketoconazole (
Figure 14c)—were entrapped in the GLS made of castor oil and gelled by HSA.
Thermal studies confirmed that there was no significant alteration of gelation phenomenon in presence of the entrapped drugs. Very stable dispersions were obtained (>3 months), with GLN presenting a mean diameter between 240 and 300 nm. High encapsulation efficiency (>98%) was measured for indomethacin and ketoconazole (
Table 2). The release profile determined by in vitro dialysis showed an immediate release of the drug from the organogel nanoparticles, due to rapid diffusion.
All these studies demonstrate the interest in the colloidal dispersions of GLN for dermo-cosmetic applications as sun protection systems and for drug delivery for lipophilic active substances.
5. Conclusions
Since lipophilic drug formulation remains the major challenge in the pharmaceutical field, recent preparations of colloidal dispersions of GLN present very interesting advantages as drug delivery formulations as well as in the cosmetic industry. These dispersions have promising stability properties, are easily formulated, and can be produced with natural organogelators or simple synthetic polymers, giving them a great range of possible industrial applications. The basic knowledge of these colloidal GLN dispersions, their properties, and characterization methods will allow the optimization of the formulation with regards to obtaining more stable dispersions according to their intended applications. Furthermore, they are still a broad area of possibilities in the synthesis of new organogelators, formulations of better and more stable dispersions, and drug immobilization. The interest for these materials will continue to grow in the future years.