Stimuli-Responsive Cationic Hydrogels in Drug Delivery Applications
Abstract
:1. Introduction
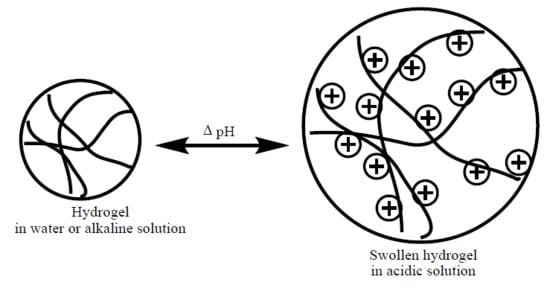
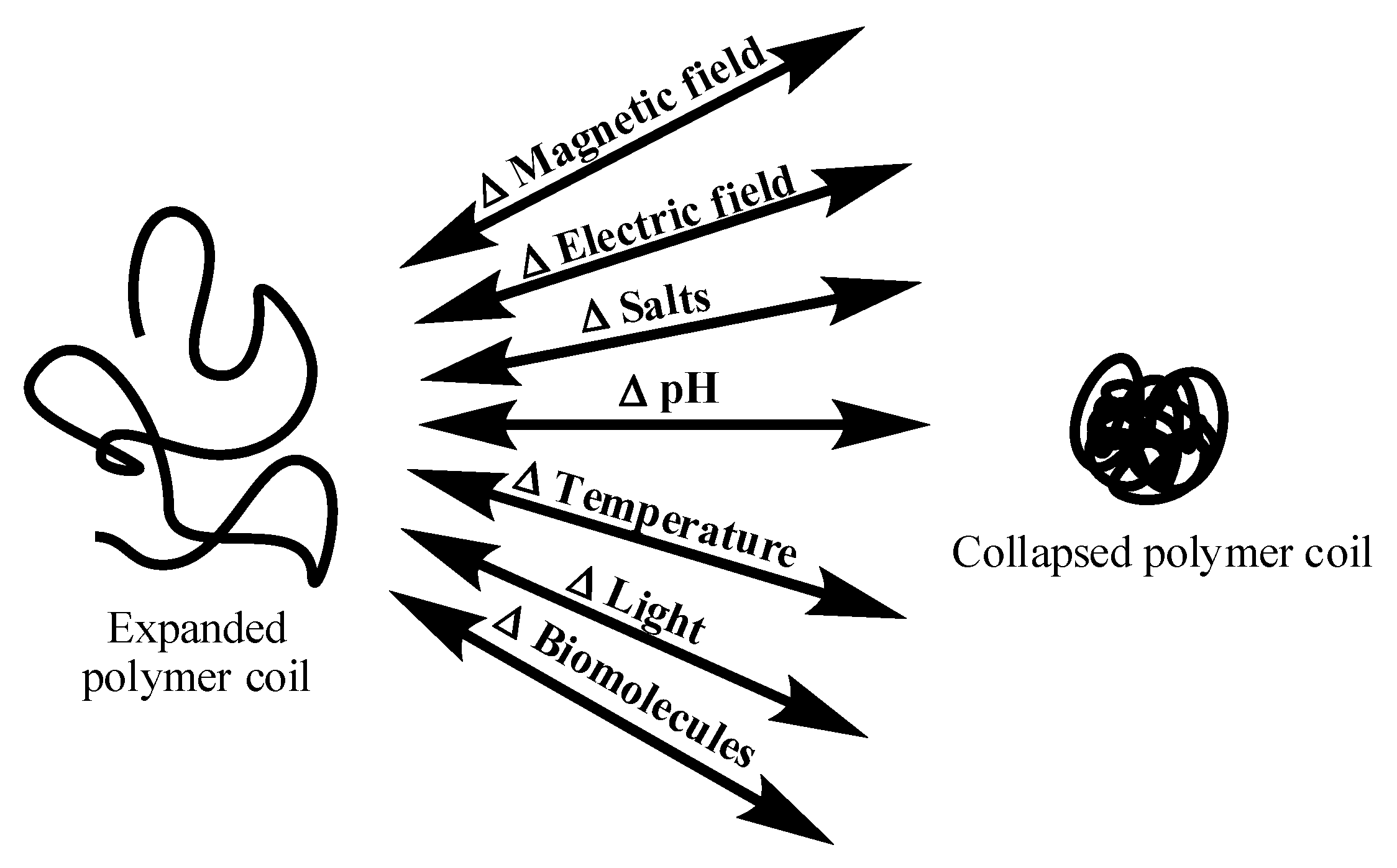
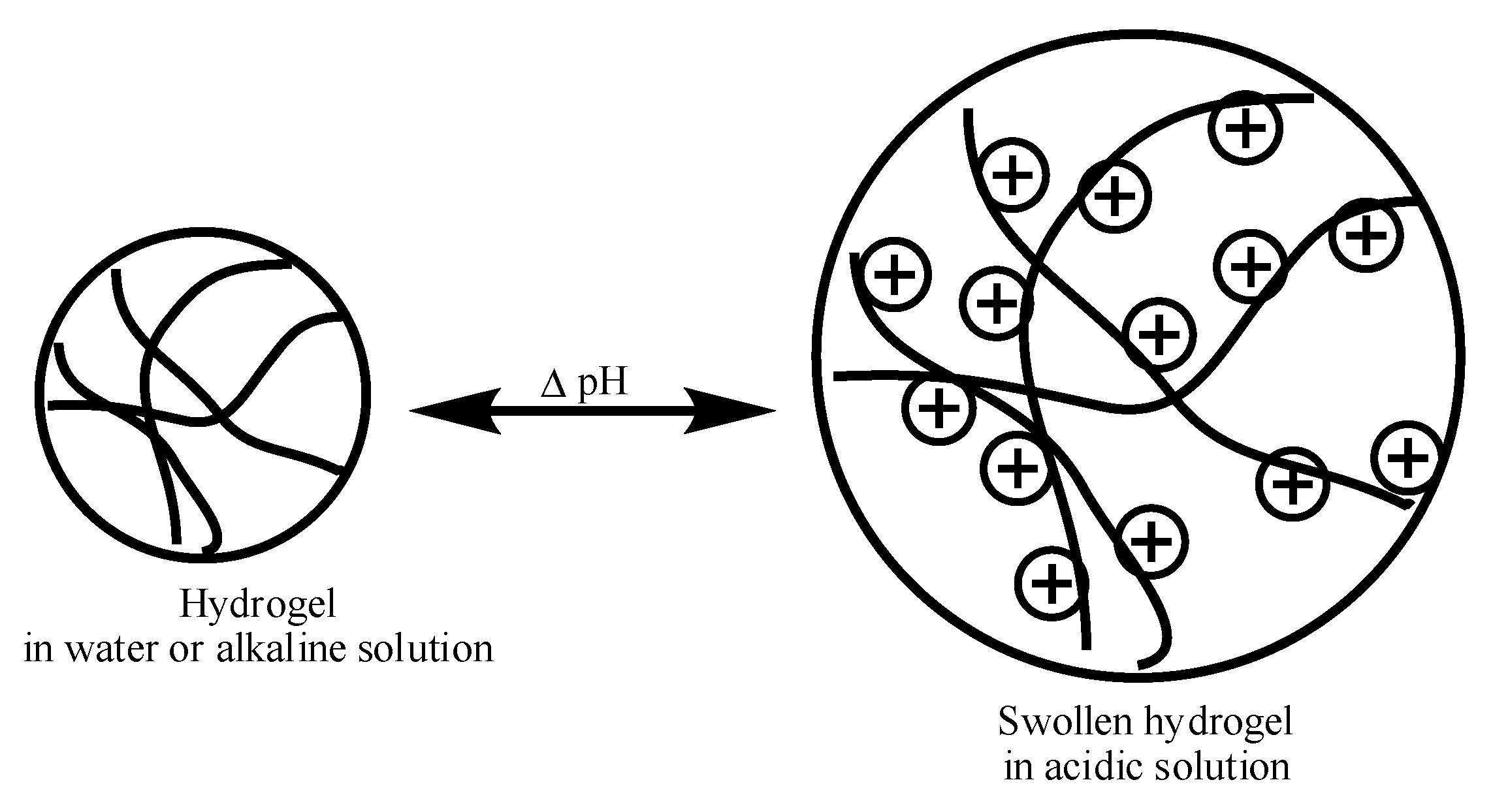
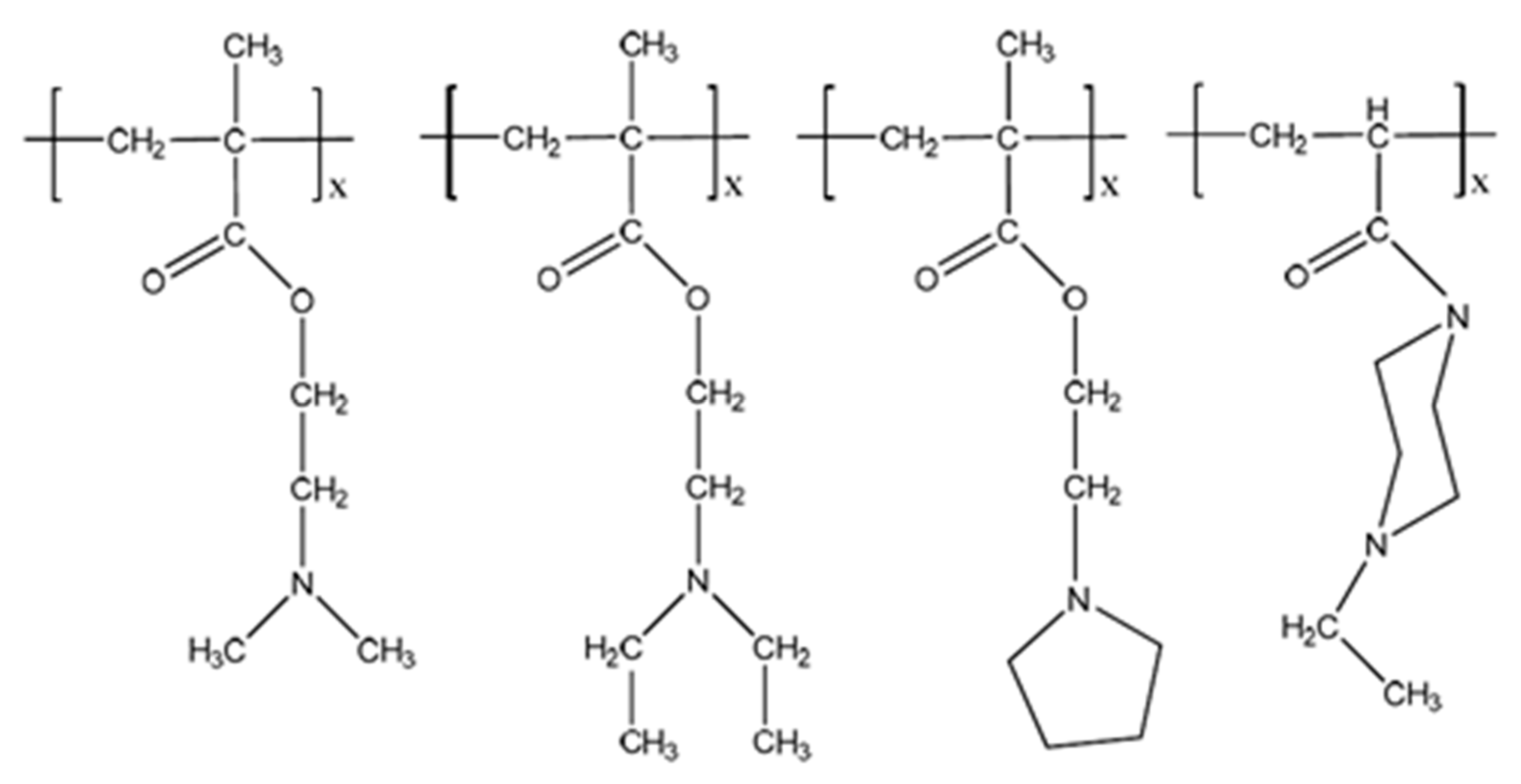
2. Swelling Behavior of Cationic Hydrogels
3. Applications of Cationic Hydrogels
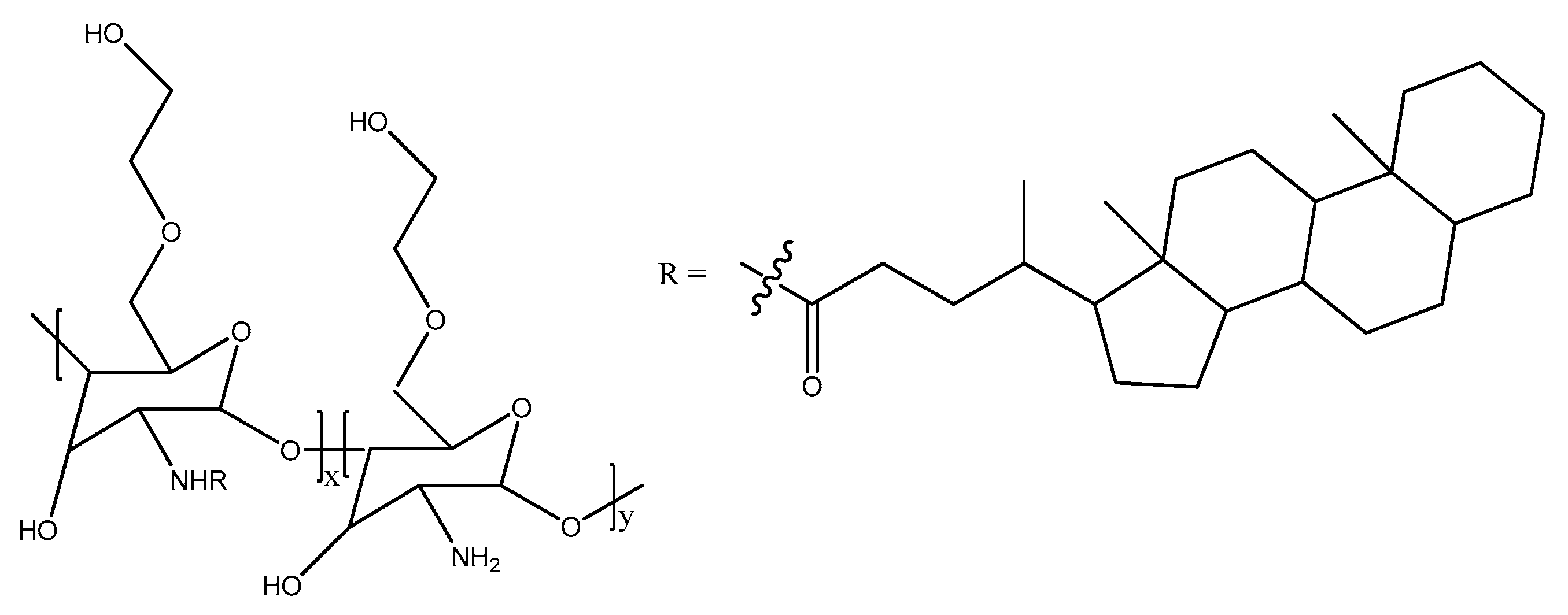
3.1. Drug Delivery Systems
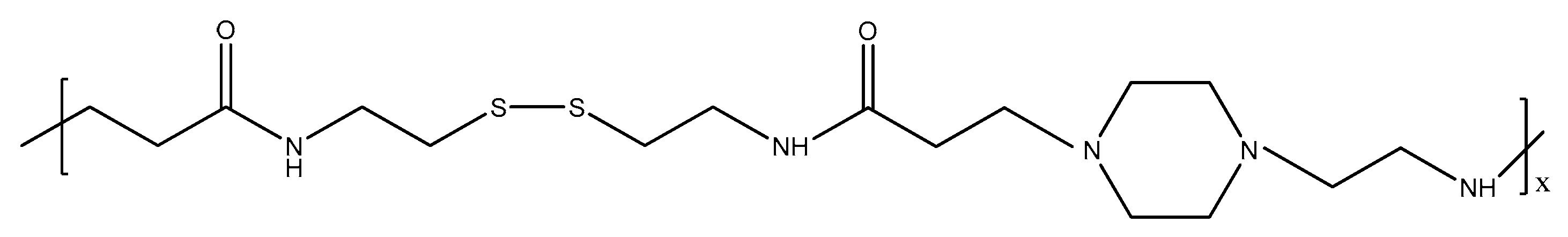
3.2. Gene Delivery Systems
- (1)
- Complexation for DNA with the cationic polymer/hydrogel. The DNA-cationic polymer/hydrogel is termed as a polyplex.
- (2)
- Addition of the polyplex to the cell containing the defective DNA for a certain period of time.
- (3)
- Release of DNA into the cytoplasm and removal of cationic polymer/hydrogel.
- (4)
- Transfer of DNA into nucleus. This step involves incubation for a period of time until the desired results are obtained.
3.3. Tissue Engineering
3.4. Nanoparticles and Microparticles Based on Cationic Polymers
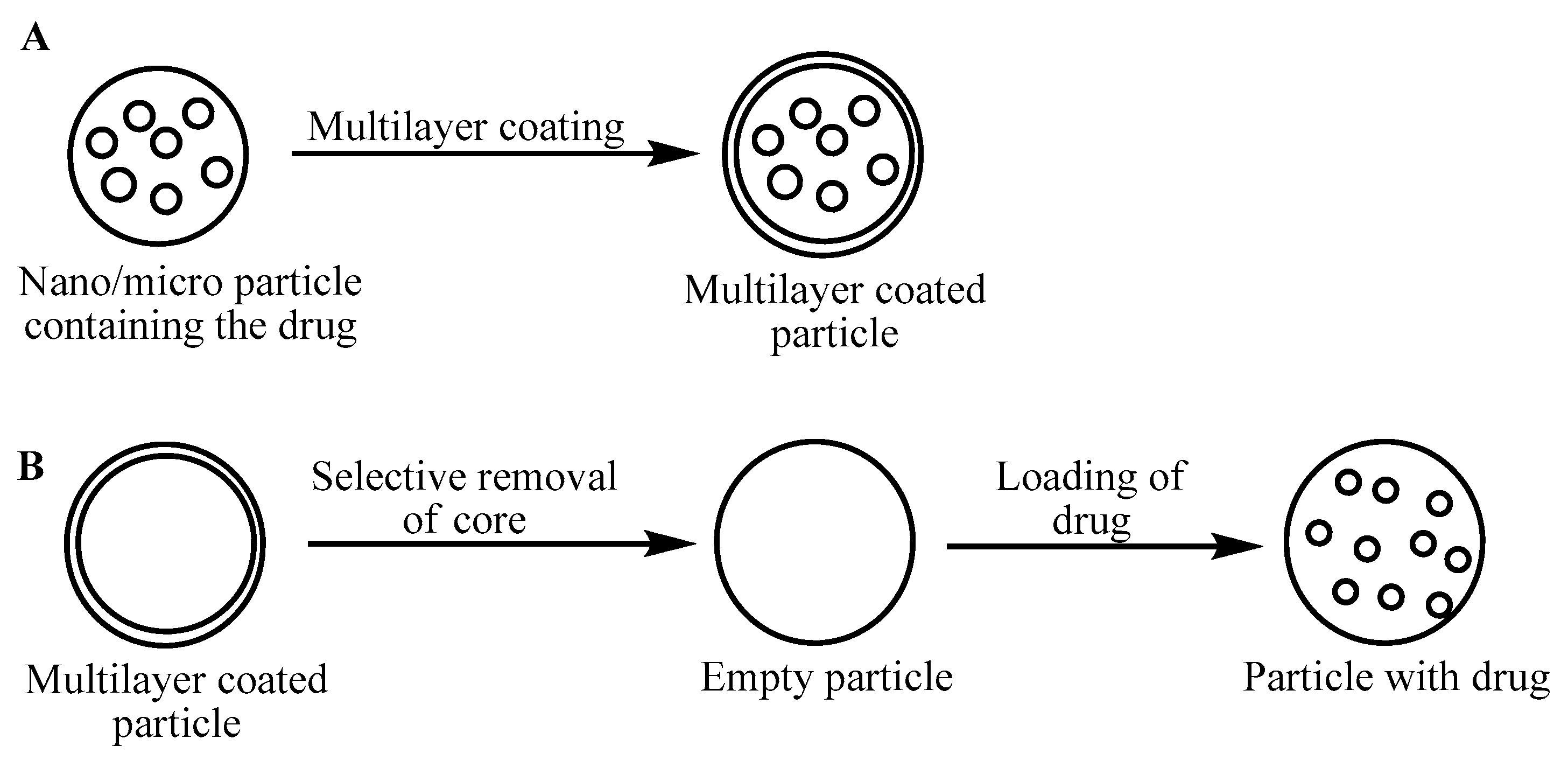
3.5. Multilayer Films or Coatings
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Roshan Deen, G.; Mah, C.H. Influence of external stimuli on the network properties of cationic poly(N-acryloyl-N′-propyl piperazine) hydrogels. Polymer 2016, 89, 55–68. [Google Scholar] [CrossRef]
- Tang, S.; Floy, M.; Bhandari, R.; Dziubla, T.; Zach Hilt, J. Development of novel N-isopropylacrylamide (NIPAAm) based hydrogels with varying content of chrysin multiacrylate. Gels 2017, 3, 40. [Google Scholar] [CrossRef]
- Stayton, P.S.; Shimoboji, T.; Long, C. Control of protein-ligand recognition using a stimuli-responsive polymer. Nature 1995, 378, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Bae, Y.H.; Kim, S.W. Biodegradable block copolymers as injectable drug delivery systems. Nature 1997, 388, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Hatefi, A.; Amsden, B. Biodegradable injectable in-situ forming drug delivery systems. J. Control Release 2002, 80, 9–28. [Google Scholar] [CrossRef]
- Pasparakis, G.; Vamvakaki, M. Multiresponsive polymers: Nano-sized assemblies, stimuli-sensitive gels and smart surfaces. Polym. Chem. 2011, 2, 1234–1248. [Google Scholar] [CrossRef]
- Ahn, S.; Kasi, R.M.; Kim, S.; Sharma, N.; Zhou, Y. Stimuli-responsive polymer gels. Soft Matter 2008, 4, 1151–1157. [Google Scholar] [CrossRef]
- Almdal, K.; Dyre, J.; Hvidt, S.; Kramer, O. Towards a phenomenological definition of the term ‘gel’. Polym. Gels Netw. 1993, 1, 5–17. [Google Scholar] [CrossRef]
- Tanaka, T. Collapse of Gels and the Critical Endpoint. Phys. Rev. Lett. 1978, 40, 820–823. [Google Scholar] [CrossRef]
- Vihola, H.; Laukkanen, A.; Tenhu, H.; Hirvonen, J. Drug release chracteristics of physically cross-linked thermosensitive poly(N-vinylcaprolactam) hydrogel particles. J. Pharm. Sci. 2008, 97, 4783–4793. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Del. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudosn, S.M. Stimuli-responsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Aguilar, M.R.; Elvira, C.; Gallardo, A.; Vázquez, B.; Román, J.S. Smart polymers and their applications as biomaterials. Top. Tissue Eng. 2007, 3, 1–27. [Google Scholar]
- Katchalsky, A. Rapid swelling and deswelling of reversible gels of polymeric acids by ionization. Cell. Mol. Life Sci. 1949, 5, 319–320. [Google Scholar] [CrossRef]
- Tanaka, T. Phase transition in gels and a single polymer. Polymer 1979, 20, 1404–1412. [Google Scholar] [CrossRef]
- Firestone, B.A.; Siegel, R.A. Dynamic pH-dependent swelling of a hydrophobic polyelectrolyte gel. Polym. Commun. 1988, 29, 204–208. [Google Scholar]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Ferruti, P.; Marchisio, M.A.; Duncan, R. Poly(amido-amine)S: Biomedical applications. Macromol. Rapid Commun. 2002, 23, 332–355. [Google Scholar] [CrossRef]
- Gan, L.H.; Gan, Y.Y.; Roshan Deen, G. Poly(N-acryloyl-N′-propyl piperazine): A new stimuli-responsive polymer. Macromolecules 2000, 33, 7893–7897. [Google Scholar] [CrossRef]
- Bulmus, V.; Ding, Z.; Long, C.J.; Stayton, P.S.; Hoffman, A.S. Site-specific polymer-streptavidin bioconjugate for pH-controlled binding and triggered release of biotin. Bioconjug. Chem. 2000, 11, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Brazel, C.S.; Peppas, N.A. Synthesis and Characterization of thermo- and chemomechanically responsive poly(isopropylacrylamide-co-methacrylic acid) hydrogels. Macromolecules 1995, 28, 8016–8020. [Google Scholar] [CrossRef]
- Roshan Deen, G.; Gan, L.H. Determination of reactivity ratios and swelling characteristics of stimuli-responsive copolymers of N-acryloyl-N′-ethyl piperazine and MMA. Polymer 2006, 47, 5025–5034. [Google Scholar] [CrossRef]
- Roshan Deen, G.; Chua, V.; Ilyas, U. Synthesis, swelling properties and network structure of new stimuli-responsive poly(N-acryloyl-N′-ethyl piperazine-co-N-isopropylacrylamide) hydrogels. J. Polym. Sci. Polym. Chem. 2012, 50, 3363–3372. [Google Scholar] [CrossRef]
- Roshan Deen, G.; Lim, E.K.; Mah, C.H.; Heng, K.M. New cationic linear copolymers and hydrogels of N-vinyl caprolactam and N-acryloyl-N′-ethyl piperazine: Synthesis, reactivity, influence of external stimuli on the LCST and swelling properties. Ind. Eng. Chem. Res. 2012, 51, 13354–13365. [Google Scholar] [CrossRef]
- Gan, L.H.; Roshan Deen, G.; Loh, X.J.; Gan, Y.Y. New stimuli-responsive copolymers of N-acryloyl-N′-alkyl piperazine and methyl methacrylate. Polymer 2001, 42, 65–69. [Google Scholar] [CrossRef]
- Roshan Deen, G.; Lee, T.T. New pH-responsive linear and crosslinked functional copolymers of N-acryloyl-N′-phenyl piperazine with acrylic acid and hydroxyethyl methcrylate: Synthesis, reactivity, and effect of steric hindrance on swelling. Polym. Bull. 2012, 69, 827–846. [Google Scholar] [CrossRef]
- Verestiuc, L.; Ivanov, C.; Barbu, E.; Tsibouklis, J. Dual-stimuli-responsive hydrogels based on poly(N-isopropylacrylamide)/chitosan semi-interpenetrating networks. Int. J. Pharm. 2004, 269, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, N.; Elvira, C.; Roman, S.J. Novel dual-stimuli-responsive polymers derived from ethylpyrrolidine. Macromolecules 2005, 38, 9298–9303. [Google Scholar] [CrossRef]
- Kurata, K.; Dobashi, A. Novel temperature and pH-responsive linear polymers and crosslinked hydrogels comprised of acidic l-α-amino acid derivatives. J. Macromol. Sci. Part A Pure Appl. Chem. 2004, 41, 143–164. [Google Scholar] [CrossRef]
- Roshan Deen, G.; Gan, Y.Y.; Gan, L.H.; Teng, S.H. New functional copolymers of N-acryloyl-N′-methyl piperazine and 2-hydroxyethyl methacrylate: Synthesis, determination of reactivity ratios and swelling characteristics of gels. Polym. Bull. 2011, 66, 301–313. [Google Scholar] [CrossRef]
- Velasco, D.; Elvira, C.; Román, J.S. New stimuli-responsive polymers derived from morpholine and pyrrolidine. J. Mater. Sci. Mater. Med. 2008, 19, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Oupicky, D.; Parker, A.L.; Seymour, L.W. Laterally stabilized complexes of DNA with linear reducible polycations: Strategy for triggered inracellular activation of DNA delivery vectors. J. Am. Chem. Soc. 2002, 124, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.B.; Kim, S.M.; Lee, Y.; Lee, W.K.; Yang, T.G.; Lee, M.J.; Suh, H.; Park, J.S. Cationic hyperbranched poly(amino ester): A novel class of DNA condensing molecule with cationic surface, biodegradable three-dimensional structure, and tertiary amine group in the interior. J. Am. Chem. Soc. 2001, 123, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, E.S.; Tanaka, T. Kinetic of discontinuous volume-phase transition of gels. J. Chem. Phys. 1988, 89, 1695–1703. [Google Scholar] [CrossRef]
- Dušek, K.; Patterson, D. Transition in swollen polymer network induced by intermolecular condensation. J. Polym. Sci. Part A-2 1986, 6, 1209–1216. [Google Scholar] [CrossRef]
- Flory, P.J. Phase equilibria in polymer systems: Swelling of network structures. In Principles of Polymer Chemistry; Cornell University: Ithaca, NY, USA, 1953. [Google Scholar]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys. 1943, 11, 521–546. [Google Scholar] [CrossRef]
- Ricka, J.; Tanaka, T. Swelling of ionic gels: Quantitative performance of the Donnan theory. Macromolecules 1984, 17, 2916–2921. [Google Scholar] [CrossRef]
- Vasheghani-Farahani, E.; Vera, J.H.; Cooper, D.G.; Weber, M.E. Swelling of ionic gels in electrolyte solutions. Ind. Eng. Chem. Res. 1990, 29, 554–560. [Google Scholar] [CrossRef]
- Patil, V.R.; AMiji, M.M. Preparation and characterization of freeze-dried chitosan-poly(ethylene oxide) hydrogels for site-specific antibiotic delivery in the stomach. Pharm. Res. 1996, 13, 588–593. [Google Scholar] [CrossRef]
- Gallardo, A.; Rodriguez, G.; Aguilar, M.R.; Fernandez, M.M.; Román, S.J. A kinetic model to explain the zero-order release of drugs from ionic polymer drug conjugates: Application to AMPS-Triflusal-derived polymeric drugs. Macromolecules 2003, 36, 8876–8880. [Google Scholar] [CrossRef]
- Kamada, H.; Tsutsumi, Y.; Yoshioka, Y. Design of a pH-Sensitive Polymeric Carrier for Drug Release and its Application in Cancer Therapy. Clin. Cancer Res. 2004, 10, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Almeda, D.; Ye, G.J.C.; Auguste, D.T. Bioresponsive matrices in drug delivery. J. Biol. Eng. 2010, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gua, S.; Qiao, Y.; Wang, W.; He, H.; Deng, L.; Xing, J.; Xu, J.; Liang, X.J.; Dong, A. Poly(ε-caprolactone)-graft-poly(2-N,N-dimethylamino) ethyl methacrylate) nanoparticles: PH dependent thermo-sensitive multifunctional carriers for gene and drug delivery. J. Mater. Chem. 2010, 20, 6935–6941. [Google Scholar] [CrossRef]
- Zhu, C.; Jung, S.; Luo, S.; Meng, F.; Zhu, X.; Park, T.G.; Zhong, Z. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA-PCL-PDMAEMA triblock copolymers. Biomaterials 2010, 31, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.N.; Du, F.S.; Cheng, J.; Dong, Y.Q.; Liang, D.H.; Ji, S.P.; Lin, S.S.; Li, Z.C. Acid-sensitive polymeric micelles based on thermoresponsive block copolymers with pendent cyclic orthoester groups. Macromolecules 2009, 42, 783–790. [Google Scholar] [CrossRef]
- Hruby, M.; Filippov, S.K.; Panke, J.; Novakova, M.; Mackova, H.; Kucka, J.; Vetvicka, D.; Ulbrich, K. Polyoxazoline thermoresponsive micelles as radionuclide delivery systems. Macromol. Biosci. 2010, 10, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Lagvinac, N.; Nichols, J.L.; Ferruti, P.; Duncan, R. Poly(amidoamine) Conjugates Containing Doxorubicin Bound via an Acid-Sensitive Linker. Macromol. Biosci. 2009, 9, 480–487. [Google Scholar] [Green Version]
- Song, Y.; Zhong, L.; Gan, W.; Zhou, J.; Zhang, L. Self-assembled micelles based on hydrophobically modified quaternized cellulose for drug delivery. Colloids Surf. B 2011, 83, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, J.; Nakayama, M.; Sakai, K.; Okano, T. Temperature-induced intracellular uptake of thermoresponsive polymeric micelles. Biomacromolecules 2009, 10, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, V.; Limer, A.J.; Haddleton, D.M.; Catalina, F.; Peinado, C. Biodegradable and thermoresponsive micelles of triblock copolymers based on 2-(N,N-dimethylamino)ethyl methacrylate and epsilon-caprolactam for controlled drug delivery. Eur. Polym. J. 2008, 44, 3853–3863. [Google Scholar] [CrossRef]
- Godbey, W.T.; Mikos, A.G. Recent progress in gene delivery using non-virial transfer complexes. J. Control Release 2001, 72, 115–125. [Google Scholar] [CrossRef]
- Lim, Y.B.; Choi, Y.H.; Park, J.S. A self-destroying polycationic polymer: Biodegradable poly(4-hydroxyl-l-proline ester). J. Am. Chem. Soc. 1999, 121, 5633–5639. [Google Scholar] [CrossRef]
- Putnam, D.; Langer, R. Poly(4-hydroxyl-l-proline ester): Low temperature polycondensation and plasmid DNA complexation. Macromolecules 1999, 32, 3658–3662. [Google Scholar] [CrossRef]
- Lim, Y.B.; Kim, C.H.; Kim, K.; Kim, S.W.; Park, J.S. Development of a safe gene delivery system using biodegrdable polymer, poly[α-(4-aminobutyl)-l-glycolic acid]. J. Am. Chem. Soc. 2000, 122, 6524–6525. [Google Scholar] [CrossRef]
- Lynn, D.M.; Langer, R. Degradable poly(β-amino esters): Synthesis, characterization, and self-assembly with plasmid DNA. J. Am. Chem. Soc. 2000, 122, 10761–10768. [Google Scholar] [CrossRef]
- Emilitri, E.; Ranucci, E.; Ferruti, P. New poly(amidoamine)s containing disulfide linkages in their main chain. J. Polym. Sci. Polym. Chem. 2005, 43, 1404–1416. [Google Scholar] [CrossRef]
- Richardson, S.C.W.; Pattrick, N.G.; Man, Y.K.S.; Ferruti, P.; Duncan, R. Poly(amido-amine)s as potential nonviral vectors: Ability to form interpolyelectrolyte complexes and to mediate transfection in vitro. Biomacromolecules 2001, 2, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Ferruti, P.; Manzoni, S.; Richardson, S.C.W.; Duncan, R.; Pattrick, N.G.; Mendichi, R.; Casolaro, M. Amphoteric linear poly(amio-amine)s as endosomolytic polymers: Correlation between physicochemical and biological properties. Macromolecules 2000, 33, 7793–7800. [Google Scholar] [CrossRef]
- Ferruti, P.; Knobloch, S.; Ranucci, E.; Duncan, R.; Gianasi, E. A Novel Modification of poly(l-lysine) leading to a soluble cationic polymer with reduced toxicity and with potential as a transfection agent. Macromol. Chem. Phys. 1998, 199, 2565–2575. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoulach, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhong, Z.; Lok, M.C.; Jiang, X.; Hennink, W.E.; Feijen, J.; Engbersen, J.F.J. Linear poly(amido amine)s with secondary and tertiary amino groups and variable amounts of disulfide linkages: Synthesis and in vitro gene transfer properties. J. Control. Rel. 2006, 116, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.L.; van der Horst, A.; van Steenbergen, M.J.; Akeroyd, N.; van Nostrum, C.F.; Schoenmakers, P.J.; Hennink, W.E. Molar-mass characterization of cationic polymers for gene delivery by aqueous size-exclusion chromatrography. Pharm. Res. 2006, 23, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Stayton, P.S.; Hoffman, A.S.; Murthy, N. Molecular engineering of proteins and polymers for targeting and intracellular delivery of therapeutics. J. Control. Release 2000, 65, 203–220. [Google Scholar] [CrossRef]
- Vanessa, I.; Afsaneh, L.; Hasana, U. Lipid and Hydrophobic Modification of Cationic Carriers on Route to Superior Gene Vectors. Soft Matter 2010, 6, 2124–2138. [Google Scholar]
- Lim, Y.B.; Han, S.-A.; Kong, H.-U.; Park, J.-S.; Jeong, B.; Kim, S.W. Biodegradable polyester, Poly[α-(4-Aminobutyl)-I-Glycolic Acid]], as a Non-Toxic Gene Carrier. Pharm. Res. 2000, 17, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.L.; Meister, G.E.; Koerber, J.T.; Pack, D.W. Partial Acetylation of Polyethylenimine Enhances in vitro Gene Delivery. Pharm. Res. 2004, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; David, J.M. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Sangram, K.S.; Mamoni, D.; Sandra, V.V.; David, L.K.; Emo, C.; Clemens, V.B.; Lorenzo, M.; Peter, D. Cationic Polymers and their Therapeutic Potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar]
- Kuo, Y.C.; Ku, N. Appplication of Polyethyleneimine-modified Scaffolds to the regeneration of Cartilaginous Tissue. Biotechnol. Prog. 2009, 25, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Burguera, E.F.; Xu, H.H.K.; Amin, N.; Ryon, H.; Arola, D.D. Fatigue and Human Umbilical Cord Stem Cell Seeding Characteristics of Calcium Phosphate-chitosan-biodegradable Fiber Scaffolds. Biomaterials 2010, 31, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Tuzlakoglu, K.; Alves, C.M.; Mano, J.F.; Reis, R.L. Production and Characterization of Chitosan Fibers and 3-D Fiber Mesh Scaffolds for Tissue Engineering Applications. Macromol. Biosci. 2004, 4, 811–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsabahy, M.; Wooley, K.L. Design of Polymeric Nanoparticles for Biomedical Delivery. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Hoffart, V.; Lamprecht, A.; Maincent, P.; Lecompte, T.; Vigneron, C.; Ubrich, N. Oral bioavailability of a Low Molecular Weight Heparin using a Polymeric Delivery system. J. Control. Release 2006, 113, 38–42. [Google Scholar] [PubMed]
- Zhang, S.; Uludağ, H. Nanoparticle Systems for Growth Factor Delivery. Pharm. Res. 2009, 26, 1561–1580. [Google Scholar] [CrossRef] [PubMed]
- Hoffart, V.; Ubrich, N.; Lamprecht, A.; Bachelier, K.; Vigneron, C.; Lecompte, T.; Hoffman, M.; Maincent, P. Microencapsulation of Low Molecular weight Heparin into polymeric Particles Designed with Biodegradabel and Nonbiodegradable Polycationic Polymers. Drug Deliv. 2003, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Mohamdi-Samani, S.; Rafiei, P.; Ashrafi, H. A Pharmokinetic Overveiw of Nanotechnology based Drug Delivery System. J. Appl. Polym. Sci. 2012, 124, 4686–4693. [Google Scholar]
- Simakova, A.; Averick, S.E.; Konkolewicz, D.; Matyjaszewski, K. Aqueous ARGET ATRP. Macromolecules 2012, 45, 6371–6379. [Google Scholar] [CrossRef]
- Mahkam, M. Modified Chitosan Crosslinked Starch Polymers for Oral Insulin Delivery. J. Bioact. Compat. Polym. 2010, 25, 406–411. [Google Scholar] [CrossRef]
- Shao, Y.; Zhu, B.; Li, J.; Liu, X.; Tan, X.; Yang, X. Novel Chitosan Microshphere-Templated Microcapsules. Mater. Sci. Eng. C 2009, 29, 936–942. [Google Scholar] [CrossRef]
- El-Shabouri, M.H. Positively Charged Nanoparticles for Improving the Oral Bioavailability of Cyclosporin-A. Int. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Zubareva, A.; Ilyina, A.; Prokhorov, A.; Kurek, D.; Efremov, M.; Varlamov, V.; Senel, S.; Ignatyev, P.; Svirshchevskaya, E. Characterization of Protein and Peptide Binding to Nanogels Form3ed by Differently Charged Chitosan Derivatives. Molecules 2013, 18, 7848–7864. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Nottelet, B.; Patterer, M.; Francois, B.; Schott, M.; Domurado, M.; Garric, X.; Domurado, D.; Coudane, J. Nanoaggregates of Biodegradable Amphiphilic Randon Polycations for delivering Water-insoluble Drugs. Biomacromolecules 2012, 13, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Khopade, A.J.; Caruso, F. Electrostatically Assembled Polyelectrolyte/Dendrimer Multilayer Films as Ultrathin Nanoreservoirs. Nano Lett. 2002, 2, 415–441. [Google Scholar] [CrossRef]
- Tong, W.; Song, X.; Gao, C. Layer-by-Layer Assembly of Micorcapsules and their Biomedical Applications. Chem. Soc. Rev. 2012, 41, 6103–6124. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Donath, E.; Mohwald, H.; Shen, J. Spontaneous Deposition of Water-Soluble Substances into Microcapsules: Phenomenon, Mechanism, and Application. Angew. Chem. Int. Ed. 2002, 41, 3789–3793. [Google Scholar] [CrossRef]
- Ye, S.; Wang, C.; Liu, X.; Tong, Z. Chitosan in Nanostructured Thin Films. J. Control. Release 2005, 106, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Donath, E.; Mohwald, H. Permeability of Ibuprofen in Various Polyelectrolyte Multilayers. Macromol. Mater. Eng. 2001, 286, 591–597. [Google Scholar] [CrossRef]
- Ai, H.; Fang, M.; Jones, S.A.; Lvov, Y.M. Electrostatic Layer-by-Layer Nanoassembly on Biological Microtemplates: Platelets. Biomacromolecules 2002, 3, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Borodina, T.; Markvicheva, E.; Kunizhev, S.; Mohawld, H.; Sukhorukov, G.B.; Kreft, O. Controlled Release of DNA from Self-degrading Microcapsules. Macromol. Rapid Commun. 2007, 28, 1894–1899. [Google Scholar] [CrossRef]
- Ai, H.; Jones, S.A.; De Villiers, M.M.; Lvov, Y.M. Nano-encapsulation of Furosemide Microcrystals for Controlled Drug Release. J. Control. Release 2003, 86, 59–68. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deen, G.R.; Loh, X.J. Stimuli-Responsive Cationic Hydrogels in Drug Delivery Applications. Gels 2018, 4, 13. https://doi.org/10.3390/gels4010013
Deen GR, Loh XJ. Stimuli-Responsive Cationic Hydrogels in Drug Delivery Applications. Gels. 2018; 4(1):13. https://doi.org/10.3390/gels4010013
Chicago/Turabian StyleDeen, G. Roshan, and Xian Jun Loh. 2018. "Stimuli-Responsive Cationic Hydrogels in Drug Delivery Applications" Gels 4, no. 1: 13. https://doi.org/10.3390/gels4010013