Development of Novel N-isopropylacrylamide (NIPAAm) Based Hydrogels with Varying Content of Chrysin Multiacrylate
Abstract
:1. Introduction
2. Results and Discussion
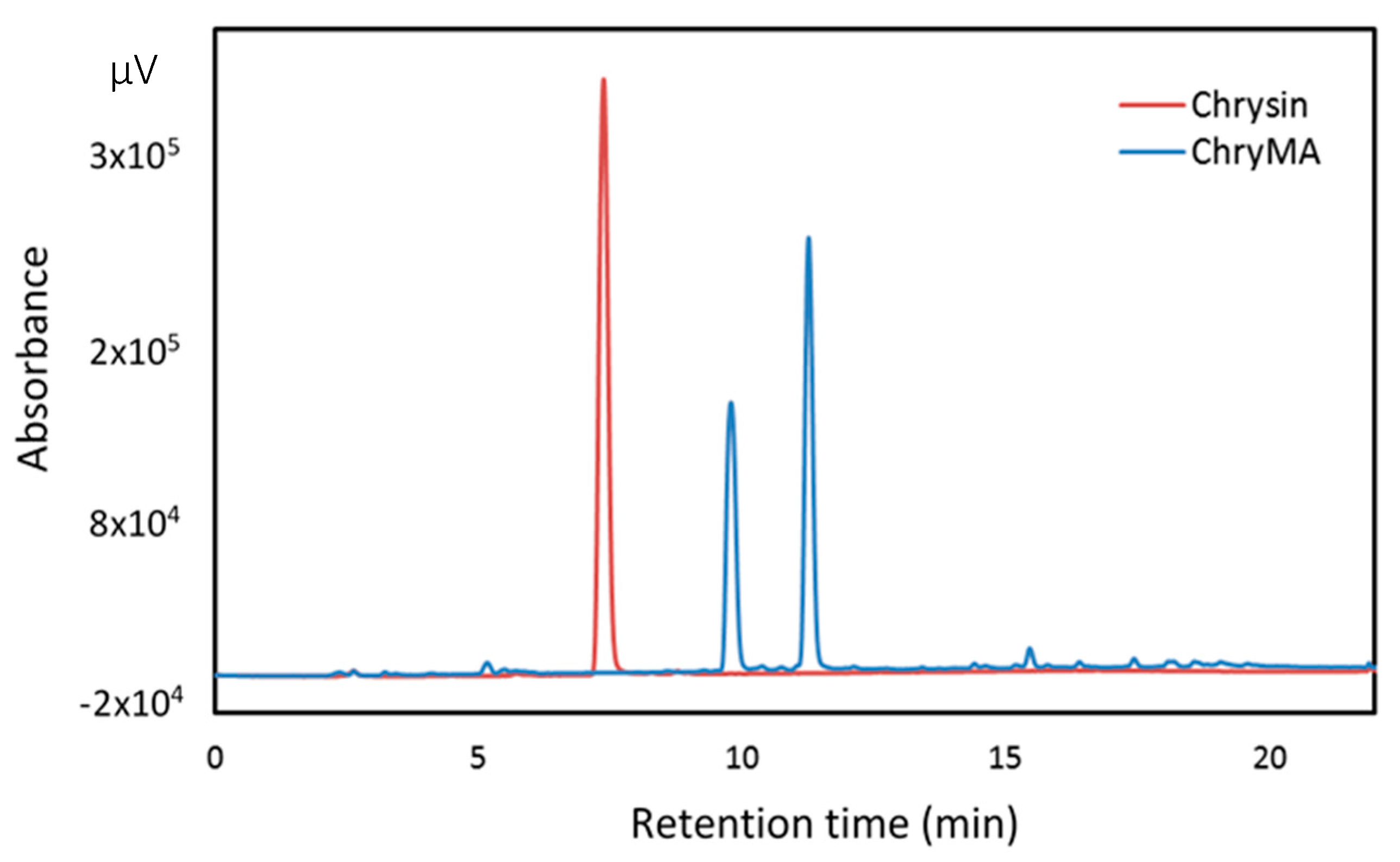
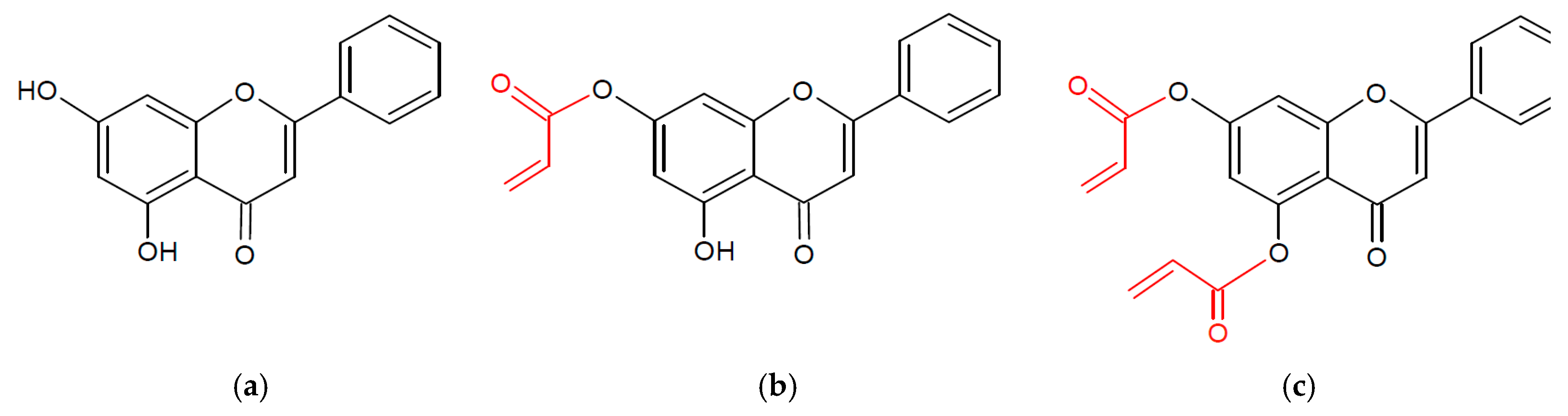
2.1. Characterization of ChryMA
2.2. Synthesis of NIPAAm-co-ChryMA Gels
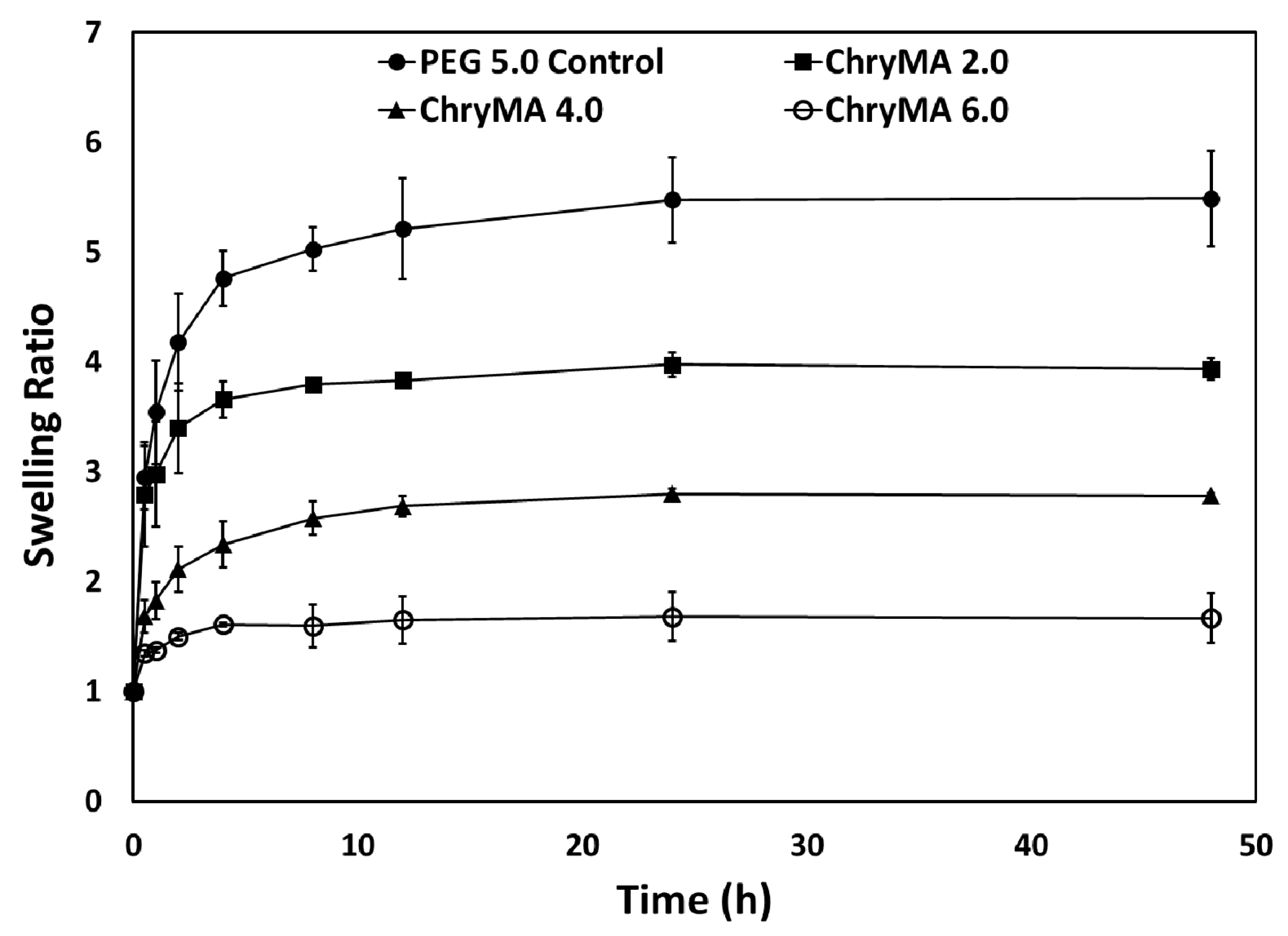
2.3. Kinetic Swelling Study
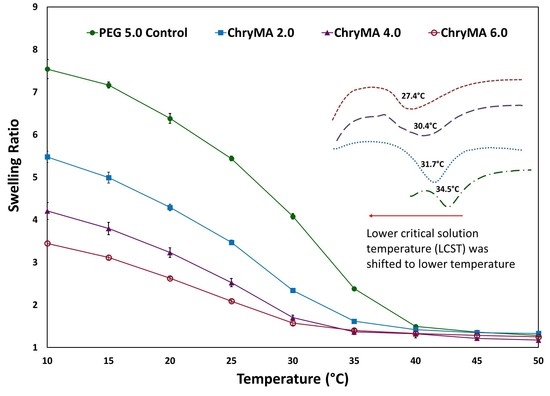
2.4. Temperature Dependent Swelling Study
2.5. LCST Measurements
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of ChryMA
4.3. Characterization of ChryMA Using High Performance Liquid Chromatography (HPLC)
4.4. Synthesis of NIPAAm-co-ChryMA Gels
4.5. Kinetic Swelling Study
4.6. Temperature Dependent Swelling Study
4.7. LCST Measurements
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lue, S.J.; Chen, C.-H.; Shih, C.-M. Tuning of lower critical solution temperature (LCST) of poly (N-isopropylacrylamide-co-acrylic acid) hydrogels. J. Macromol. Sci. Part B 2011, 50, 563–579. [Google Scholar] [CrossRef]
- Lee, W.-F.; Hsu, C.-H. Thermoreversible hydrogels: 3. Synthesis and swelling behavior of the (N-isopropylacrylamide-co-trimethylacrylamidopropyl ammonium iodide) copolymeric hydrogels. Polymer 1998, 39, 5393–5403. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Synthesis and Characterization of Thermo-and Chemomechanically Responsive Poly (N-isopropylacrylamide-co-methacrylic acid) Hydrogels. Macromolecules 1995, 28, 8016–8020. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.B.; Kim, S.J.; Lee, Y.M. Rapid temperature/pH response of porous alginate-g-poly (N-isopropylacrylamide) hydrogels. Polymer 2002, 43, 7549–7558. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, Y.; Tanaka, T. Volume phase transition in a non-ionic gel. In Proceedings of the AIP Conference, Steamboat Springs, CO, USA, 23–30 May 1984. [Google Scholar]
- Ogata, T.; Nonaka, T.; Kurihara, S. Permeation of solutes with different molecular size and hydrophobicity through the poly (vinyl alcohol)-graft-N-isopropylacrylamide copolymer membrane. J. Membr. Sci. 1995, 103, 159–165. [Google Scholar] [CrossRef]
- Hoffman, A.S. Environmentally sensitive polymers and hydrogels. MRS Bull. 1991, 16, 42–46. [Google Scholar] [CrossRef]
- Schild, H.G. Poly (N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Kuckling, D.; Wohlrab, S. Synthesis and characterization of biresponsive graft copolymer gels. Polymer 2002, 43, 1533–1536. [Google Scholar] [CrossRef]
- Safont, B.; Vitas, A.; Peñas, F. Biodegradation of phenol in a draft-tube spouted bed bioreactor with biomass attached to hydrogel particles. In Water Resources Management V; WIT Press: Southampton, UK, 2009; pp. 147–156. [Google Scholar]
- Ali, W.; Gebert, B.; Hennecke, T.; Graf, K.; Ulbricht, M.; Gutmann, J.S. Design of thermally responsive polymeric hydrogels for brackish water desalination: Effect of architecture on swelling, deswelling, and salt rejection. ACS Appl. Mater. Interfaces 2015, 7, 15696–15706. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Park, H.-K.; Park, H.; Lee, S.-H. Snapshot of phase transition in thermoresponsive hydrogel PNIPAM: Role in drug delivery and tissue engineering. Macromol. Res. 2016, 24, 297–304. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Díez-Peña, E.; Frutos, P.; Frutos, G.; Quijada-Garrido, I.; Barrales-Rienda, J.M. The influence of the copolymer composition on the diltiazem hydrochloride release from a series of pH-sensitive poly [(N-isopropylacrylamide)-co-(methacrylic acid)] hydrogels. AAPS PharmSciTech 2004, 5, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Walle, U.K.; Galijatovic, A.; Walle, T. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem. Pharmacol. 1999, 58, 431–438. [Google Scholar] [CrossRef]
- Comalada, M.; Ballester, I.; Bailón, E.; Sierra, S.; Xaus, J.; Gálvez, J.; de Medina, F.S.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure–activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Wolfman, C.; Viola, H.; Paladini, A.; Dajas, F.; Medina, J.H. Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coerulea. Pharmacol. Biochem. Behav. 1994, 47, 1–4. [Google Scholar] [CrossRef]
- Zeng, Y.-B.; Yang, N.; Liu, W.S.; Tang, N. Synthesis, characterization and DNA-binding properties of La (III) complex of chrysin. J. Inorg. Biochem. 2003, 97, 258–264. [Google Scholar] [CrossRef]
- Nielsen, S.; Breinholt, V.; Justesen, U.; Cornett, C.; Dragsted, L.O. In vitro biotransformation of flavonoids by rat liver microsomes. Xenobiotica 1998, 28, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Champ, S.; Huglin, M.B. Network and swelling parameters of chemically crosslinked thermoreversible hydrogels. Polymer 2001, 42, 3665–3669. [Google Scholar] [CrossRef]
- Velada, J.L.; Liu, Y.; Huglin, M.B. Effect of pH on the swelling behaviour of hydrogels based on N-isopropylacrylamide with acidic comonomers. Macromol. Chem. Phys. 1998, 199, 1127–1134. [Google Scholar] [CrossRef]
- Yoo, M.; Sung, Y.K.; Lee, Y.M.; Cho, C.S. Effect of polyelectrolyte on the lower critical solution temperature of poly (N-isopropyl acrylamide) in the poly (NIPAAm-co-acrylic acid) hydrogel. Polymer 2000, 41, 5713–5719. [Google Scholar] [CrossRef]
- Kim, S.; Healy, K.E. Synthesis and characterization of injectable poly (N-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules 2003, 4, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hoffman, A.S. Temperature-induced phase transition behaviors of random vs. graft copolymers of N-isopropylacrylamide and acrylic acid. Macromol. Rapid Commun. 1995, 16, 175–182. [Google Scholar] [CrossRef]
- Jones, M. Effect of pH on the lower critical solution temperatures of random copolymers of N-isopropylacrylamide and acrylic acid. Eur. Polym. J. 1999, 35, 795–801. [Google Scholar] [CrossRef]
- Zhang, J.; Peppas, N.A. Synthesis and characterization of pH-and temperature-sensitive poly (methacrylic acid)/poly (N-isopropylacrylamide) interpenetrating polymeric networks. Macromolecules 2000, 33, 102–107. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Pulsatile local delivery of thrombolytic and antithrombotic agents using poly (N-isopropylacrylamide-co-methacrylic acid) hydrogels. J. Control. Release 1996, 39, 57–64. [Google Scholar] [CrossRef]
- Tang, S.; Bhandari, R.; Delaney, S.P.; Munson, E.J.; Dziubla, T.D.; Hilt, J.Z. Synthesis and characterization of thermally responsive N-isopropylacrylamide hydrogels copolymerized with novel hydrophobic polyphenolic crosslinkers. Mater. Today Commun. 2017, 10, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Guan, X.X.; Zheng, J.; Guo, H.; Wu, K.; Liang, L.; Lu, M. Biodegradable, thermoresponsive PNIPAM-based hydrogel scaffolds for the sustained release of levofloxacin. RSC Adv. 2016, 6, 32967–32978. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Xue, W.; Hamley, I.W. Thermoreversible swelling behaviour of hydrogels based on N-isopropylacrylamide with a hydrophobic comonomer. Polymer 2002, 43, 3069–3077. [Google Scholar] [CrossRef]
- Singh, R.; Deshmukh, S.A.; Kamath, G.; Sankaranarayanan, S.K.R.S.; Balasubramanian, G. Controlling the aqueous solubility of PNIPAM with hydrophobic molecular units. Comput. Mater. Sci. 2017, 126, 191–203. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Liu, Z.; Ju, X.; Shi, K.; Xie, R.; Wang, W.; Cheng, Z.; Chu, L. Gamma-Cyclodextrin-Recognition-Responsive Characteristics of Poly (N-isopropylacrylamide)-Based Hydrogels with Benzo-12-crown-4 Units as Signal Receptors. Macromol. Chem. Phys. 2016, 218. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Sun, G.M.; Wu, D.Q.; Chu, C.C. Synthesis and characterization of partially biodegradable and thermosensitive hydrogel. J. Mater. Sci. Mater. Med. 2004, 15, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Cristea, M.; Ascenzi, P.; Fundueanu, G. Lower critical solution temperature versus volume phase transition temperature in thermoresponsive drug delivery systems. Express Polym. Lett. 2011, 5, 839–848. [Google Scholar] [CrossRef]
- Boutris, C.; Chatzi, E.; Kiparissides, C. Characterization of the LCST behaviour of aqueous poly (N-isopropylacrylamide) solutions by thermal and cloud point techniques. Polymer 1997, 38, 2567–2570. [Google Scholar] [CrossRef]
- Gupta, P.; Jordan, C.T.; Mitov, M.I.; Butterfield, D.A.; Hilt, J.Z.; Dziubla, T.D. Controlled curcumin release via conjugation into PBAE nanogels enhances mitochondrial protection against oxidative stress. Int. J. Pharm. 2016, 511, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Authimoolam, S.P.; Hilt, J.Z.; Dziubla, T.D. Quercetin conjugated poly (β-amino esters) nanogels for the treatment of cellular oxidative stress. Acta Biomater. 2015, 27, 194–204. [Google Scholar] [CrossRef] [PubMed]

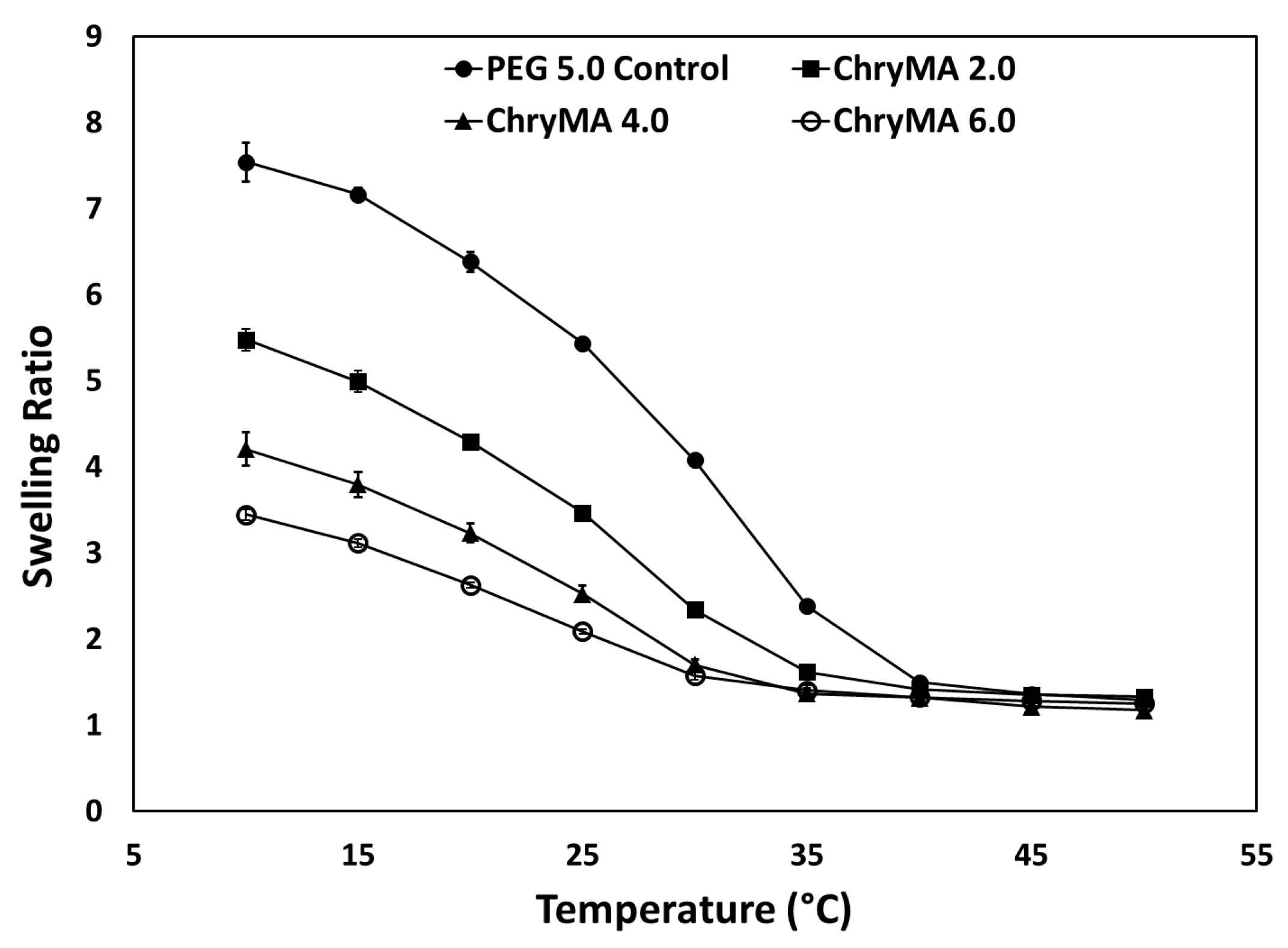
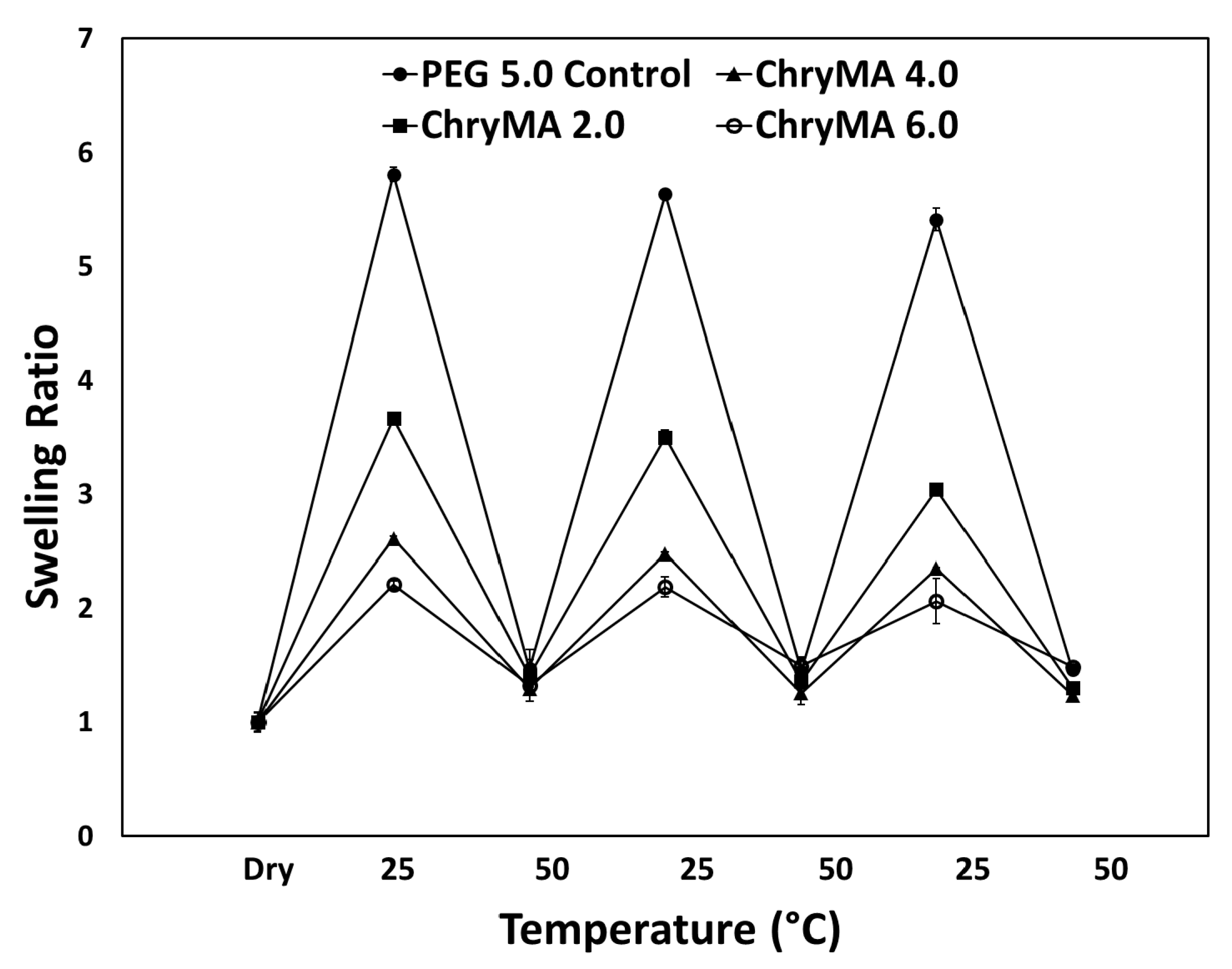
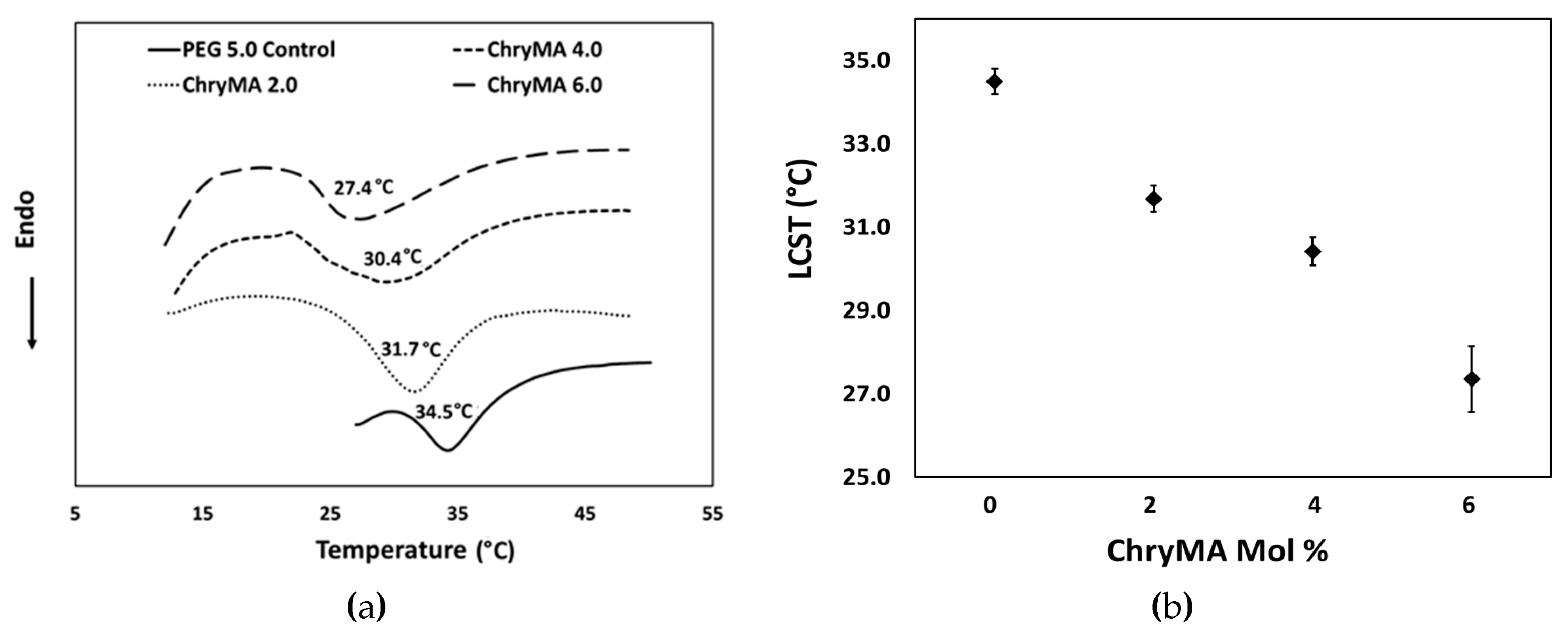
| Sample | NIPAAm mol % | ChryMA mol % | PEG400DMA mol % | Equilibrium q (25 °C) | LCST (°C) |
|---|---|---|---|---|---|
| Control | 95.0 | 0.0 | 5.0 | 5.5 | 34.5 |
| ChryMA 2.0 | 93.0 | 2.0 | 5.0 | 3.8 | 31.7 |
| ChryMA 4.0 | 91.0 | 4.0 | 5.0 | 2.6 | 30.4 |
| ChryMA 6.0 | 89.0 | 6.0 | 5.0 | 1.7 | 27.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Floy, M.; Bhandari, R.; Dziubla, T.; Hilt, J.Z. Development of Novel N-isopropylacrylamide (NIPAAm) Based Hydrogels with Varying Content of Chrysin Multiacrylate. Gels 2017, 3, 40. https://doi.org/10.3390/gels3040040
Tang S, Floy M, Bhandari R, Dziubla T, Hilt JZ. Development of Novel N-isopropylacrylamide (NIPAAm) Based Hydrogels with Varying Content of Chrysin Multiacrylate. Gels. 2017; 3(4):40. https://doi.org/10.3390/gels3040040
Chicago/Turabian StyleTang, Shuo, Martha Floy, Rohit Bhandari, Thomas Dziubla, and J. Zach Hilt. 2017. "Development of Novel N-isopropylacrylamide (NIPAAm) Based Hydrogels with Varying Content of Chrysin Multiacrylate" Gels 3, no. 4: 40. https://doi.org/10.3390/gels3040040